Abstract
Inhibitors of the mammalian target of rapamycin (MTOR) belong to a family of drugs with potent immunosuppressive, antiangiogenic, and antiproliferative properties. De novo or worsening proteinuria can occur during treatment with these agents, but the mechanism by which this occurs is unknown. We generated and characterized mice carrying a podocyte-selective knockout of the Mtor gene. Although Mtor was dispensable in developing podocytes, these mice developed proteinuria at 3 weeks and end stage renal failure by 5 weeks after birth. Podocytes from these mice exhibited an accumulation of the autophagosome marker LC3 (rat microtubule-associated protein 1 light chain 3), autophagosomes, autophagolysosomal vesicles, and damaged mitochondria. Similarly, human podocytes treated with the MTOR inhibitor rapamycin accumulated autophagosomes and autophagolysosomes. Taken together, these results suggest that disruption of the autophagic pathway may play a role in the pathogenesis of proteinuria in patients treated with MTOR inhibitors.
The mammalian target of rapamycin (MTOR) is an evolutionarily conserved serine-threonine kinase that interacts with regulatory associated protein of MTOR (Rptor) or Rptor independent companion of MTOR (Rictor) to form mTORC1 and mTORC2 complexes, respectively. In turn, mTORC1 and mTORC2 regulate different aspects of MTOR function. mTORC1 is a key regulator of cellular metabolism, including protein translation, ribosomal biogenesis, cell growth and proliferation, and suppression of autophagy in response to amino acids, growth factors, and elevated cellular ATP levels.1 mTORC2 is regulated primarily by growth factors to promote actin cytoskeletal rearrangement, cell survival, and cell cycle progression.2 In mammalian cells, rapamycin and other MTOR inhibitors associate with the FKBP12 protein, and together they directly bind MTOR to prevent the RPTOR-MTOR interaction and thus inhibit mTORC1 function.3 In certain cell types, including the podocyte, chronic inhibition of MTOR by rapamycin also results in downregulation of mTORC2 functions.4–6 Although this mechanism of action has not been completely elucidated, data in podocytes suggest that prolonged rapamycin treatment directly downregulates MTOR and Rictor, both of which are required for mTORC2 function.6
Sirolimus (rapamycin) was originally proposed as an immunosuppressant to prevent rejection of solid organ transplants. There were expectations that MTOR inhibitors would replace nephrotoxic calcineurin inhibitors (CNIs). In one prospective trial, patients treated with sirolimus or switched to sirolimus from CNIs had comparable rates of biopsy-confirmed acute allograft rejection and 2-year graft survival to those treated with CNIs.7 In addition, sirolimus-treated patients had fewer malignancies and a better estimated GFR (eGFR) at 24 months if their baseline eGFR was >40 ml/min. Because of their antiproliferative and antiangiogenic effects, indications for MTOR inhibitors have expanded to include treatment of various cancers such as renal cell carcinoma, nonmalignant conditions such as autosomal dominant polycystic kidney disease (AD-PKD), and primary glomerulopathies.8–12
Despite its potential advantages in the transplant setting, evidence that sirolimus causes de novo or worsening proteinuria is unequivocal. In one randomized clinical trial in which patients with AD-PKD received sirolimus or placebo, the group receiving an MTOR inhibitor had a significantly higher median urine protein/creatinine ratio.9 Similarly, in a recent open-label randomized clinical trial in which 503 renal transplant patients were randomized to an everolimus-based CNI-free regime or standard CNI therapy, those taking everolimus had a significantly higher 24-hour urine protein excretion.13 Although subnephrotic increases in proteinuria may result from glomerular or tubular injury, the small incidence of reported cases of patients developing full-blown nephrotic syndrome after treatment with rapamycin14 suggests that the glomerular filtration barrier is affected, at least in this subset of patients.
Several in vitro and patient biopsy studies have addressed a role for MTOR in the glomerulus. One group described thrombotic microangiopathic glomerular lesions in renal biopsies from five patients who developed proteinuria when treated with sirolimus.15 These lesions were associated with downregulation of vascular endothelial growth factor A (VEGFA) expression in podocytes, a molecular mechanism that has been associated with thrombotic microangiopathy in patients with pre-eclampsia16 and in those treated with anti-VEGFA agents.17 Another small case series describes three instances of de novo FSGS in patients treated with sirolimus, characterized by focal loss of PAX2, synaptopodin, and VEGFA.14 Although not all patients with proteinuria who take sirolimus have a distinct glomerular lesion, Stallone et al. performed a biopsy study showing that sirolimus treatment was associated with decreased expression of synaptopodin, podocin, CD2AP, and nephrin in podocytes.18 Cell culture studies support the results of these biopsy studies and further suggest a role for MTOR in regulating actin and slit-diaphragm–associated proteins in the podocyte.6 Finally, genetic deletion of Rptor alone or both Rictor and Rptor from podocytes results in glomerular injury in mice by an unknown mechanism.19 These data suggest that inhibition of MTOR signaling within the podocyte may play a complex role to promote proteinuria in patients.
Given the well recognized proteinuric effect of MTOR inhibitors, we were interested in understanding its mechanism. To explore the role of MTOR in vivo, we developed a mouse model with a podocyte-selective deletion of the Mtor gene (Mtor pod-KO).
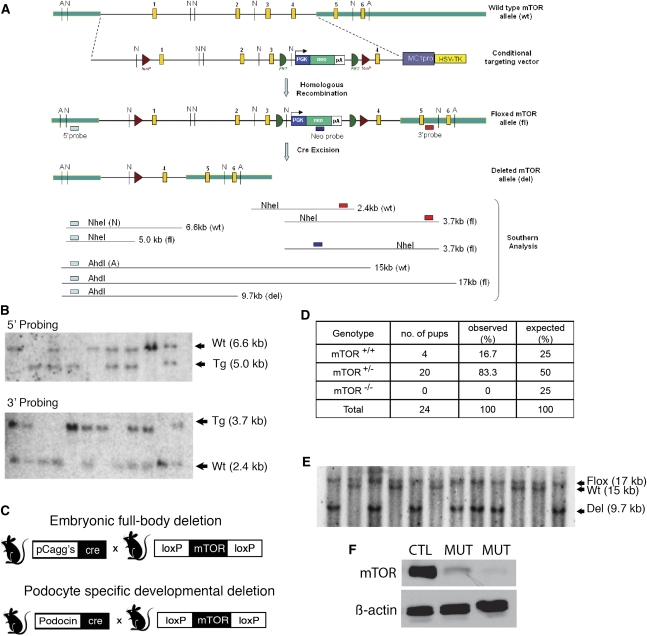
A conditional Mtor allele was generated using BAC recombineering to place loxP sites around the first three exons of the Mtor gene (Figure 1A). The successful generation of a floxed Mtor allele was confirmed by Southern blot (Figure 1B). In this mouse, Cre-mediated excision results in a null Mtor allele, abolishing function of both mTORC1 and mTORC2 complexes. Because chronic use of mTOR inhibitors downregulates both mTORC1 and mTORC2 functions, we chose to delete the Mtor gene—as opposed to its partners Rptor or Rictor—to obtain the most complete knockdown and simulate the clinical effects of MTOR inhibitors.
Figure 1.
Generation of podocyte-selective knockout of the Mtor gene. (A) BAC recombineering construct. LoxP sites (triangles) are inserted around the first three exons of the Mtor gene. The neomycin selection cassette is used to select for positive embryonic stem cell clones, then removed by FLPe recombinase-mediated excision. Cre-mediated excision results in a null Mtor allele. (B) Southern blot analysis confirms correctly targeted alleles. (C) A null Mtor allele (Mtordel) is generated by breeding floxed mice to a pCaggs-Cre driver strain that deletes at the one cell stage (germline deletion). A podocyte-selective knockout is generated by breeding Mtorflox/del mice to a Podocin-Cre driver strain. (D) Table showing correlation between genotype and survival. As predicted, Mtordel/del (Mtor−/−) mice die in utero. (E) Southern blot analysis of renal cortical genomic DNA confirms the presence of a deleted Mtor allele in Mtor pod-KO mice. (F) Western blot analysis of isolated glomerular protein confirms downregulation of Mtor protein in the glomeruli from Mtor pod-KO mice compared with Mtorwt/flox mice. Each lane contains glomeruli pooled from four mice. Wt, wild-type allele; Tg, transgenic Mtorflox allele.
To confirm functional deletion of the Mtor gene, we bred the Mtorflox/flox mouse to a pCaggsCre-driver strain that deletes the gene at the one cell embryo stage, giving a germline deletion of Mtor (Mtordel) (Figure 1C). Heterozygous Mtorwt/del mice appear healthy but have reduced Mtor protein levels (Supplemental Figure 1). Homozygous Mtordel/del mice die in utero, similar to the conventional knockout (Figure 1D).20,21 In all subsequent breeding, one Mtordel allele was utilized to enhance the degree of Cre-mediated excision in the podocyte.
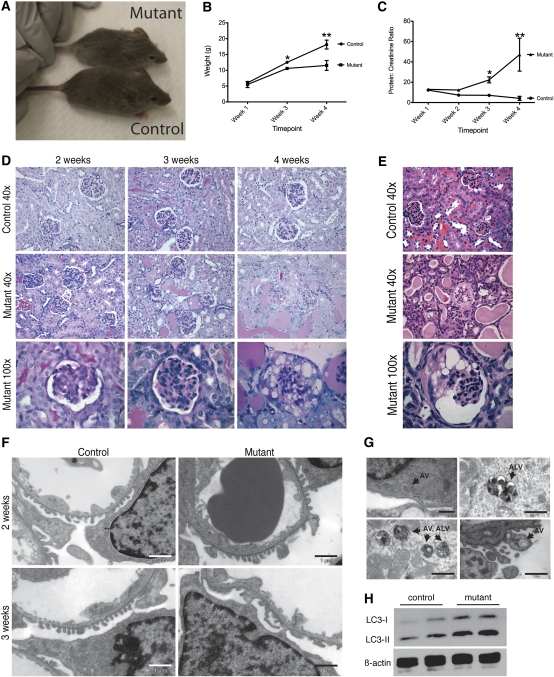
To generate an Mtor pod-KO, Podocin-Cre mice were bred to mice carrying one conditional allele (Mtorflox) and one deleted (Mtordel) allele (Figure 1C). Glomerular deletion of Mtor was verified by analysis of genomic DNA isolated from the renal cortex and identification of the deleted band (Figure 1E). Further confirmation was obtained by Western blot analysis of glomerular protein showing downregulation of Mtor (Figure 1F). Mice of all genotypes were born in expected Mendelian frequency (Supplemental Table 1) and mutants appeared as healthy as wild-type or mixed genotype controls at birth. Interestingly, no overt phenotype was observed until 3 weeks of age, suggesting that Mtor is dispensable in developing podocytes. By 4 weeks of age, however, whole animal growth restriction was evident in Mtor pod-KO mice (11.54 g [Mtor pod-KO] versus 18.13 g [control]; P=0.03) (Figure 2, A and B). The protein/creatinine ratio was significantly increased in 3-week-old mutants (22.32 mg/mg for Mtor pod-KO mice) compared with controls (7.117 mg/mg) (P=0.025) (Figure 2C). Glomeruli from 2- to 3-week-old mutant mice were histologically similar to controls but proteinaceous casts were observed in the tubules of mutant mice (Figure 2D). However, by 4 weeks of age, glomeruli in mutant mice showed dramatic pathologic changes with areas of sclerosis and numerous vacuolated podocytes (Figure 2D). By 5 weeks of age, complete destruction of the glomerular tuft was seen, with distended Bowman’s space and widespread vacuolization of podocytes (Figure 2E). These histologic findings are similar to those reported in mice lacking both mTorc1 and mTorc2 in their podocytes (Rptor/Rictor pod-KO).19 However, unlike Rptor/Rictor pod-KO, the phenotype in Mtor pod-KO mice is fully penetrant and strain independent, which is likely due to a more complete excision and knockdown of gene function.
Figure 2.
Disease course and glomerular histology in Mtor pod-KO mice. (A) Mtor pod-KO mice appear smaller than their control littermates at 3 weeks of age. (B) Mtor pod-KO mice are significantly growth restricted by 4 weeks of age (*P<0.01; **P<0.05). (C) Protein/creatinine ratios are significantly increased by 3 weeks of age in Mtor pod-KO mice compared with wild-type (*P<0.01; **P<0.05). (D) At 3 weeks of age, glomeruli look similar in mutants and controls although proteinaceous casts are visible in tubules (periodic acid–Schiff stain, magnification ×40). By 4 weeks of age, glomerular scarring and vacuolization of podocytes are evident. (E) By 5 weeks of age, glomeruli are end stage with widespread vacuolization of podocytes. (F) Electron micrographs from control and mutant glomeruli at 3 weeks of age show focal foot process effacement in mutants compared with controls. The endothelium and glomerular basement membrane appear largely intact. Scale bars represent 1 μm. (G) Electron micrographs of glomeruli from 2-week-old and 3-week-old Mtor pod-KO mice show frequent double-membraned cytoplasmic vesicles characteristic of autophagosomes. Scale bar in the top left panel represents 500 nm. All other scale bars represent 1 μm. (H) Isolated glomeruli from Mtor pod-KO mice show increased levels of LC3-II compared with controls. AV, autophagosomal vesicles; ALV, autophagolysosomal vesicles.
Electron micrographic (EM) studies showed focal effacement of foot processes in mutant mice beginning at 3 weeks of age although the endothelium and glomerular basement membranes were preserved (Figure 2F). Nonetheless, by 5 weeks of age, profound structural changes were observed in podocytes, endothelial and mesangial compartments, consistent with light microscopic findings (not shown).
Despite the presence of foot process effacement, previous studies indicate that this is unlikely to be the primary mechanism of glomerular injury because other transgenic mouse models with even more marked foot process defects do not exhibit such a dramatic disease course or the vacuolated podocyte phenotype. For example, mice lacking the adaptor proteins, Nck1/2, in their podocytes do not form any foot processes. Although they are born with severe proteinuria, their renal injury progresses more slowly than that seen in Mtor pod-KO mice.22 In keeping with structural changes observed by EM, podocin expression was decreased at 3 weeks in glomeruli of Mtor pod-KO mice (Supplemental Figure 2A). However, Wt1 and Nphs1 expression were unaffected at this time point (Supplemental Figure 2B).
Given the observations of decreased VEGFA levels in renal biopsies from patients treated with MTOR inhibitors and regulation of VEGFA by mTORC1 through upregulation of hypoxia-inducible factors,15,23 we next examined whether reduced Vegfa in podocytes might be driving the disease in Mtor pod-KO mice. In our model, Vegfa levels were similar in mutants and controls at the onset of disease (between 2 and 3 weeks of age). However, by 3 weeks of age, Vegfa levels were visibly reduced but not absent (Supplemental Figure 1C). Despite this finding, a primary reduction of Vegfa alone in podocytes results in a very different phenotype and disease course in mice, including marked endothelial swelling with mesangiolysis,17,24 and, in the case of developmental deletion, complete loss of the glomerular endothelium. Similarly, reduction of Vegfa by approximately 75% of normal levels results in dramatic mesangiolysis by 3 weeks of age.25 None of these pathologic findings were observed in Mtor pod-KO mice.
If primary cytoskeletal defects and Vegfa deficiency are unlikely to be the initiating events of the glomerular injury in Mtor pod-KO mice, what explains the dramatic phenotype? MTOR is known to regulate and inhibit many cellular processes, including autophagy (reviewed by Zoncu et al.1). Autophagy is a lysosomal-dependent cellular survival response to starvation or lack of growth factors in which cells degrade cellular constituents from proteins to entire organelles, such as mitochondria, to provide a supply of nutrients under conditions of stress. A basal level of autophagy is, however, necessary to remove damaged organelles, excessive lipids, and long-lived or misfolded proteins. Terminally differentiated, nondividing cells such as neurons have an elevated basal level of autophagy.26 Similarly to neurons, the basal level of autophagy also seems to be increased in podocytes.27,28 In the podocyte, it has been suggested that autophagy may be required to protect the cell from injury. In this regard, deletion of Atg5, a key component of the autophagic pathway, results in late onset of glomerular disease in mice at 20–24 months of age, presumably due to the accumulation of damaged organelles and ubiquitinated protein complexes.27 Active autophagy is characterized by the presence of double-membraned cytoplasmic vesicles known as autophagosomal vesicles (AV), which can be seen on electron micrographs.29 Importantly, AVs were readily observed in podocytes from Mtor pod-KO mice (Figure 2G) beginning at 2 weeks of age but were not seen in controls. To quantitate the number of AVs, we isolated protein from the glomeruli of wild-type or Mtor pod-KO mice. LC3 (rat microtubule-associated protein 1 light chain 3) is the mammalian homolog of the essential yeast autophagy protein Atg8 and is the most commonly used molecular marker of the autophagosome. There are two LC3 isoforms generated by post-translational modification: LC3-I and LC3-II. LC3-I localizes to the cytoplasmic compartment, whereas LC3-II localizes to the autophagosome membrane as well as the cytoplasm. LC3-II is increased in glomeruli isolated from mutant animals (Figure 2H) consistent with an increase in AVs.
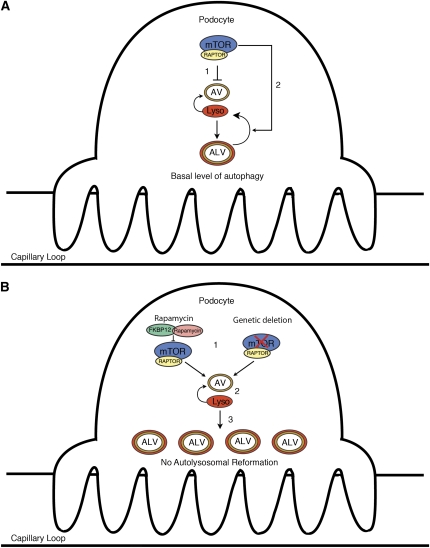
After engulfment of the intracellular targets, the AV fuses with lysosomes to generate the autophagolysosomal vesicle (ALV), permitting degradation of intracellular organelles. The process is successfully terminated after reformation of the autophagosome and lysosomes from the ALV (Figure 3A). Failure to regenerate these components will disrupt the autophagic cycle, with consequent accumulation of acidophilic ALVs and damaged organelles such as mitochondria, ultimately leading to cell death. A recent cell culture study showed that MTOR plays a role in both phases of the autophagic cycle and that inhibition of MTOR therefore has two distinct effects on the process. First, MTOR inhibition causes enhanced autophagosome formation, followed by failure of MTOR-dependent lysosomal reformation, which is accompanied by decreased clearance of damaged organelles due to accumulation of autophagolysosomes.30 In this sense, autophagy stimulated by naturally occurring endogenous factors or events such as starvation or growth factor withdrawal has a very different effect on the cell than autophagy that is initiated through pharmacologic or genetic inhibition of MTOR. Although starvation or loss of growth factor signals initiate autophagy through inhibition of MTOR, continued starvation, paradoxically, leads to upregulation of MTOR activity, establishing a negative feedback loop that is important for autolysosomal vesicle recycling and cell survival (Figure 3A). In the setting of rapamycin or a knockout of the Mtor gene, this negative feedback loop is blocked and autophagic vesicles will accumulate, will fail to recycle, and the process will arrest, resulting in toxicity to the cell. On the basis of these data, we posit that disruption of both steps of the autophagic cycle contribute to the development of dramatic glomerular injury observed in Mtor pod-KO mice.
Figure 3.
Model explaining the effect of MTOR inhibition in podocytes. (A) Physiologic level of MTOR activity inhibits autophagy (step 1), and maintains it at a basal level (step 2) in podocytes. MTOR reactivation allows autophagolysosomal reformation and the cycle of autophagy to complete itself. (B) MTOR inhibition (step 1) disrupts the autophagic pathway at two points. First, it relieves chronic suppression, resulting in activation and enhanced autophagy (step 2). However, MTOR inhibition will also lead to suppression of the reformation of lysosomes and autophagosomes, ultimately resulting in an accumulation of ALVs (step 3), damaged intracellular organelles such as mitochondria, and cell death. Lyso, lysosome; AV, autophagosomal vesicle; ALV, autophagolysosomal vesicle.
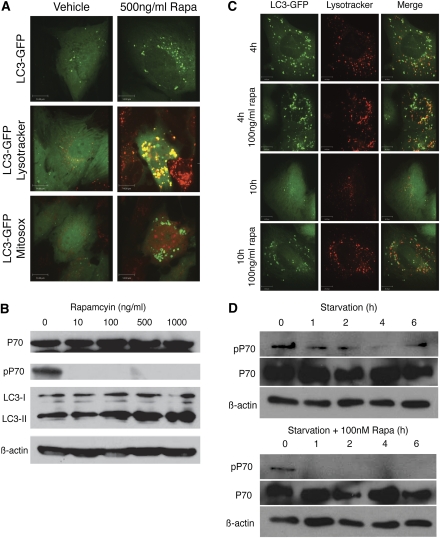
We tested this model in human podocytes treated with rapamycin. Human podocytes were stably transfected with an LC3-GFP construct. After exposure to rapamycin, accumulation of fluorescent ringed structures was observed (Supplemental Movie 1 and Figure 4A) consistent with an increased number of AVs and enhanced level of autophagy. Western blot analysis confirmed an upregulation of the AV-associated isoform LC3-II in the rapamycin-treated human podocytes. These findings were associated with coincident inhibition of phosphorylated S6K, a marker of mTORC1 activity (Figure 4B).
Figure 4.
Chronic MTOR inhibition disrupts the autophagic pathway in podocytes in vitro. (A) Human podocytes stably transfected with LC3-GFP and treated with rapamycin exhibit increased fluorescent-tagged ringed structures characteristic of autophagosomes. The AVs colocalize with the lysosomal marker Lysotracker Red. Podocytes exposed to rapamycin also demonstrate increased superoxide activity as shown by Mitosox Red, suggestive of mitochondrial damage. (B) Western blot analysis confirms downregulation of phospho-S6K (pS6K) and inhibition of mTOR activity in podocytes exposed to rapamycin along with upregulation of the autophagosome-associated LC3 isoform. (C) Podocytes exposed to starvation show only a transient increase in ALVs. In contrast, rapamycin-treated cells exhibit prolonged and marked accumulation of ALVs. ALVs are marked by co-localization of the lysosomal dye (Lysotracker Red) and GFP-LC3 (green). (D) Western blot analysis shows recovery of mTOR activity after 6 hours of starvation as evidenced by increasing levels of phospho-S6K. In contrast, rapamycin-treated cells show continued suppression of phospho-S6K.
To determine whether ALVs accumulate after MTOR inhibition (i.e., arrest of the autophagic cycle), we studied distribution of LC3-GFP puncta and Lysotracker, a lysosomal marker. After 5 days of exposure to rapamycin, there was almost complete co-localization of LC3-GFP and acidophilic Lysotracker Red (Figure 4A). In addition, there was evidence of enhanced Mitosox staining indicative of superoxide activity derived from mitochondria in podocytes treated with rapamycin. This suggests an accumulation of dysfunctional mitochondria that would normally be cleared by autophagy (Figure 4A). Consistent with the model in Figure 3A, ALV accumulation in podocytes is transient under starved conditions, coinciding with a transient downregulation of MTOR activity (Figure 4, C and D). At 4 hours of starvation, the autophagosome and lysosomes often co-localized. After 10 hours of starvation, there was almost complete clearance of LC3-GFP–labeled vesicles and restoration of lysosomal size. Although phosphorylated S6K is downregulated after 4 hours of starvation alone, it recovers by 6 hours, thereby demonstrating reactivation of mTORC1.
By contrast, administration of rapamycin results in massive accumulation of ALVs in podocytes as demonstrated by LC3 and Lysotracker markers (Figure 4C) and robust suppression of phosphorylated S6K that does not recover even by 6 hours (Figure 4D). These findings were recapitulated in vivo in the Mtor pod-KO mouse model (Figure 2). Specifically, LC3-II is increased in glomeruli isolated from mutant animals (Figure 2H) and EM studies revealed large intravesicular structures characteristic of AVs and ALVs together with accumulation of damaged mitochondria in podocytes of mutant mice (Figure 2G) (data not shown) consistent with a substantive defect in lysosomal reformation.
Our data support a model whereby MTOR inhibition in podocytes results in enhanced initiation of autophagy followed by failure to regenerate key components of this process (Figure 3B). Why might this be important? Although reduced autophagy can lead to cell damage in podocytes, activation and dysregulation of autophagy are also likely to result in susceptibility to glomerular disease in patients. This is notably apparent in patients with lysosomal storage diseases, such as Fabry’s disease, aspartylglucosaminuria, or Scheie’s disease, in which failure of lysosomes to acidify causes failure of lysosomal reformation30 and these patients are also prone to developing proteinuria.31 In addition to the kidney, there are a number of other pathologic states, including cystic fibrosis,32 certain myopathies,33 and neurodegenerative diseases,26 characterized by dysfunctional or incomplete autophagy causing tissue damage.
Our results suggest that dysregulation of autophagy may be an important component in the pathogenesis of proteinuria and glomerular injury in patients treated with MTOR inhibitors and perhaps other types of glomerular disease. Although actin cytoskeletal defects and reduction of Vegfa may contribute to this damage, the Mtor pod-KO phenotype does not resemble the phenotypes observed in mouse models with primary defects in either of these pathways. In turn, these data suggest that strategies to protect the cell from enhanced and/or dysregulated autophagy may be useful in protecting the kidney during treatment with MTOR inhibitors.
Finally, it is important to consider these results in light of recent data demonstrating a critical role for Mtor activation in the development and progression of diabetic nephropathy.19,34 Haploinsufficiency for Rptor slowed the development of glomerular changes in diabetic mice, suggesting that rapalogs may be useful agents to treat diabetic nephropathy. Our findings highlight the importance of tight MTOR regulation in podocytes and suggest that the use of rapalogs to treat renal disease must be considered with caution.
Concise Methods
Generation of Mtor Pod-KO
Podocin-Cre recombinase transgenic mice were used as previously described.35 A detailed targeting strategy was designed for the Mtor gene using BAC recombineering36 to generate a floxed Mtor gene. The first loxP site was introduced into the unconserved 5′ flanking region of the Mtor promoter and the second loxP site into an unconserved region in intron 3. Correctly targeted clones analyzed by Southern blot were used for aggregation to produce chimeras. After identification of F1 offspring carrying the targeted allele, the neomycin selection cassette was removed by breeding Mtorwt/flox neo-in mice to a mouse carrying the FLPe recombinase transgene.37 Heterozygous Mtorwt/flox mice were bred to mice carrying a pCaggs-Cre transgene to generate Mtorwt/del mice and then bred to the Podocin-Cre driver mouse strain. Mtorwt/del Podocin-Cre mice were bred to Mtorflox/flox mice to obtain Mtorflox/del Podocin-Cre double-transgenic mice.
Phenotypic Analysis
Spot urine was collected from mice 1, 2, 3, and 4 weeks of age. We used a urine dipstick (Chemstrip7; Roche Diagnostics Corp) to detect the presence or absence of protein in the urine. A standard colorimetric assay for urine protein and urine creatinine was performed according to the manufacturer’s instructions (Sigma) to estimate urine protein/creatinine ratios.
Histologic Analysis and Immunofluorescence
Kidneys from mice 1, 2, 3, and 4 weeks of age were dissected and fixed in 10% formalin in PBS and then embedded in paraffin for histologic analysis. Sections 4 μm in thickness were cut and stained with periodic acid–Schiff or hematoxylin and eosin and then examined and photographed with a DC200 Leica camera and Leica DMLB microscope (Leica Microsystems Inc).
Immunofluorescent tissue staining for podocin was performed using a rabbit anti-podocin antibody (P0372; Sigma).
In Situ Hybridization
Kidneys from mice at 2, 3, and 4 weeks of age were dissected and washed briefly in RNAse-free PBS and fixed overnight in diethyl pyrocarbonate–treated 4% paraformaldehyde. These tissues were then transferred to 15% sucrose for 24 hours followed by 30% sucrose for 24 hours, after which they were embedded in Tissue-Tek OCT and snap frozen. Sections 10 μm in thickness were cut on a Leica Jung cryostat (model CM3050; Leica Microsystems Inc) and transferred to Superfrost microscope slides (Fischer Scientific Co.). Digoxigenin-labeled probes were prepared according to the Roche Molecular Biochemicals protocol (Roche Molecular Biochemicals). Probes used for in situ analysis were Nphs1, Vegfa, and Wt1. Further details regarding the in situ protocol can be obtained on request.
Cell Culture
The immortalized human podocyte cell line was a kind gift from Moin Saleem (University of Bristol, Bristol, UK) and was previously characterized.38 Cells were maintained in RPMI 1640 medium supplemented with 10% FBS and insulin-transferrin-selenium at 33°C.
Human podocytes were co-transfected with a pCMV-GFP-LC3 expression vector (Cell Biolabs Inc) and a marker plasmid expressing a puromycin-resistant gene. Two days after transfection, cells were selected in 2 mg/L of puromycin (Sigma) until resistant colonies of cells appeared. GFP positive clones were selected. Cells were treated with 50–1000 ng/ml of rapamycin (R878; Sigma) in RPMI.
Lysotracker Red (Molecular Probes) or Mitosox Red (Invitrogen) was incubated with GFP-LC3 stably transfected podocytes at concentrations of 75 nM and 5 μM, respectively, for 20 minutes. After 20 minutes, cells were washed with PBS and visualized by confocal microscopy.
Untransfected human podocytes were used for Western blotting. Cells were treated with vehicle, 10, 100, 500, and 1000 ng/ml rapamycin for 5 days and were then lysed using RIPA buffer (5 ml 1M tris-HCl pH 7.4, 30 ml 5M NaCl, 5 ml 20% Triton-X, 5 ml 10% sodium deoxycholate, 0.5 ml 20% SDS, 50 ml ddH2O). Protein was isolated and concentration was determined by Bradford assay (BioRad).
Isolation of Mouse Glomeruli
Glomeruli were isolated from 3-week-old mice using Dynabead (Invitrogen) magnetic bead perfusion as previously described.39,40 Glomeruli from four mice were pooled in each sample and homogenized in radioimmunoprecipitation assay buffer. Protein concentration was determined by Bradford assay (BioRad).
Western Blotting
Protein electrophoresis was performed on protein isolated from mouse glomeruli or cultured human podocytes. Antibodies against MTOR (#2972; Cell Signaling), LC3 (PM046; MBL), phospho-P70S6K (#9205; Cell Signaling,), total P70S6K (#9202; Cell Signaling), and β-actin (ab8227; Abcam) were used. A goat anti-rabbit IgG-horseradish peroxidase secondary antibody was used for all of the above primary antibodies (sc-2054; Santa Cruz).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Doug Holmyard and Ken Harpal for electron microscopy and histologic stains and Anne-Claude Gingras, John Brumell, and the entire Quaggin laboratory for helpful discussions. We also thank Jeffrey Zaltzman whose thoughtful question stimulated this study.
This work was supported by the Kidney Foundation of Canada (KFOC110014), Canadian Institutes of Health Research (CIHR MOP62931 and MOP77756), and CIHR Team Grant/Terry Fox TFF105268 to S.E.Q. D.P.C. is supported by an American Society of Nephrology Student Scholar Grant. S.E.Q. holds the Gabor-Zellerman Chair in Renal Research in the Department of Medicine at the University of Toronto.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011070690/-/DCSupplemental.
References
- 1.Zoncu R, Efeyan A, Sabatini DM: mTOR: From growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12: 21–35, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacinto E, Loewith R, Schmidt A, Lin S, Rüegg MA, Hall A, Hall MN: Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 6: 1122–1128, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Guertin DA, Sabatini DM: The pharmacology of mTOR inhibition. Sci Signal 2: pe24, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Zeng Z, Sarbassov D, Samudio IJ, Yee KW, Munsell MF, Ellen Jackson C, Giles FJ, Sabatini DM, Andreeff M, Konopleva M: Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood 109: 3509–3512, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM: Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22: 159–168, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Vollenbröker B, George B, Wolfgart M, Saleem MA, Pavenstädt H, Weide T: mTOR regulates expression of slit diaphragm proteins and cytoskeleton structure in podocytes. Am J Physiol Renal Physiol 296: F418–F426, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Schena FP, Pascoe MD, Alberu J, del Carmen Rial M, Oberbauer R, Brennan DC, Campistol JM, Racusen L, Polinsky MS, Goldberg-Alberts R, Li H, Scarola J, Neylan JF; Sirolimus CONVERT Trial Study Group: Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT trial. Transplantation 87: 233–242, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Tumlin JA, Miller D, Near M, Selvaraj S, Hennigar R, Guasch A: A prospective, open-label trial of sirolimus in the treatment of focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 1: 109–116, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wüthrich RP: Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 363: 820–829, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Walz G, Budde K, Mannaa M, Nürnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Hörl WH, Obermüller N, Arns W, Pavenstädt H, Gaedeke J, Büchert M, May C, Gschaidmeier H, Kramer S, Eckardt KU: Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 363: 830–840, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Perico N, Antiga L, Caroli A, Ruggenenti P, Fasolini G, Cafaro M, Ondei P, Rubis N, Diadei O, Gherardi G, Prandini S, Panozo A, Bravo RF, Carminati S, De Leon FR, Gaspari F, Cortinovis M, Motterlini N, Ene-Iordache B, Remuzzi A, Remuzzi G: Sirolimus therapy to halt the progression of ADPKD. J Am Soc Nephrol 21: 1031–1040, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinto Marin A, Redondo Sanchez A, Espinosa Arranz E, Zamora Aunon P, Castelo Fernandez B, Gonzalez Baron M: mTOR pathway inhibition in renal cell carcinoma [published online ahead of print March 4, 2010]. Urol Oncol doi:10.1016/j.urolonc.2009.11.008 [DOI] [PubMed]
- 13.Budde K, Becker T, Arns W, Sommerer C, Reinke P, Eisenberger U, Kramer S, Fischer W, Gschaidmeier H, Pietruck F; ZEUS Study Investigators: Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: An open-label, randomised, controlled trial. Lancet 377: 837–847, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Letavernier E, Bruneval P, Mandet C, Duong Van Huyen JP, Péraldi MN, Helal I, Noël LH, Legendre C: High sirolimus levels may induce focal segmental glomerulosclerosis de novo. Clin J Am Soc Nephrol 2: 326–333, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Sartelet H, Toupance O, Lorenzato M, Fadel F, Noel LH, Lagonotte E, Birembaut P, Chanard J, Rieu P: Sirolimus-induced thrombotic microangiopathy is associated with decreased expression of vascular endothelial growth factor in kidneys. Am J Transplant 5: 2441–2447, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA: Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stallone G, Infante B, Pontrelli P, Gigante M, Montemurno E, Loverre A, Rossini M, Schena FP, Grandaliano G, Gesualdo L: Sirolimus and proteinuria in renal transplant patients: Evidence for a dose-dependent effect on slit diaphragm-associated proteins. Transplantation 91: 997–1004, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Gödel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, Debreczeni-Mór A, Lindenmeyer MT, Rastaldi MP, Hartleben G, Wiech T, Fornoni A, Nelson RG, Kretzler M, Wanke R, Pavenstädt H, Kerjaschki D, Cohen CD, Hall MN, Rüegg MA, Inoki K, Walz G, Huber TB: Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest 121: 2197–2209, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami M, Ichisaka T, Maeda M, Oshiro N, Hara K, Edenhofer F, Kiyama H, Yonezawa K, Yamanaka S: mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol 24: 6710–6718, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, Um SH, Brown EJ, Cereghini S, Thomas G, Kozma SC: Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol 24: 9508–9516, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li HP, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T: Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440: 818–823, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Land SC, Tee AR: Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J Biol Chem 282: 20534–20543, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE: Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eremina V, Cui S, Gerber H, Ferrara N, Haigh J, Nagy A, Ema M, Rossant J, Jothy S, Miner JH, Quaggin SE: Vascular endothelial growth factor a signaling in the podocyte-endothelial compartment is required for mesangial cell migration and survival. J Am Soc Nephrol 17: 724–735, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Mizushima N, Levine B, Cuervo AM, Klionsky DJ: Autophagy fights disease through cellular self-digestion. Nature 451: 1069–1075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartleben B, Gödel M, Meyer-Schwesinger C, Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstädt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB: Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 120: 1084–1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narita M, Young AR, Arakawa S, Samarajiwa SA, Nakashima T, Yoshida S, Hong S, Berry LS, Reichelt S, Ferreira M, Tavaré S, Inoki K, Shimizu S, Narita M: Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science 332: 966–970, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ost A, Svensson K, Ruishalme I, Brännmark C, Franck N, Krook H, Sandström P, Kjolhede P, Strålfors P: Attenuated mTOR signaling and enhanced autophagy in adipocytes from obese patients with type 2 diabetes. Mol Med 16: 235–246, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, Mi N, Zhao Y, Liu Z, Wan F, Hailey DW, Oorschot V, Klumperman J, Baehrecke EH, Lenardo MJ: Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature 465: 942–946, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischer EG, Moore MJ, Lager DJ: Fabry disease: A morphologic study of 11 cases. Mod Pathol 19: 1295–1301, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina D, Settembre C, Gavina M, Pulze L, Giardino I, Pettoello-Mantovani M, D’Apolito M, Guido S, Masliah E, Spencer B, Quaratino S, Raia V, Ballabio A, Maiuri L: Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat Cell Biol 12: 863–875, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Grumati P, Coletto L, Sabatelli P, Cescon M, Angelin A, Bertaggia E, Blaauw B, Urciuolo A, Tiepolo T, Merlini L, Maraldi NM, Bernardi P, Sandri M, Bonaldo P: Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat Med 16: 1313–1320, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Rüegg MA, Hall MN, Kwiatkowski DJ, Rastaldi MP, Huber TB, Kretzler M, Holzman LB, Wiggins RC, Guan KL: mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest 121: 2181–2196, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding M, Cui S, Li C, Jothy S, Haase V, Steer BM, Marsden PA, Pippin J, Shankland S, Rastaldi MP, Cohen CD, Kretzler M, Quaggin SE: Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med 12: 1081–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Liu P, Jenkins NA, Copeland NG: A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res 13: 476–484, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodríguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM: High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet 25: 139–140, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui S, Li C, Ema M, Weinstein J, Quaggin SE: Rapid isolation of glomeruli coupled with gene expression profiling identifies downstream targets in Pod1 knockout mice. J Am Soc Nephrol 16: 3247–3255, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.