Abstract
Renal tubular atrophy accompanies many proteinuric renal diseases, suggesting that glomerular proteinuria injures the tubules. However, local or systemic inflammation and filtration of abnormal proteins known to directly injure tubules are also present in many of these diseases and animal models; therefore, whether glomerular proteinuria directly causes tubular injury is unknown. Here, we examined the renal response to proteinuria induced by selective podocyte loss. We generated mice that express the diphtheria toxin receptor exclusively in podocytes, allowing reproducible dose-dependent, specific ablation of podocytes by administering diphtheria toxin. Ablation of <20% of podocytes resulted in profound albuminuria that resolved over 1–2 weeks after the re-establishment of normal podocyte morphology. Immediately after the onset of albuminuria, proximal tubule cells underwent a transient burst of proliferation without evidence of tubular damage or increased apoptosis, resulting in an increase in total tubular cell numbers. The proliferative response coincided with detection of the growth factor Gas6 in the urine and phosphorylation of the Gas6 receptor Axl in the apical membrane of renal tubular cells. In contrast, ablation of >40% of podocytes led to progressive glomerulosclerosis, profound tubular injury, and renal failure. These data suggest that glomerular proteinuria in the absence of severe structural glomerular injury activates tubular proliferation, potentially as an adaptive response to minimize the loss of filtered proteins.
Podocytes, mesangial cells, and glomerular endothelial cells together contribute to the formation and maintenance of the kidney ultrafiltration barrier that serves to retain the majority of plasma proteins within the vascular space.1–5 This barrier can be compromised by injury to any of these cells, resulting in glomerular proteinuria that has in turn been associated with progressive renal dysfunction. Of these cells, podocyte damage is the most problematic because podocytes provide a critical component of the filtration barrier and seem to lack the ability to proliferate in response to cell loss. Multiple causes of podocyte dysfunction have been identified, including genetic defects in components of the slit diaphragm, toxins, deposition of abnormal proteins in the glomerular basement membrane (GBM), and immune-mediated injury.6–8
There have been numerous animal models developed to study the effect of glomerular injury on kidney function.9,10 Typically, these models involve injection of general cellular toxins such as adriamycin or nephritic serum containing antibodies directed against either the GBM or podocyte-specific antigens. In most cases, there is widespread damage to multiple cell types in the glomerulus with a substantial inflammatory response, making it difficult to determine which components of the subsequent kidney injury are related specifically to podocyte injury and which are related to the ensuing response. One of the key responses to glomerular damage is albuminuria, and several studies have suggested that glomerular albuminuria can lead to subsequent tubule damage.11,12 In vitro studies in which albuminuria was modeled by culturing tubular cells in the presence of albumin have led to conflicting results. Whereas some groups demonstrated that albumin can cause oxidative stress13 and programmed cell death,14,15 other studies showed that albumin is one of the major serum survival factors for renal tubular cells and can serve to scavenge reactive oxygen species.16,17
To induce selective podocyte loss via a nonimmune mechanism, we utilized mice that contained the transgene for diphtheria toxin receptor (DTR) downstream of a polyadenylation signal flanked by loxP sites (iDTR mouse18). Because mice normally lack the DTR, expression of the Cre recombinase in a podocyte-specific manner (Pod-Cre19) results in DTR expression selectively in podocytes, allowing subsequent podocyte ablation induced by diphtheria toxin (DT) to be performed in a dose-dependent manner without concomitant injury to other glomerular components. A similar approach has been successfully used for selective podocyte ablation in the rat.20 In these studies, we demonstrate that structural tubular injury occurs only when podocyte loss is sufficient to cause glomerular damage, and is not seen in the setting of isolated glomerular albuminuria. In fact, modest podocyte loss results in marked albuminuria that is accompanied by a proliferative response of proximal tubule cells without evident tubular injury, leading to an increase in total proximal tubule cell numbers. We propose that the proteinuria that accompanies selective podocyte loss, in the absence of more generalized glomerular injury, leads to an increase in proximal tubule cell numbers that may provide an adaptive response to limit the loss of filtered proteins in the urine.
Results
iDTR+/−;Pod-Cre+/− Mice Express DTR in Podocytes
iDTR+/−;Pod-Cre+/− mice were found to be viable and fertile. To examine the expression pattern of DTR, multiple organs were isolated from double transgenic (iDTR+/−;Pod-Cre+/−)18,19 and control mice (iDTR+/−, Pod-Cre+/−, and wild type). These included heart, lung, liver, pancreas, small intestine, spleen, kidney, and skeletal muscle. Immunofluorescence microscopy demonstrated that DTR expression was found only in the glomeruli of the iDTR+/−;Pod-Cre+/− mice, with anti-Wt1 and antinephrin staining confirming co-localization restricted to podocytes (Figure 1, A and B and data not shown). No DTR expression was detected in any other organ of iDTR+/−;Pod-Cre+/− mice or in any organ of iDTR+/- or Pod-Cre+/− mice. Analysis of DTR immunostaining of multiple kidney sections from iDTR+/−;Pod-Cre+/− mice demonstrated that 99.5% of Wt1+ podocytes co-expressed the DTR.
Figure 1.
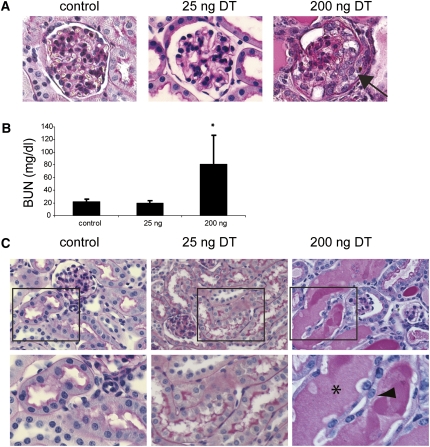
DTR expression in podocytes. Immunofluorescent staining with anti-DTR (green) and anti-Wt1 (pink) was performed to evaluate kidney sections. (A) Podocytes of the single transgenic iDTR+/− mouse do not express DTR (arrow, podocyte expressing Wt1). (B) Podocytes from iDTR+/−;Pod-Cre+/− express DTR only in podocytes (arrow, podocyte double positive for DTR and Wt1). (C) After 25 ng DT, podocytes numbers are reduced. The white arrow indicates a DTR+/Wt1+ podocyte that survived the initial ablation by DT. (D) Podocytes are undetectable after treatment with 200 ng DT. Paraffin sections at 3 μm. Original magnification, ×400.
DT Exposure Selectively Ablates Podocytes in iDTR+/−;Pod-Cre+/− Mice
It has been demonstrated that binding of one molecule of DT to the DTR will trigger cell death.21,22 Increasing the dose of DT injected should therefore result in progressively greater podocyte loss. The rate of podocyte ablation was tested in 4- to 5-week-old mice (18–20 g body wt) exposed to DT doses ranging from 25 to 200 ng. Double immunostaining using anti-Wt1 and anti-DTR antibodies was performed to calculate the ablation rate. One week after a single dose of 25 ng DT there was a 19.6% decrease in podocyte numbers (from 11.6±3.2 per glomerular cross-section to 9.3±2.4, n=11 mice, P<0.001), whereas 50 ng DT led to 46.1% podocyte loss (6.8±1.1 podocytes per glomerular cross-section, n=7 mice, P<0.001) and 200 ng DT resulted in complete loss of Wt1-expressing cells (n=3 mice, P<0.001).
Podocytes Fail to Proliferate after Partial Ablation
Semi-quantitative analysis of Wt1-expressing cells in the glomeruli of mice treated with 200 ng DT reveals a progressive decline in podocyte numbers beginning after the first day (Figure 2A). Transmission electron microscopy (EM) of these glomeruli revealed that there are no detectable changes 24 hours after DT injection (Figure 2B), whereas effacement of podocyte foot processes was obvious by day 3 (when total podocyte numbers are reduced by 75%). On day 5, retraction of foot processes with microvillous transformation of the podocytes had occurred, leading to podocyte detachment and complete denudation of the GBM by day 7 at which time <1 podocyte/glomerulus was detectable by Wt1 or DTR staining (Figure 2, A and B). Transmission electron microscopy performed after 25-ng DT injection revealed focal podocyte effacement in >80% of the glomeruli examined on day 7, but surviving podocytes remained attached and foot process morphology returned to normal by 4 weeks (Figure 2C).
Figure 2.
Reduction of podocyte numbers after DT treatment. (A) Podocyte numbers were estimated after Wt1 immunostaining. Treatment with 200 ng DT resulted in a time-dependent loss of Wt1+ podocytes. ***P<0.005 compared with control. n=3 mice for each group. (B) EM analysis after 200 ng DT injection. (a) Untreated control glomeruli demonstrate normal foot processes (arrow). (b) Podocytes retain normal foot processes 1 day after DT injection (arrow). (c) Foot process effacement is evident 3 days after DT injection (arrow), along with microvillous transformation (arrowhead). (d) GBM is denuded 5 days after injection (arrow) with microvillous transformation of the podocytes (arrowhead). Original magnification, ×10,000. CL, capillary lumen. (C) (a) EM analysis of a control glomerulus showing normal ultrastructure of the foot processes (arrowhead). (b) Focal areas of foot process effacement are evident 7 days after 25 ng DT treatment (arrowhead). (c) Foot process morphology is normal 30 days after 25 ng DT treatment (arrowhead). (D) ELISA quantification of albumin loss in the urine. *P<0.05, ***P<0.005, and #P<0.0001 versus control. (E) Wt1 positive cell numbers were counted per glomerular cross-section at the indicated times after 25 ng DT injection. n=44, 7, and 11 for control, day 7, and day 30, respectively. P<0.0001 by one-way ANOVA.
Consistent with the initial loss of foot process integrity after administration of either 25 ng or 200 ng DT, the concentration of albumin in the urine increased to a similar degree in both groups beginning 3–5 days after DT treatment (Figure 2D). In the mice treated with 200 ng DT, albuminuria persisted until their death on day 9–14, whereas those treated with 25 ng DT exhibited a peak of albuminuria between days 5 and 7 with resolution by days 9 and 10. Normalization of albumin concentration to creatinine revealed equal proteinuria on day 5 with an increase in the normalized urine albumin value on day 7 in the group treated with 200 ng DT due to a marked decrease in urinary creatinine concentration that correlated with the acute renal failure and oliguria present at that time point (Supplemental Figure 1).
To determine if resolution of foot process effacement in the group treated with 25 ng DT was due to proliferation of surviving podocytes, mice were given bromodeoxyuridine (BrdU) daily in their drinking water for up to 28 days after DT injection, and then sections were stained for BrdU, Wt1, and DTR. Neither BrdU+Wt1+ nor BrdU+DTR+ cells were detected along the GBM (data not shown). Staining for Ki-67 also failed to detect any podocyte proliferation after DT treatment. Consistent with the absence of detectable podocyte proliferation, podocyte numbers fell to a nadir of 9.3±2.4 per glomerular cross-section by 7 days after 25-ng DT injection and then remained constant for the following 3 weeks (Figure 2E). The absence of detectable podocyte proliferation or restoration of podocyte numbers in adult mice during the 30 days after treatment with 25 ng DT suggests that podocyte hypertrophy is the primary feature that accounts for the reconstitution of normal podocyte morphology and foot process function in these animals.
Tubular Injury and Decline of Renal Function Correlates with Glomerular Damage
Light microscopic and functional changes closely mirrored these podocyte events. After treatment with 25 ng DT, most glomeruli were normal at the light microscopic level with rare glomeruli showing focal areas of glomerulosclerosis (Figure 3A and data not shown), and BUN values remained normal (Figure 3B). However, 200 ng DT led to development of glomerular pseudocysts, crescent formation, collapse of the glomerular tuft, and glomerulosclerosis (Figure 3A), with markedly increased BUN values and animal death by day 10–14 days after treatment (Figure 3B). Mice receiving 50–100 ng DT exhibited an intermediate phenotype with the presence of glomerulosclerosis beginning at 1 week after treatment and progressively worsening by 4 weeks (Supplemental Figure 2A). Interestingly, DTR+/α-smooth muscle actin+ cells were occasionally found in these crescents (Supplemental Figure 2B). To note, administration of as much as 400 ng/d DT for 6 consecutive days had no detectable adverse effect on control mice (wild-type, iDTR+/-, or Pod-Cre+/− single transgenic mice) as indicated by normal survival, normal histology of various organs, absence of albuminuria, and normal renal function (data not shown).
Figure 3.
Extensive podocyte ablation leads to glomerulosclerosis and tubular injury. (A) Representative glomerular histology 7 days after control, 25 ng DT injection, or 200 ng DT injection. Arrow, crescent formation. Original magnification, ×400. (B) BUN values 1 week after DT treatment. n=13, 7, and 15 for control, 25 ng group, and 200 ng group, respectively. *P<0.001 versus control. (C) Representative tubular histology 7 days after control, 25 ng DT injection, or 200 ng DT injection. In the 200 ng–treated animals, there is extensive cast formation (asterisk) and epithelial simplification (arrowhead).
Although the concentration of urinary albumin on days 5 and 7 was comparable between mice receiving 25 and 200 ng DT (Figure 2D), histologic changes in the tubulointerstitial compartment were markedly different and strongly correlated with those in the glomerulus. The tubules of mice treated with 25 ng DT had preserved cellular architecture with no interstitial changes observed and only occasional proteinaceous casts on day 7 (Figure 3C), with no detectable casts or tubular changes present on day 14. In contrast, tubular damage was extensive on day 7 in those mice treated with 200 ng DT, consisting of marked cast formation, tubular cell flattening. and interstitial damage (Figure 3C). Again, animals receiving a 50-ng dose of DT exhibited a less rapid progression of the tubular changes, but had obvious cast formation, tubular simplification, and interstitial changes that progressively worsened over the 4 weeks after DT (Supplemental Figure 2A). These findings demonstrate that the degree of tubular injury and renal failure correlates closely with the severity of glomerular damage but not with the level of proteinuria.
Podocyte Damage Stimulates Proliferation of Proximal Tubular Cells
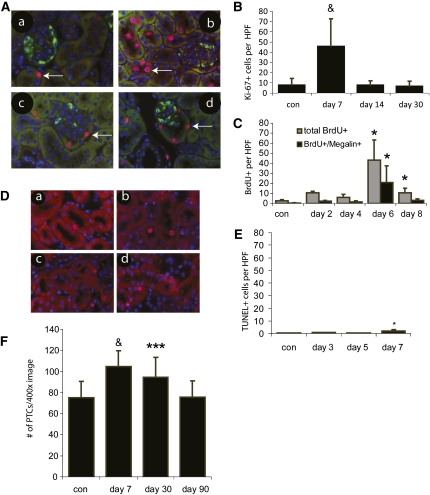
Although no BrdU+ or Ki67+ podocytes were detected in the glomerular tufts of the DT-treated mice, large numbers of tubular cells were found to be positive for proliferation markers in mice treated with either 25 or 200 ng DT (Figure 4A and data not shown). In addition, tubular cells at the urinary pole of Bowman’s capsule were also found to be proliferating (Figure 4, A, C, and D). To further characterize the tubular proliferation in response to podocyte ablation, a time course of the proliferative response was established in mice treated with 25 ng DT. The rate of tubular proliferation was found to increase after day 5, peak on days 6–7, and return to baseline after day 8 (Figure 4, B and C). This timing correlates closely with the onset (days 4–5) and resolution (day 8) of albuminuria seen with this dose of DT (Figure 2D). Approximately 50% of the proliferating cells 1 week after partial podocyte ablation were in the proximal tubule based on co-staining with megalin, with the remaining proliferating cells found in the interstitial compartment, other tubular segments, and rarely in glomeruli (nonpodocytes) (Figure 4C).
Figure 4.
Tubular proliferation after low-level podocyte ablation. (A) Ki-67 immunostaining to identify cell proliferation (a-d) double immunostaining (anti-Ki-67 [red] and anti-DTR [green]) to identify proliferating cells after DT treatment. (a) Control (arrows indicate Ki-67+ tubular cells). (b) Multiple tubular cells are Ki-67+ 1 week after 25 ng DT treatment. (c and d) Two and 4 weeks after 25 ng DT, proliferating tubular cells are still detected in the urinary pole of Bowman’s capsule (arrows). Original magnification, ×400. (B) Quantification of Ki-67+ tubular cells after administration of 25 ng DT to DTR+/−;Pod-Cre+/− mice. n=4, 3, 6, and 3 for control, day 7, day 14, and day 30, respectively. &P<0.001 versus control. (C) Quantification of BrdU-positive cells as well as BrdU+Megalin+ cells after 25 ng DT injection. n=4 for no DT group, n=3 for days 2, 4, 6, and 8. *P<0.05 versus control. (D) Terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling+ (TUNEL) cells from (a) control, (b) 3 days after DT treatment, (c) 5 days after DT treatment, and (d) 7 days after 25 ng DT treatment. (E) Quantification of TUNEL+ cells counted from randomly chosen ×400 fields in renal cortex. n=5 each. *P<0.05 versus control. Panels B, C, and E are plotted with the same y-axis scale to present the relative levels of proliferation versus apoptosis. (F) Counting of proximal tubular cells per ×400 field reveals an increase in proximal tubule cells at 7 and 30 days after DT treatment. By day 90, however, proximal tubular cells returned to baseline. n=4, 6, 4, and 5 for control, day 7, day 30, and day 90, respectively. &P<0.001 versus control, ***P<0.005 versus control.
Addition of albumin has been shown to induce apoptosis of cultured tubular cells.23 Thus, it was possible that the tubular proliferation occurred in response to albuminuria-induced apoptosis and reduced tubular cell numbers. However, terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling staining at 3, 5, and 7 days after podocyte ablation with 25 ng DT revealed that there was no increase in tubular cell apoptotic rates on days 3 and 5 (i.e., preceding the proliferation observed on days 6–7) and only a modest increase in apoptotic rates on day 7 (Figure 4, D and E). Furthermore, counting the nuclei of brush border positive tubular cells revealed a significant increase of PTC by day 7 (P<0.001) and day 30 (P<0.005) after 25 ng DT compared with baseline (Figure 4F). At 3 months after 25-ng DT injection, a time when albuminuria has completely resolved, tubular cell numbers had returned to normal.
Activation of Tubular Cell Axl Coincides with Proteinuria-Induced Proliferation
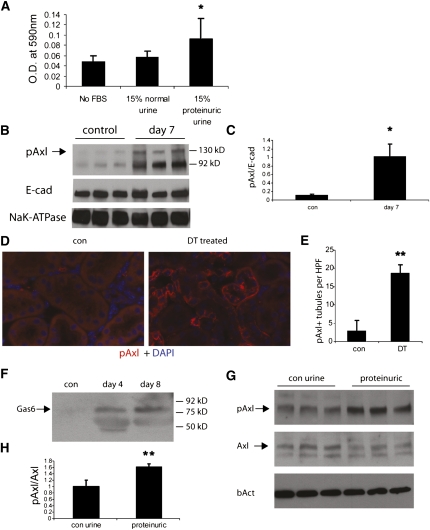
To determine if glomerular proteinuria results in exposure of tubular cells to growth factors present in the serum, we examined the effect of proteinuric urine on tubular cell proliferation. Urine collected from mice 7 days after treatment with 25 ng DT significantly stimulated cell proliferation of cultured proximal tubular cells, whereas control urine had no effect (Figure 5A). To examine the mechanism of this effect, we isolated membrane microsomes from total kidney homogenates of both controls and mice treated with 25 ng DT to determine if they exhibited differential tyrosine kinase receptor activation. Incubation of the microsomes on an array that can detect phosphorylation of 39 different receptor tyrosine kinases (RTKs) (mouse RTK array; R&D Systems, Minneapolis, MN) revealed increased phosphorylation of the PDGF receptor-α (PDGFR-α) and the Gas6 receptor (Axl) in the microsomes from DT-treated mice (Supplemental Figure 3). Western blot analysis of microsome fractions with two separate phospho-specific antibodies (site 754 and 742) against PDGFR-α failed to confirm activation of this receptor in kidneys from DT-treated mice (data not shown). However, phospho-Axl levels were reproducibly increased in microsomes isolated from DT-treated kidneys 7 days after DT injection (Figure 5B, quantified in Figure 5C), correlating with the peak of cell proliferation at this time. Immunofluorescence staining of kidney sections from control and DT-treated mice revealed that Axl phosphorylation occurs primarily in tubular cells, with detection of the activated receptor on both the apical and basolateral membranes (Figure 5, D and E).
Figure 5.
Increased Axl phosphorylation after podocyte damage. (A) MTT assay for cell proliferation. n=3 each. *P<0.05 versus No FBS control. (B) Western blotting of microsomal proteins shows increased Axl phosphorylation in DT-treated kidneys. Blotting against E-cadherin and Na,K-ATPase was performed to confirm equal loading. (C) Quantification of microsomal blotting results as shown in panel A (pAxl levels normalized to E-cadherin levels). n=3. *P<0.05. (D) Immunofluorescence staining of kidney sections for pAxl in iDTR+/−;Pod-Cre+/− under control conditions (con) and 7 days after 25 ng DT. Both slides were processed identically. (E) Counting of all pAxl+ tubules per cross-section reveals a significant increase after DT treatment compared with untreated control mice. **P<0.01. HPF, high power field using ×40 objective lens. (F) urine was obtained from iDTR+/−;Pod-Cre+/− mice under control conditions and 4 and 8 days after treatment with 25 ng DT, and equal protein (5 μg) loaded and immunoblotted for Gas6 (predicted molecular mass is 85 kD). (G) Urine from iDTR+/−;Pod-Cre+/− mice 7 days after DT treatment stimulates Axl phosphorylation. *P<0.05. (H) Quantification of Western blot results as shown in G. n=3. **P<0.01.
Western blotting of urine samples collected from DT-treated mice detected the presence of the Axl ligand, Gas6, during the proteinuric period between days 4 and 8, but not in control urine (Figure 5F). Consistent with the possibility that Gas6 in the proteinuric urine can activate tubular cell Axl, incubation of murine proximal tubular cells (MPT cells) with urine from DT-treated mice induced a 1.8-fold increase in Axl phosphorylation as compared with control, nonproteinuric urine (Figure 5G, quantified in Figure 5H).
Discussion
The experiments performed in this study were designed to determine the glomerular and tubular response to selective podocyte ablation. Analysis of the data led us to three conclusions that we believe are pertinent to our understanding of the pathogenesis of CKD after glomerular injury in humans. First, the glomerulus and the tubules seem to have the ability to adapt to a modest loss of podocytes (<20%) that allows resolution of the proteinuria without a subsequent loss of surviving podocytes or progressive glomerular or tubular injury. Second, the loss of large numbers of podocytes (>40%) exceeds these adaptive responses and leads to sustained proteinuria and progressive glomerulosclerosis. Finally, tubular cell injury in response to podocyte loss correlates with the degree of secondary glomerular injury (crescent formation and glomerulosclerosis), rather than the degree of proteinuria.
In agreement with Wharram et al.,20 who reported a similar model of podocyte ablation in the rat, data from this study demonstrate that ablation of <20% of podocytes results in transient albuminuria followed by resolution. In most epithelial cell populations, this reparative response involves activation of proliferation to increase cell numbers, as is seen in the proximal tubule after ischemia/reperfusion injury.24,25 However, consistent with prior studies in adult animals, we found no evidence for adaptive proliferation of surviving podocytes or a recovery of podocyte numbers along the GBM during the 4 weeks after ablation with 25 ng DT. Even though podocyte numbers never recovered after partial ablation, the morphology of the podocytes returned to normal over several weeks and albuminuria resolved. This suggests that the early adaptive response to partial podocyte loss may involve hypertrophy of surviving podocytes to cover a greater area of GBM.
The second conclusion is that loss of a larger number of podocytes (>40% in our study) seems to exceed the capacity for glomerular adaptation and results in progressive glomerular damage. In this setting, some surviving podocytes lose Wt1 expression and contribute to the formation of cellular crescents. We found occasional examples of cells within crescents that were positive for BrdU or Ki-67 and continued to express DTR even though they had lost Wt1 expression. Although we found no evidence that these DTR+ cells ever re-express Wt1 or contribute to the podocyte population along the GBM, these data suggest that podocytes are not truly postmitotic, rather they are prevented from proliferating due to some aspect of their microenvironment.
The third finding is that albuminuria due to podocyte loss without persistent structural glomerular damage did not lead to significant tubular injury or progressive renal dysfunction within the timeframe of our study. This is consistent with data from patients with minimal change disease, in whom massive albuminuria occurs after foot process fusion yet tubular cells remain structurally intact unless ensuing hypotension leads to acute tubular necrosis or the glomeruli develop the structural changes of focal glomerulosclerosis.
Because the longest duration of tubule exposure to albuminuria was approximately 5 days in our 25-ng DT ablation model, we cannot rule out the possibility that sustained albuminuria is eventually problematic for tubular cell survival. However, we can clearly say that even a brief period of glomerulosclerosis/crescent formation (group treated with 200 ng DT) leads to severe tubular damage that is not seen with albuminuria alone. This observation is consistent with the findings of Kriz and LeHir, who showed that tubular injury in experimental proteinuric diseases correlates with the anatomic finding of occlusion of urine passage from Bowman’s space into the proximal tubule.26
On the basis of our in vitro and in vivo findings, it is our belief that serum factors other than albumin that are present in the proteinuric glomerular ultrafiltrate induce the observed tubule cell proliferation. There are multiple candidate factors present in serum that are known to have a proliferative effect on tubular cells, including Hgf, Egf, Igf1, and Vegf. On the basis of our non-biased approach, an additional mitogen, Gas6, is now implicated in this response. Gas6 is a secreted protein that can exist in the free state or complexed with soluble Axl (sAxl, the extracellular domain of the Axl receptor). Binding of Gas6 to full-length Axl on the surface of cells results in receptor phosphorylation and activation of anti-apoptotic and pro-proliferative signaling pathways such as the PI3K cascade.27,28 Consistent with the possibility that urinary Gas6 is playing a significant role in the tubular cell response to albuminuria, we observed that phosphorylated Axl (pAxl) is present at the apical membrane of proximal tubular cells from proteinuric kidneys. Interestingly, pAxl is also present at the basolateral membrane in these kidneys. It is unclear whether this is due to increased local production of Gas6 in the renal interstitium in response to the proteinuria or due to megalin-dependent transcytosis of the intact ligand.29,30 This second possibility is of particular interest in light of the observation by Theilig et al. that proteinuria causes tubular cell proliferation in a megalin-dependent fashion.31
In conclusion, the iDTR+;Pod-Cre+ mouse provides an excellent model to study the effects of selective podocyte loss in the pathogenesis of glomerular injury. Our data indicate that selective loss of a small number of podocytes leads to transient proteinuria with subsequent resolution despite the absence of podocyte proliferation. The proteinuria itself seems to stimulate proliferation of tubular cells without evidence of tubule injury, scarring, or loss of kidney function. However, more substantial loss of podocytes results in scarring and crescent formation, coinciding with severe tubular injury and kidney failure. More extensive studies are required to determine the exact mechanism of tubule proliferation and to ascertain whether the increase in cell numbers provides an adaptive advantage by increasing the number of cells available for reabsorption of abnormally filtered proteins and other small molecules.
Concise Methods
Mouse Care and Genotyping
Mouse protocols were approved by the Yale University Institutional Animal Care and Use Committee. The iDTR mouse was kindly provided by Dr. Waisman (Mainz, Germany)18 and the Podocin-Cre from Dr. Lawrence Holzman (University of Pennsylvania).19 The iDTR mice were genotyped by PCR using primers 5′-gaccatgaagctgctgccgtc-3′ (DTR sense) and 5′-tcagtgggaattagtcatgc-3′ (DTR antisense). The Podocin-Cre mice were genotyped by PCR using primers 5′-acagctccaccaagacacag-3′ (human podocin promoter, sense) and 5′-tccggttattcaacttgcacc-3′ (Cre, antisense).
DT Treatment
DT was dissolved in sterile 1×PBS at 50 μg/ml. The stock solution was then aliquoted and frozen at −80°C until use. DT solution was thawed and diluted to 100 ng/ml, 250 ng/ml, 500 ng/ml, or 1000 ng/ml for intraperitoneal injections. To test the dose-response curve of DT treatment, both male and female double transgenic mice aged 4–5 weeks were used (18–20 g body wt).
BrdU Administration
BrdU was first dissolved in DMSO at 50 mg/ml. Aliquots were frozen at −20°C till use. BrdU was administered in drinking water at 200 μg/ml unless otherwise stated. BrdU water was changed every 3 days.
Urine Analysis and BUN Quantification
Urine albumin levels were quantified by ELISA using a kit purchased from Bethyl Laboratories following the manufacturer's protocol. Serum BUN values were performed by the Mouse Metabolic Phenotyping Center at Yale University using the COBAS Integra system (Roche, Indianapolis, IN).
Podocyte and Proximal Tubular Cell Quantification
Podocyte numbers were estimated by counting the number of Wt1-positive cells per glomerular cross-section in 20 randomly selected glomeruli/kidney as described by Shibata et al.32 The mean of these counts thus represents the average number of podocytes per glomerular cross-section, rather than the total number of podocytes per glomerulus.
To count proximal tubular cells, slides were stained by periodic acid–Schiff (PAS) and counterstained with hematoxylin. At least 10 random fields from the renal cortex were imaged with one glomerulus in the center of the picture. Image J software was used to count nuclei of pink (proximal tubular brush border stained by PAS) cells per image. An average of 10 images was used for each mouse (n=5 mice for each group).
Histology and Fluorescent Immunostaining
After sacrifice, kidneys were gently sliced into two pieces, fixed in 1×PBS buffered formalin for 12 hours, embedded in paraffin, and sectioned at 3 μm. To review histology, slides were stained by PAS. Immunostaining on paraffin embedded tissue was performed as described33 using rabbit polyclonal antibody against Wt1 (C-19; Santa Cruz Biotechnology, CA), rabbit anti-Ki-67 (Abcam, Cambridge, MA), rabbit anti-nephrin (kindly provided by Dr. Lawrence Holzman, University of Pennsylvania), phosphor-Axl (Y779; US Biologics), and goat anti-DTR (R&D Systems).
Microsome Preparation
Total kidneys were minced into small pieces before being homogenized in homogenization buffer (1× PBS supplemented with phosphatase inhibitors [50 mM sodium fluoride, 15 mM sodium pyrophosphate]) and protease inhibitors (Roche, Indianapolis, IN) using a Potter-Elvehjem Teflon tissue grinder with a loose-fitting Teflon pestle as described.34 The membrane fraction was collected from the supernatant after 2000 × g centrifugation for 10 minutes. Microsomes were concentrated by centrifugation at 100,000 × g for 1 hour. The microsome pellet was then resuspended in 500 μl homogenization buffer and stored at −80°C until use.
Protein Array of Phosphorylated RTKs
The mouse RTK array kit (catalog #ARY014; R&D Systems) was used to screen for kinases being activated in DT-treated kidneys following the manufacturer’s instructions. A total of four arrays with four different samples (two control and two podocyte damaged) were used.
Western Blotting and Quantification
Western blotting was performed following standard protocols. The following antibodies were used: anti-phospho-tyrosine kinase and anti-E-cadherin (Cell Signaling Technology, Inc), anti-phospho-Axl (US Biological), and anti-Gas6 (R&D Systems). Quantification of Western blot results was done using Image J software.
In Vitro Activation of Axl
MPT cells were serum starved overnight, washed, and then incubated in 15% urine (diluted in serum-free culture medium) from either untreated control iDTR+/−;Pod-Cre+/− mice or proteinuric urine on day 7 after treatment with 25 ng DT. Cells were lysed after 2 hours, proteins separated by SDS-PAGE, and immunoblotted following standard protocols.
MTT Assay
Cell proliferation was quantified following the modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method based on the protocol described by the Wallert and Provost.35 Briefly, MTT was dissolved in 1×PBS at 4 mg/ml and filter sterilized before use. Proximal tubular cells of approximately 75% confluence cultured in six-well plates were serum starved for 24 hours and then supplied with serum-free medium versus serum-free medium supplemented with 15% normal urine or proteinuric urine. Eight hours later, MTT solution was added to each well and incubated for 3.5 hours before being terminated for spectrophotometer reading at 590 nm. Experiments were carried out in triplicate for each condition and repeated twice.
Statistical Analyses
Data were presented at mean ± SD throughout the text unless otherwise specified. Data were analyzed using either the t test or ANOVA where appropriate, with P<0.05 regarded as statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Ali Waisman (Mainz, Germany) for providing the iDTR mouse and Dr. Lawrence Holzman (University of Pennsylvania) for providing the podocin-Cre mouse and anti-nephrin antibody. The Yale Mouse Metabolic Phenotyping Center performed the BUN and creatinine measurements.
This study was supported by the following grants from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health: U24 DK76169 (Yale Mouse Metabolic Phenotyping Center); DK075464 (J.K.G.); HL073742, DK061846, and DK072442 (D.S.K.); and DK065109 (L.G.C.). A.S., an undergraduate student from Brown University, was supported by a summer student program from the George M. O'Brien Kidney Center at Yale University (NIH/NIDDK P30DK079310).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011040396/-/DCSupplemental.
References
- 1.Tryggvason K, Wartiovaara J: How does the kidney filter plasma? Physiology (Bethesda) 20: 96–101, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P: Actin up: Regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428–437, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Gubler MC: Podocyte differentiation and hereditary proteinuria/nephrotic syndromes. J Am Soc Nephrol 14[Suppl 1]: S22–S26, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Johnstone DB, Holzman LB: Clinical impact of research on the podocyte slit diaphragm. Nat Clin Pract Nephrol 2: 271–282, 2006 [DOI] [PubMed] [Google Scholar]
- 6.LeHir M, Kriz W: New insights into structural patterns encountered in glomerulosclerosis. Curr Opin Nephrol Hypertens 16: 184–191, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Nosadini R: Hypertension and renal complications in type 2 diabetes. Semin Vasc Med 2: 109–119, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Shah SN, He CJ, Klotman P: Update on HIV-associated nephropathy. Curr Opin Nephrol Hypertens 15: 450–455, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Fogo AB: Animal models of FSGS: lessons for pathogenesis and treatment. Semin Nephrol 23: 161–171, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Lu TC, He JC, Klotman P: Animal models of HIV-associated nephropathy. Curr Opin Nephrol Hypertens 15: 233–237, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Erkan E, Devarajan P, Schwartz GJ: Apoptotic response to albumin overload: Proximal vs. distal/collecting tubule cells. Am J Nephrol 25: 121–131, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Donadelli R, Zanchi C, Morigi M, Buelli S, Batani C, Tomasoni S, Corna D, Rottoli D, Benigni A, Abbate M, Remuzzi G, Zoja C: Protein overload induces fractalkine upregulation in proximal tubular cells through nuclear factor kappaB- and p38 mitogen-activated protein kinase-dependent pathways. J Am Soc Nephrol 14: 2436–2446, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Shalamanova L, McArdle F, Amara AB, Jackson MJ, Rustom R: Albumin overload induces adaptive responses in human proximal tubular cells through oxidative stress but not via angiotensin II type 1 receptor. Am J Physiol Renal Physiol 292: F1846–F1857, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Ohse T, Inagi R, Tanaka T, Ota T, Miyata T, Kojima I, Ingelfinger JR, Ogawa S, Fujita T, Nangaku M: Albumin induces endoplasmic reticulum stress and apoptosis in renal proximal tubular cells. Kidney Int 70: 1447–1455, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Caruso-Neves C, Pinheiro AA, Cai H, Souza-Menezes J, Guggino WB: PKB and megalin determine the survival or death of renal proximal tubule cells. Proc Natl Acad Sci USA 103: 18810–18815, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iglesias J, Abernethy VE, Wang Z, Lieberthal W, Koh JS, Levine JS: Albumin is a major serum survival factor for renal tubular cells and macrophages through scavenging of ROS. Am J Physiol 277: F711–F722, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Iglesias J, Levine JS: Albuminuria and renal injury—Beware of proteins bearing gifts. Nephrol Dial Transplant 16: 215–218, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, Waisman A: A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods 2: 419–426, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Yamaizumi M, Mekada E, Uchida T, Okada Y: One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell 15: 245–250, 1978 [DOI] [PubMed] [Google Scholar]
- 22.Yamaizumi M, Uchida T, Takamatsu K, Okada Y: Intracellular stability of diphtheria toxin fragment A in the presence and absence of anti-fragment A antibody. Proc Natl Acad Sci USA 79: 461–465, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erkan E, Devarajan P, Schwartz GJ: Mitochondria are the major targets in albumin-induced apoptosis in proximal tubule cells. J Am Soc Nephrol 18: 1199–1208, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV: Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 115: 1743–1755, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin F, Moran A, Igarashi P: Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney, J Clin Invest 115: 1756–1764, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kriz W, LeHir M: Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67: 404–419, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Goruppi S, Ruaro E, Varnum B, Schneider C: Requirement of phosphatidylinositol 3-kinase-dependent pathway and Src for Gas6-Axl mitogenic and survival activities in NIH 3T3 fibroblasts. Mol Cell Biol 17: 4442–4453, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goruppi S, Ruaro E, Varnum B, Schneider C: Gas6-mediated survival in NIH3T3 cells activates stress signalling cascade and is independent of Ras. Oncogene 18: 4224–4236, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Pohl M, Kaminski H, Castrop H, Bader M, Himmerkus N, Bleich M, Bachmann S, Theilig F: Intrarenal renin angiotensin system revisited: role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem 285: 41935–41946, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marinó M, Andrews D, Brown D, McCluskey RT: Transcytosis of retinol-binding protein across renal proximal tubule cells after megalin (gp 330)-mediated endocytosis. J Am Soc Nephrol 12: 637–648, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Theilig F, Kriz W, Jerichow T, Schrade P, Hähnel B, Willnow T, Le Hir M, Bachmann S: Abrogation of protein uptake through megalin-deficient proximal tubules does not safeguard against tubulointerstitial injury. J Am Soc Nephrol 18: 1824–1834, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, Miyoshi J, Takai Y, Fujita T: Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med 14: 1370–1376, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Charles AK, Mall S, Watson J, Berry PJ: Expression of the Wilms’ tumour gene WT1 in the developing human and in paediatric renal tumours: an immunohistochemical study. Mol Pathol 50: 138–144, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biemesderfer D, DeGray B, Aronson PS: Active (9.6 s) and inactive (21 s) oligomers of NHE3 in microdomains of the renal brush border. J Biol Chem 276: 10161–10167, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Provost J, Wallert M: MTT Proliferation Protocol, Moorehead, MN, Minnesota State University, 2007 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.