Abstract
Levels of proinflammatory cytokines associate with risk for developing type 2 diabetes but whether chronic inflammation contributes to the development of diabetic complications, such as ESRD, is unknown. In the 1990s, we recruited 410 patients with type 2 diabetes for studies of diabetic nephropathy and recorded their characteristics at enrollment. During 12 years of follow-up, 59 patients developed ESRD (17 per 1000 patient-years) and 84 patients died without ESRD (24 per 1000 patient-years). Plasma markers of systemic inflammation, endothelial dysfunction, and the TNF pathway were measured in the study entry samples. Of the examined markers, only TNF receptors 1 and 2 (TNFR1 and TNFR2) associated with risk for ESRD. These two markers were highly correlated, but ESRD associated more strongly with TNFR1. The cumulative incidence of ESRD for patients in the highest TNFR1 quartile was 54% after 12 years but only 3% for the other quartiles (P<0.001). In Cox proportional hazard analyses, TNFR1 predicted risk for ESRD even after adjustment for clinical covariates such as urinary albumin excretion. Plasma concentration of TNFR1 outperformed all tested clinical variables with regard to predicting ESRD. Concentrations of TNFRs moderately associated with death unrelated to ESRD. In conclusion, elevated concentrations of circulating TNFRs in patients with type 2 diabetes at baseline are very strong predictors of the subsequent progression to ESRD in subjects with and without proteinuria.
Activation of the innate immune system and chronic low-grade inflammation are important components of the pathogenesis of type 2 diabetes (T2D).1,2 Risk of T2D increases with concentrations of circulating proinflammatory cytokines, such as TNFα and markers of systemic low-grade inflammation, such as IL-6 and C-reactive protein3–5; however, the mechanisms behind these associations are not fully explained.6 These observations prompted us to question whether chronic inflammation may also contribute to the development of complications of T2D.
TNFα is a pleiotropic cytokine that plays an essential role in mediating inflammatory processes.7–9 It is a transmembrane homotrimeric protein generated by many cells, including fat, endothelial, and white blood cells. Subsequently, TNFα and its receptors are shed from the cell surface by a disintegrin and metalloproteinase 17. In plasma, TNFα appears as free or bound to circulating TNF receptor 1 (TNFR1) and TNF receptor 2 (TNFR2) (collectively referred to as markers of the TNF pathway).
Hasegawa et al. were the first to implicate TNFα in the pathogenesis of diabetic nephropathy.7 Experimental studies of the roles of the TNF pathway in the development of diabetic nephropathy and other kidney diseases were recently reviewed.7–10 In addition to these laboratory studies, investigations in humans with T2D have associated the level of circulating markers of the TNF pathway with the risk of abnormal urinary albumin excretion, impaired renal function, and cardiovascular death.5,11–13 However, the authors of these studies did not seek to separate the effect of free TNFα from that of total TNFα or to investigate the independent effects of each of the TNFRs. Moreover, none of them studied ESRD, the ultimate outcome of diabetic nephropathy.
In this 8- to 12-year follow-up study of a cohort of patients with T2D, we examine the associations of the risk of ESRD or death unrelated to ESRD with circulating markers of the TNF pathway (free and total TNFα, TNFR1, and TNFR2), markers of endothelial dysfunction that are downstream from the TNF pathway (intercellular adhesion molecule-1 [ICAM-1], vascular cell adhesion molecule-1 [VCAM-1], plasminogen activator inhibitor-1 [PAI-1]), and markers of systemic inflammation (IL-6 and CRP).
Results
Characteristics of Patients according to Outcome
Between 1991 and 1995, a cohort with T2D was recruited into the Joslin Study of the Genetics of Type 2 Diabetes and Kidney Complications. Eligibility criteria included residence in Massachusetts, T2D diagnosed between ages 35 and 64 years, and age 40–69 years at examination. Of 600 patients selected, 509 were enrolled in the study and examined. For this study, only those with stored baseline plasma available for measuring creatinine and inflammatory markers were eligible. Furthermore, patients with evidence of nephropathy unrelated to diabetes and patients with stage 4 and stage 5 CKD14 were excluded. Thus, there were 410 patients, 85% of whom defined themselves as Caucasian.
At the end of 2004, 267 of the 410 patients (65.1%) remained alive after 8–12 years of follow-up. ESRD had developed in 59 patients (14.4%) and death had followed for 51 of them. Of the 59 with ESRD, 40 were registered with the US Renal Data System (USRDS) and 19 were not but died of renal failure as a primary or secondary cause. The two subgroups of patients with ESRD were almost identical with regard to clinical characteristics (Supplemental Table 1) and the distributions of plasma concentrations of the plasma markers (Supplemental Table 2); thus, they were analyzed as one group of patients with ESRD. The remaining 84 patients (20.5%) died without ESRD. Baseline characteristics are summarized in Table 1 according to three outcomes: alive, ESRD, and deceased without ESRD. Aside from differences among outcome groups with regard to characteristics related to long duration diabetes, the only difference was the much higher BP and albumin excretion rate (AER) in patients with an ESRD outcome. This group also had the lowest estimated GFR (eGFR).
Table 1.
Baseline characteristics of patients with T2D according to outcome during 8–12 years of follow-up
| Baseline Characteristic | Outcome | P Value | ||||
|---|---|---|---|---|---|---|
| Alive (n=267) | ESRD (n=59) | Deceased (n=84) | Alive Compared with ESRD | Alive Compared with Deceased | ESRD Compared with Deceased | |
| Male | 54.7 | 47.5 | 65.6 | 1.00 | 0.89 | 0.35 |
| Age (yr) | 54±10 | 59±8 | 60±7 | 0.003 | <10−5 | 1.00 |
| Body mass index (kg/m2) | 29.7±5.9 | 31.5±7.1 | 29.4±6.5 | 1.00 | 1.00 | 1.00 |
| Systolic BP (mmHg) | 134±17 | 143±20 | 134±18 | 0.013 | 1.00 | 0.21 |
| Serum cholesterol (mg/dl)a | 229±49 | 241±56 | 227±45 | 1.00 | 1.00 | 1.00 |
| Duration of diabetes (yr) | 12±8 | 17±7 | 16±8 | 10−4 | 10−3 | 1.00 |
| HbA1c | 8.3±1.7 | 8.9±1.6 | 8.6±1.6 | 0.65 | 1.00 | 1.00 |
| AER (μg/min) | 20 (12, 66) | 623 (321, 1579) | 72 (22, 217) | <10−30 | <10−7 | <10−30 |
| eGFR (ml/min per 1.73 m2) | 100±27 | 61±31 | 90±30 | <10−30 | 0.09 | <10−6 |
| Treated with insulin | 51.5 | 86.4 | 73.8 | <10−4 | 0.004 | 0.75 |
| Treated with RASi/AHTN | 45.1 | 64.3 | 50.7 | 0.11 | 1.00 | 1.00 |
Data are mean ± SD, median (25th, 75th percentiles), or percentage. The 3×2 chi-squared or ANOVA test was used. Bonferroni correction for the number of clinical variables (n=11). RASi/AHTN, renin-angiotensin system inhibitors and/or other antihypertensive agent.
To convert values for cholesterol to millimoles per liter, multiply by 0.02586.
Results for the nine markers in baseline plasma are summarized in Table 2 according to outcome. Concentrations of markers of endothelial dysfunction and systemic inflammation were similar in all three groups and were not further examined. In contrast, concentrations of markers of the TNF pathway (free TNFα, total TNFα, TNFR1, and TNFR2) were highest in the ESRD group and intermediate for those who died without ESRD. Bonferroni corrected differences between the ESRD group and the group alive without ESRD were highly significant. Differences between those who died without ESRD and the alive group were significant only for TNFR1 and TNFR2. Notable is the high intercorrelation among TNF pathway markers (Supplemental Table 3), particularly of TNFR1 with TNFR2 (Spearman correlation coefficient r= 0.90, P<0.001). This correlation was not due to cross-reactivity between the antibodies used for assay (Supplemental Figure 1).
Table 2.
Baseline concentrations of plasma markers of inflammation in patients with T2D according to outcome during 8–12 years of follow-up
| Baseline Plasma Marker | Outcome | P Valuea | ||||
|---|---|---|---|---|---|---|
| Alive (n=267) | ESRD (n=59) | Deceased (n=84) | Alive Compared with ESRD | Alive Compared with Deceased | ESRD Compared with Deceased | |
| ICAM-1 (ng/ml) | 171 (141, 197) | 184 (159, 233) | 181 (147, 229) | 1.00 | 1.00 | 1.00 |
| VCAM-1 (ng/ml) | 438 (358, 542) | 519 (451, 652) | 481 (424, 572) | 0.33 | 1.00 | 1.00 |
| PAI-1 (ng/ml) | 15.6 (11.0, 24.3) | 15.2 (9.0, 24.5) | 16.6 (11.8, 21.1) | 0.99 | 1.00 | 1.00 |
| IL-6 (pg/ml) | 1.6 (1.0, 2.3) | 2.3 (1.7, 3.6) | 2.3 (1.3, 3.4) | 1.00 | 0.05 | 1.00 |
| CRP (mg/L) | 3.0 (1.2, 6.5) | 4.8 (1.9, 7.8) | 4.2 (1.5, 7.7) | 1.00 | 1.00 | 1.00 |
| Free TNFα (pg/ml) | 3.9 (2.8, 5.5) | 8.3 (5.2, 11.1) | 4.9 (3.3, 7.6) | 0.002 | 1.00 | 0.83 |
| Total TNFα (pg/ml) | 10.3 (7.1, 14.4) | 23.0 (17.5, 29.4) | 14.1 (9.3, 21.3) | 0.02 | 1.00 | 0.35 |
| TNFR1 (pg/ml) | 1184 (1005, 1446) | 2424 (2137, 3704) | 1588 (1174, 2066) | <10−12 | 0.01 | <10−5 |
| TNFR2 (pg/ml) | 2273 (1898, 2708) | 4745 (3735, 7018) | 2969 (2225, 3841) | <10−11 | 0.02 | <10−4 |
Data are median (25th, 75th percentiles).
Adjusted for significant covariates in Table 1 (age, systolic BP, AER, eGFR, insulin treatment, and renoprotective treatment) and Bonferroni corrected for the number of markers and groups compared (n=3×9).
Markers of the TNF Pathway and Risk of ESRD: Univariate Analyses
To explore how ESRD risk varied with baseline concentrations of TNF pathway markers, we examined incidence rates according to quartiles of their distributions (Table 3). For each marker, most ESRD cases occurred in the fourth quartile and few or none occurred in the second or first quartile. Whereas this gradient was steepest for TNFR1 (84/1000 person-years in the fourth quartile and zero cases in the first), it was almost as steep for TNFR2 but clearly less steep for free TNFα and total TNFα. We next examined how the risk gradients for the quartiles of TNFR1 and TNFR2 were modified by the patients’ albuminuria status (normoalbuminuria, microalbuminuria, or proteinuria) determined before recruitment (see Concise Methods). Interestingly, the shape of the gradient across quartiles was the same in each albuminuria category (rising sharply across the third and fourth quartiles) although the quartile-specific incidence rate of ESRD increased as the abnormality of albumin excretion increased (results for TNFR1 are shown in Supplemental Table 4).
Table 3.
Incidence rate of ESRD in patients with T2D during 8–12 years of follow-up according to quartiles of the distributions of baseline plasma concentrations of markers of the TNF pathway
| Quartilea | Number of Patients | Incidence Rateb | |||
|---|---|---|---|---|---|
| Free TNFα | Total TNFα | TNFR1 | TNFR2 | ||
| Q1 | 102 | 3 (3) | 0 | 0 | 0 |
| Q2 | 102 | 7 (7) | 3 (3) | 1 (1) | 2 (2) |
| Q3 | 103 | 12 (11) | 11 (11) | 5 (5) | 5(5) |
| Q4 | 101 | 49 (38) | 68 (45) | 84 (53) | 78 (52) |
| P for trendc | <10−11 | <10−12 | <10−12 | <10−12 | |
Quartile boundaries are as follows. Free TNFα (pg/ml): 3.0 for the 25th percentile, 4.3 for the 50th percentile, and 6.7 for the 75th percentile; total TNFα (pg/ml): 8.1 for the 25th percentile, 12.5 for the 50th percentile, and 17.2 for the 75th percentile; TNFR1 (pg/ml): 1049 for the 25th percentile, 1310 for the 50th percentile, and 1837 for the 75th percentile; TNFR2 (pg/ml): 2017 for the 25th percentile, 2527 for the 50th percentile, and 3363 for the 75th percentile.
Per 1000 person-years. Number of events is indicated in parentheses.
Bonferroni correction was applied.
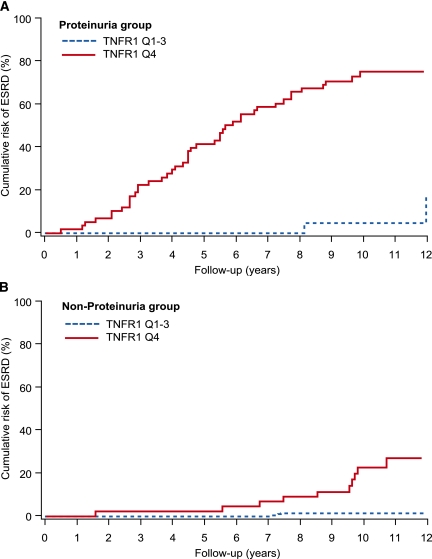
To further explore what influence the severity of albuminuria had on the relationship between TNFR1 concentration and the risk of ESRD, we examined the temporal pattern of occurrence of ESRD during follow-up according to proteinuria status and TNFR1 quartile. Among patients with proteinuria, the cumulative risk of ESRD for the highest quartile of TNFR1 rose steeply from the start of observation and at a constant rate throughout follow-up (Figure 1A). By contrast, among patients without proteinuria (normoalbuminuria and microalbuminuria combined), the majority of whom had normal renal function at baseline, the first cases of ESRD appeared after a delay of 6 years (Figure 1B). Thereafter, the cumulative risk in the highest quartile rose steeply, roughly paralleling the steep increase seen in those with proteinuria (Figure 1A). The 12-year cumulative risk of ESRD for the highest TNFR1 quartile was 54%, whereas it was 3% for the other quartiles. When quartiles were defined by the distribution of TNFR2, similar results were obtained (data not shown).
Figure 1.
Cumulative risk of ESRD in patients with T2D during 12 years of follow-up according to quartiles of plasma TNFR1 at baseline examination and proteinuria status.
Markers of the TNF Pathway and Risk of ESRD: Multivariate Analyses
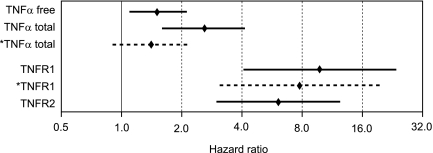
We assessed the independent effect of each TNF marker on the risk of ESRD by adding it to a Cox proportional hazard model of the influential clinical characteristics: hemoglobin A1c (HbA1c), AER, and eGFR (see Concise Methods). In this model, each of the TNF pathway markers remained significant (Supplemental Table 5). A summary of the results for this set of models is illustrated in Figure 2 by the hazard ratios (HRs) and 95% confidence intervals (95% CIs) for each of the TNF pathway markers after adjustment for clinical covariates. The effects of TNFR1 and TNFR2 were similar but much stronger than that of total TNFα. When both total TNFα and TNFR1 were included in the model, only the effect of TNFR1 remained significant (see broken lines in Figure 2). Similar results were obtained when total TNFα and TNFR2 were included in the model (data not shown). Finally, when both TNFR1 and TNFR2 were included in the model, the effect of TNFR1 remained significant (HR, 4.7; 95% CI, 1.3–17.0), whereas the effect of TNFR2 did not (HR, 2.2; 95% CI, 0.7–6.4).
Figure 2.
Effect of each TNF pathway marker on the risk of ESRD in T2D patients during 8–12 years of follow-up. Data are estimates of HRs for an increase by one quartile in the distribution of the marker concentration. The estimates are from a Cox proportional hazard model that adjusted for AER, eGFR, and HbA1c. Other clinical covariates were not significant and did not confound the effects of the receptors. Diamonds and solid lines represent point estimates and 95% CIs for individual markers. The third and fifth lines in the figure are broken and marked by an asterisk to indicate that they represent point estimates and 95% CIs when both total TNFα and TNFR1 were included in the model. For additional details of these analyses, see Supplemental Table 5.
To explore whether the severity of albuminuria modified the multivariate results, we stratified the Cox analyses according to proteinuria strata at enrollment (as in Figure 1, A and B). Within each stratum, baseline AER and TNFR1 emerged as the main determinants of time to ESRD. However, the magnitude of their effects was independent of where the patients were on the spectrum of albuminuria, indicating lack of interaction between the effect of AER and TNFR1. The effect of baseline eGFR was muted within the individual strata of proteinuria, but differences in eGFR between the strata profoundly influenced the delay in appearance of ESRD (Figure 1, A and B).
Predictors of the Risk of ESRD in T2D
The strong influence of TNFR1 as a determinant of time to ESRD prompted a comparison of this plasma marker with clinical characteristics as a predictor of ESRD in Cox proportional hazard models. On the basis of the results in Table 4, this meant a comparison of TNFR1 with AER in patients without proteinuria and with AER and eGFR in patients with proteinuria. The ability of a Cox proportional hazard model to predict an outcome is usually measured by the C-index. In patients without proteinuria, the C-index for TNFR1 was 0.93 (95% CI, 0.89, 0.97) whereas that for AER was only 0.78 (95% CI, 0.64, 0.91), and the difference had a P value of P=0.048. In patients with proteinuria, the C-index for TNFR1 was 0.84 (95% CI, 0.78, 0.90) whereas that for AER was 0.62 (95% CI, 0.53, 0.71) and that for eGFR was 0.78 (95% CI, 0.71, 0.84). The improvement of prediction with TNFR1 relative to AER was highly significant (P<0.001) and that to eGFR had a P value of P=0.02.
Table 4.
Multivariate Cox proportional hazard models of the risk of ESRD in patients with T2D with clinical predictors and the plasma concentration of a TNF marker stratified according to the presence of proteinuria
| Nonproteinuria | Proteinuria | |||
|---|---|---|---|---|
| HRa (95% CI) | P Value | HRa (95% CI) | P Value | |
| Clinical predictorb | ||||
| HbA1c | 1.56 (0.86, 2.82) | 0.14 | 1.24 (0.96, 1.61) | 0.10 |
| AER | 2.23 (1.11, 4.48) | 0.02 | 2.52 (1.14, 5.56) | 0.02 |
| eGFR | 1.10 (0.72, 1.67) | 0.67 | 1.37 (1.11, 1.69) | 0.004 |
| Individual markerc | ||||
| free TNFα | 2.22 (1.20, 4.12) | 0.01 | 1.21 (0.81, 1.81) | 0.34 |
| total TNFα | 2.53 (1.25, 5.13) | 0.01 | 2.61 (1.42, 4.81) | 0.002 |
| TNFR1 | 7.11 (2.13, 23.69) | 0.0004 | 7.05 (2.23, 22.30) | 0.0018 |
| TNFR2 | 3.82 (1.59, 9.20) | 0.0008 | 5.88 (2.10, 16.43) | 0.0013 |
Effect measures are expressed as the HR for a one-quartile increase in the distribution of each covariate except for eGFR, for which it is a one-quartile decrease. Quartile definitions are based on the full cohort.
Clinical predictors examined together with TNFR1.
Individual TNF markers examined together with all three clinical predictors.
Markers of the TNF Pathway and Risk of Mortality
An analysis of markers of the TNF pathway as determinants of time to death without ESRD was performed in parallel with the analysis of determinants of ESRD. Only the TNFRs contributed significantly to CVD and all-cause mortality and their effects were equivalent. However, these effects were small compared with their effect on time to onset of ESRD (Supplemental Tables 6 and 7). Regarding the question of whether to include the 19 cases of ESRD based on death certificates with USRDS registered cases or to include them with the “deceased without ESRD” group, model 2 in Supplemental Table 7 includes them in the deceased without ESRD group. The result is to introduce associations that were weaker or absent in the other 87 patients who died without ESRD.
Discussion
In our study, the risk of ESRD in T2D was strongly associated with elevated concentrations of circulating TNFR1 and TNFR2, but not with the other TNF pathway markers (free or total TNFα). Furthermore, it was not associated with markers of endothelial dysfunction (ICAM-1, VCAM-1, PAI-1) or markers of systemic inflammation (IL-6, CRP). Although associations between renal function loss and certain markers of the TNF pathway have been described in individuals with diabetes15,16 and individuals without diabetes,13 those associations were much weaker and some interpreted a higher circulating concentration of TNFR2 as a proxy for TNFα. Thus, our finding of extremely strong associations of ESRD with circulating TNFR1 and TNFR2 that are independent of its associations with other markers is entirely novel. Similarly novel is the observation that this association is stronger in patients without proteinuria than those with proteinuria. Thus, a high concentration of a circulating TNFR is a new predictor of ESRD in T2D that is more revealing than proteinuria.
Before considering what mechanisms might underlie the associations of ESRD with high concentrations of circulating TNFR1 and TNFR2, several aspects of the finding must be reviewed. First, although TNFR1 and TNFR2 have very different biologic effects when engaged, their concentrations are highly correlated (Spearman correlation coefficient r=0.9). The possibility of artifact due to cross-reactivity of the antibodies used in the ELISA has been excluded (Supplemental Figure 1), but such high correlation means that a statistical determination of whether one receptor is more causally related to the risk of ESRD than another is unlikely. The answer to that question will rest on further research on their biology.
Second, persistence of the very strong effect of either receptor after controlling for proteinuria status, AER, and eGFR points to how informative the TNFRs are as indicators of the risk of ESRD. Their importance is further underlined by the independence of their effects from the other clinical covariates and from plasma concentrations of markers of systemic inflammation and of endothelial injury. Third, it is worth mentioning that the predictive effect of ESRD was based on the single measurement of both TNFR1 as well as albuminuria, and this fact may be of potentially high relevance in future diagnostic algorithms.
How may elevated concentrations of circulating TNFRs be related to the risk of ESRD in patients with T2D? Three hypotheses can be considered. One is that their elevated concentrations are a manifestation of a disease process present in the kidneys that leads to ESRD.17 This possibility is made unlikely by the prospective study design and by their strong effect in patients regardless of proteinuria status.
The second possibility is that elevated concentrations of TNFRs contribute directly to renal injury and progressive renal function decline to ESRD. This alternative is analogous to the process by which high serum cholesterol contributes to the development of coronary atherosclerosis. Further support for this interpretation is provided by the findings in our companion manuscript.18 Although this is our preferred interpretation, a clinical trial of a modifier of the concentrations of circulating TNFRs is required to prove causality.10,19
The third possibility is that variation in plasma concentrations of TNFRs reflects variation in an unknown causal factor (e.g., the plasma concentration of TNFα). Although plausible, our multivariate analyses excluded all forms of circulating TNFα as contributors to the risk of ESRD independent of their correlation with TNFRs. However, we acknowledge that a weak effect of free or total TNFα on the risk of ESRD may be undetected in a study group of this size. For the same reason, we may have failed to detect weak interactions between TNFRs and the effects of glycemic control, obesity, lipid abnormalities, hypertension, systemic inflammation, or endothelial injury on the risk of ESRD.
In addition to their etiologic implications, our findings also have significant diagnostic implications. Patients whose concentrations of circulating TNFR1 were in quartiles one and two had an extremely low risk of developing ESRD during 12 years of follow-up, regardless of all other covariates. On the other hand, patients whose concentrations of TNFR1 were in the highest quartile had an enormously increased risk of ESRD. It is noteworthy that a single measurement of TNFR1 carried information about the risk of outcomes that occur many years in the future.
It is interesting that the distribution of circulating concentrations of this receptor in T2D is quite similar to what we report in an accompanying manuscript on type 1 diabetes.18 In contrast to this study, the patients in our other study were young, had normal renal function, did not have proteinuria, and were not obese. Although some reports demonstrated that TNFRs may be related to those clinical characteristics,20 our two studies indicate that the concentrations of TNFR1 and TNFR2 are not markedly influenced by these clinical covariates. Moreover, they are a stable patient characteristic as demonstrated in our other work.18 Perhaps, this feature of the receptor accounts for its outstanding diagnostic performance. Currently, however, measurement of TNFRs relies on cumbersome ELISA assays. A more convenient test must be developed for use in a clinical setting to assess a patient’s long-term risk of declining renal function and ESRD.
In contrast to the strong association of TNFRs with the risk of ESRD, the associations of these receptors with mortality unrelated to ESRD were weaker, although statistically significant. In several other studies,12,21–24 concentrations of circulating TNFα and TNFR2 predicted cardiovascular events in individuals with and without diabetes. However, the other studies did not control for confounders such as abnormalities in renal function and elevated urinary albumin excretion or misclassification of causes of deaths.
Finally, we should consider the limitations of our study and their possible effect on the interpretation of the findings. The first concern is how we handled outcomes with less than certain documentation. Although ESRD in the 40 incidence cases registered in the USRDS is unambiguous, its presence may be questioned in the additional 19 patients who had renal failure identified as a primary or secondary cause on death certificates but were not registered in the USRDS for a variety of reasons (e.g., refusal to accept dialysis, moribund status, death too soon after the first dialysis to meet criteria for registration, and so forth). However, we found that the 19 unregistered and 40 registered ESRD cases were more similar to each other with regard to baseline clinical characteristics and plasma markers than they were to those alive or deceased without ESRD (Supplemental Tables 1 and 2). For this reason, we combined the two subgroups and reported the results for the combined group of ESRD outcomes. The second concern is misclassification of patients due to inaccuracies in the assessment of baseline clinical characteristics (i.e., due to biologic variation). Such a random misclassification could account for the lack of a significant effect of the BP measurement and the moderate effect of AER obtained during baseline examination on risk of ESRD. However, such misclassification could not account for the very strong effect of plasma concentrations of TNFRs on risk of ESRD. Finally, our study was conducted among patients with diabetes, 85% of whom were Caucasian. Therefore, it is not certain that similar findings will be obtained in non-Caucasians or among patients without diabetes.
In summary, we explored the role of inflammation-related plasma markers as determinants of ESRD in T2D. Only TNFRs 1 and 2 were associated with the risk of ESRD independently of all relevant clinical covariates. The association was present regardless of whether urinary albumin excretion was in the proteinuria or nonproteinuria range. As a predictor of time to ESRD, plasma concentration of TNFR1 outperformed all clinical predictors. Baseline plasma concentrations of TNFRs were also moderately associated with death unrelated to ESRD. In conclusion, elevated concentrations of circulating TNFRs in participants with T2D are strongly associated with the subsequent progression to ESRD. A single determination of the concentration of TNFR1 predicts the 12-year risk of ESRD in patients with or without proteinuria.
Concise Methods
Study Patients and Their Examination
The Joslin Clinic cares for approximately 12,000 adult patients with T2D, and the majority of patients remain under its care for a long period of time. A random sample of patients with T2D who were attending the Joslin Clinic in 1991 was surveyed with a mailed questionnaire about their diabetes and the occurrence of diabetes in their families. Between 1991 and 1995, responders were recruited into the Joslin Study of the Genetics of Type 2 Diabetes and Kidney Complications. Eligibility criteria included residence in Massachusetts, T2D diagnosed between ages 35 and 64 years, and age 40–69 years at examination. Of the 600 patients selected, 509 patients were examined and enrolled into the study.
The Joslin Diabetes Center Institutional Review Board approved the study protocol and informed consent procedures. All patients had a baseline examination that coincided with a routine clinic visit. Trained recruiters performed a physical examination that included standardized measurements of BP and collected samples of urine and blood for DNA and biochemical determinations (stored at −80°C). Questionnaires were supplemented with data from medical records and clinical laboratory database.
Only patients with stored baseline plasma available for measuring creatinine and inflammatory markers were eligible for this study. Furthermore, patients with evidence of nephropathy unrelated to diabetes and patients with stage 5 CKD (defined as an eGFR<15 ml/min per 1.73 m2 using the Modified Diet in Renal Disease formula)14 were excluded. This left 410 patients, 85% of whom defined themselves as Caucasian.
Measurement of Inflammation-Related Plasma Markers
All markers were assayed in 2009–2010 by immunoassays. According to the manufacturers’ protocols, we used ELISA to measure TNFR1, TNFR2, and IL-6 (cat# DRT100, DRT200, and HS600B; R&D Systems, Minneapolis, MN) and total TNFα (cat# KAC1751; Invitrogen, Carlsbad, CA). We confirmed the specificity of assays of TNFR1 and TNFR2 in the ELISA assays by showing the absence of increased signal in the TNFR1 ELISA after the addition of the known amounts of purified TNFR2 and vice versa (data not shown), and further by flow cytometry (Supplemental Figure 1). Anti-TNFR2 antibodies are specific for TNFR2, whereas anti-TNFR1 may cross-react slightly with TNFR2 (<7%).
Concentrations of other markers were measured on the Luminex platform by a multiplex particle-enhanced, sandwich-type immunoassay with a laser detection system based on flow cytometry. We measured free TNFα with adipokine-panel B (cat# HADK2-61K-B; Linco-Millipore, Billerica, MA) and PAI-1, ICAM-1, and VCAM-1 with human sepsis-apoptosis panel (cat# HSEP-63K; Linco-Millipore). CRP was measured with N High Sensitivity CRP by nephelometry (cat#QIY21; Siemens Healthcare, Canton, MA). Assay protocols have been previously described.25
Assessment of Diabetic Nephropathy at Baseline
In the urine sample obtained during the baseline examination, the concentrations of albumin (in grams) and creatinine (in milligrams) were determined and the albumin/creatinine ratio calculated (ACR). The ACR value was converted to an AER according to a previously published formula.26 This AER was used in the univariate and multivariate analyses.
In addition to the baseline urine, we retrieved the results of urinalyses performed on these patients’ urine during the preceding 2-year interval from the Joslin Clinical computer database. These data were used to stratify patients according to degree of albuminuria based on the geometric mean of the results during that interval. For the 410 patients, 1420 ACR measurements and 65 Albustix determinations were retrieved. Albustix readings were reported when patients had very high urinary albumin concentrations; to determine 2-year medians, they were considered equal to ACR 2500. The protocol for measuring the urinary ACR in the random urine samples obtained during the baseline examination and clinic visits and converting it to an AER has been described previously.26 For each patient, the geometric mean AER for the preceding 2-year interval was determined to assign an albuminuria status as follows: normoalbuminuria (AER <30 mg/min), microalbuminuria (AER 30–300 mg/min), and macroalbuminuria/proteinuria (AER >300 mg/min). The criteria were published previously.16,17,27
In 2009, plasma creatinine was measured in stored baseline samples at the University of Minnesota with the Roche enzymatic assay (Prod no. 11775685) on a Roche/Hitachi Mod P analyzer. GFR was estimated from plasma concentrations of creatinine using the IDMS-traceable Modified Diet in Renal Disease formula.28
Ascertainment of ESRD and Mortality
All patients were queried against rosters of the USRDS and the National Death Index (NDI) covering all events up to the end of 2004. The USRDS maintains a roster of US patients receiving renal replacement therapy, and it includes dates of dialysis and transplantation.29 The NDI is a comprehensive roster of deaths in the United States, and it includes date and causes of death.30,31 ESRD was defined by a match with the USRDS roster (n=40) or a listing of renal failure (n=19) among the causes of death on an NDI death certificate. The onset of ESRD was given the date of first dialysis or transplantation or the date of death for those captured by death certificate. If ESRD did not develop and a date of death was obtained from NDI, the outcome was defined as “deceased without ESRD” (n=84). All other patients were considered alive (n=267). The majority of patients had at least one measurement of serum creatinine during the preceding several years, and only four had stage 4 CKD and none had stage 5 CKD.
Statistical Analyses
Analyses were performed with SAS software (version 9.2; SAS Institute, Cary, NC). Differences among the three outcome groups were tested by using the chi-squared test for categorical variables, as well as ANOVA and Tukey’s t test for continuous variables. For plasma markers, we added a Bonferroni correction for the number of markers considered. Follow-up data were analyzed as incidence rates of ESRD or death without ESRD and tested for trend across quartiles of each marker distribution. To take competing risk into account, we estimated cause-specific cumulative incidences using a SAS macro provided by the Mayo Clinic.32,33 All of the clinical characteristics in Table 1 were considered for the multivariate analysis of determinants of ESRD (or death without ESRD) compared with survival without ESRD using Cox proportional hazard models. Reduced cause-specific models were selected by minimizing Schwartz’s (Bayesian) information criterion and consisted only of significant covariates (age and AER for the deceased group and HbA1c, AER, and eGFR for the ESRD group). The other clinical characteristics considered (Table 1) did not confound the effect of TNF pathway markers, and thus they were not retained in the final models. The independent effects of all of the variables were evaluated uniformly by a one-quartile difference in the reduced models. To assess predictive performance, we compared models built from clinical covariates or markers according to the general discrimination index (C-index)34 obtained with a SAS macro provided by the Mayo Clinic.35,36 C-indexes were compared using bootstrap estimates of standard errors of the differences. P<0.05 was considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Paul W. Eggers from the National Institute of Diabetes and Digestive and Kidney Diseases for help in matching the Joslin study roster against the USRDS.
This study was supported by a Juvenile Diabetes Research Foundation research grant (1-2008-1018) and grants from the National Institutes of Health (DK041526 and DK067638) to A.S.K.; an American Diabetes Association mentor-based fellowship (7-03-MN-28) to M.A.N.; a grant from the Uehara Memorial Foundation and a personal grant from Yasuhiko Tomino, Juntendo University, Tokyo, Japan, to T.G.; a Juvenile Diabetes Research Foundation fellowship grant (3-2009-397) to J.S.; grants from the National Institutes of Health to T.N.M. (DK077111 and HL065095) and X.C. (KO1 AR054984); and a graduate student fellowship from CONACYT Fundacion Mexico en Harvard to F.R.
The analyses and opinions expressed in this manuscript do not represent the position of the USRDS or the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Do We Now Have a Prognostic Biomarker for Progressive Diabetic Nephropathy?” on pages 376–377.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011060627/-/DCSupplemental.
References
- 1.Hotamisligil GS, Spiegelman BM: Tumor necrosis factor alpha: A key component of the obesity-diabetes link. Diabetes 43: 1271–1278, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Pickup JC, Crook MA: Is type II diabetes mellitus a disease of the innate immune system? Diabetologia 41: 1241–1248, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM: C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286: 327–334, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF: Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 52: 812–817, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Tinker L, Song Y, Rifai N, Bonds DE, Cook NR, Heiss G, Howard BV, Hotamisligil GS, Hu FB, Kuller LH, Manson JE: A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med 167: 1676–1685, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Donath MY, Shoelson SE: Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 11: 98–107, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa G, Nakano K, Sawada M, Uno K, Shibayama Y, Ienaga K, Kondo M: Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int 40: 1007–1012, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Navarro JF, Mora C: Role of inflammation in diabetic complications. Nephrol Dial Transplant 20: 2601–2604, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Navarro JF, Mora-Fernandez C: The role of TNF-alpha in diabetic nephropathy: Pathogenic and therapeutic implications. Cytokine Growth Factor Rev 17: 441–450, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Ernandez T, Mayadas TN: Immunoregulatory role of TNFalpha in inflammatory kidney diseases. Kidney Int 76: 262–276, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Keller C, Katz R, Cushman M, Fried LF, Shlipak M: Association of kidney function with inflammatory and procoagulant markers in a diverse cohort: A cross-sectional analysis from the Multi-Ethnic Study of Atherosclerosis (MESA). BMC Nephrol 9: 9, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E: Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation 101: 2149–2153, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Tonelli M, Sacks F, Pfeffer M, Jhangri GS, Curhan G: Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int 68: 237–245, 2005 [DOI] [PubMed] [Google Scholar]
- 14.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 15.Lin J, Hu FB, Rimm EB, Rifai N, Curhan GC: The association of serum lipids and inflammatory biomarkers with renal function in men with type II diabetes mellitus. Kidney Int 69: 336–342, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin J, Hu FB, Mantzoros C, Curhan GC: Lipid and inflammatory biomarkers and kidney function decline in type 2 diabetes. Diabetologia 53: 263–267, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarro JF, Milena FJ, Mora C, Leon C, Garcia J: Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: Effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am J Nephrol 26: 562–570, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, Cullere X, Johnson AC, Crabtree G, Smiles AM, Mayadas TN, Warram JH, Krolewski AS: Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 23: 516–524, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tansey MG, Szymkowski DE: The TNF superfamily in 2009: New pathways, new indications, and new drugs. Drug Discov Today 14: 1082–1088, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Chu NF, Spiegelman D, Rifai N, Hotamisligil GS, Rimm EB: Glycemic status and soluble tumor necrosis factor receptor levels in relation to plasma leptin concentrations among normal weight and overweight US men. Int J Obes Relat Metab Disord 24: 1085–1092, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Tracy RP, Rubin SM, Harris TB, Pahor M: Inflammatory markers and cardiovascular disease (the Health, Aging and Body Composition [Health ABC] Study). Am J Cardiol 92: 522–528, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Knight EL, Rimm EB, Pai JK, Rexrode KM, Cannuscio CC, Manson JE, Stampfer MJ, Curhan GC: Kidney dysfunction, inflammation, and coronary events: A prospective study. J Am Soc Nephrol 15: 1897–1903, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB: Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med 351: 2599–2610, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Shai I, Schulze MB, Manson JE, Rexrode KM, Stampfer MJ, Mantzoros C, Hu FB: A prospective study of soluble tumor necrosis factor-alpha receptor II (sTNF-RII) and risk of coronary heart disease among women with type 2 diabetes. Diabetes Care 28: 1376–1382, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Niewczas MA, Ficociello LH, Johnson AC, Walker W, Rosolowsky ET, Roshan B, Warram JH, Krolewski A: Serum concentrations of markers of TNFalpha and Fas-mediated pathways and renal function in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol 4: 62–70, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krolewski AS, Laffel LM, Krolewski M, Quinn M, Warram JH: Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus. N Engl J Med 332: 1251–1255, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS: Regression of microalbuminuria in type 1 diabetes. N Engl J Med 348: 2285–2293, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration: Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Agodoa LY, Eggers PW: Renal replacement therapy in the United States: Data from the United States Renal Data System. Am J Kidney Dis 25: 119–133, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention, National Center for Health Statistics: Data Access—National Death Index. Available at: http://www.cdc.gov/nchs/ndi.htm Accessed October 10, 2010
- 31.Cowper DC, Kubal JD, Maynard C, Hynes DM: A primer and comparative review of major US mortality databases. Ann Epidemiol 12: 462–468, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Bergstralh E: SAS Macro That Performs Cumulative Incidence in Presence of Competing Risks. Available at: http://mayoresearch.mayo.edu/mayo/research/biostat/upload/comprisk.sas Accessed February 3, 2010
- 33.Gooley TA, Leisenring W, Crowley J, Storer BE: Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med 18: 695–706, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA: Evaluating the yield of medical tests. JAMA 247: 2543–2546, 1982 [PubMed] [Google Scholar]
- 35.Kremers W: SAS Macro That Calculates the c-Statistic (Concordance, Discrimination Index) for Survived Data with Time Dependent Covariates and Corresponding SE and 100(1−α)% CI. Available at: http://mayoresearch.mayo.edu/mayo/research/biostat/upload/survcstd.sas Accessed November 24, 2009
- 36.Kremers WK: Concordance for Survival Time Data: Fixed and Time-Dependent Covariates and Possible Ties in Predictor and Time. Technical Report Series No. 80. Rochester, MN, Department of Health Science Research, Mayo Clinic, 2007 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.