Abstract
TGF-β1 upregulates microRNA-192 (miR-192) in cultured glomerular mesangial cells and in glomeruli from diabetic mice. miR-192 not only increases collagen expression by targeting the E-box repressors Zeb1/2 but also modulates other renal miRNAs, suggesting that it may be a therapeutic target for diabetic nephropathy. We evaluated the efficacy of a locked nucleic acid (LNA)–modified inhibitor of miR-192, designated LNA–anti-miR-192, in mouse models of diabetic nephropathy. LNA–anti-miR-192 significantly reduced levels of miR-192, but not miR-194, in kidneys of both normal and streptozotocin-induced diabetic mice. In the kidneys of diabetic mice, inhibition of miR-192 significantly increased Zeb1/2 and decreased gene expression of collagen, TGF-β, and fibronectin; immunostaining confirmed the downregulation of these mediators of renal fibrosis. Furthermore, LNA–anti-miR-192 attenuated proteinuria in these diabetic mice. In summary, the specific reduction of renal miR-192 decreases renal fibrosis and improves proteinuria, lending support for the possibility of an anti-miRNA–based translational approach to the treatment of diabetic nephropathy.
Diabetic nephropathy (DN) is a major microvascular complication of diabetes and the leading cause of ESRD, which can manifest despite tight glycemic control and various therapeutic interventions.1 There is thus an imperative need to identify additional biomarkers and novel targets for better management of DN, which is clinically manifested as microalbuminuria, proteinuria, and progressive glomerular dysfunction. The key pathologic features of DN include podocyte loss, mesangial cell (MC) hypertrophy, glomerular basement membrane thickening, and tubulointerstitial fibrosis due to the increased deposition of extracellular matrix (ECM) proteins such as collagens and fibronectin (FN).2–4 TGF-β1 is increased in several renal cells in diabetes, including MCs, and mediates these profibrotic events, hypertrophy, and cell survival.3,5,6 Therefore, TGF-β has been evaluated as a major target for DN treatment. However, this approach could have drawbacks due to the multifunctional role of TGF-β. Hence, further evaluation of the subtle molecular mechanisms by which TGF-β regulates fibrotic events in renal cells can lead to more effective translational approaches for DN treatment.
MicroRNAs (miRNAs) are small noncoding RNAs that are increasingly recognized as critical players in gene regulation and various diseases.7–10 Interestingly, a cluster of miRNAs is reported to be highly expressed in the kidney, and recent studies show that key miRNAs are upregulated in the kidneys of diabetic mice.11–19 Podocyte-specific deletion of Dicer, a key enzyme in the miRNA biogenesis pathway, depicted proteinuria, renal dysfunction, and several abnormalities.20–22 Deletion of Dicer from proximal tubules could protect against renal ischemia-reperfusion injury.23 However, the in vivo functional relevance of specific miRNAs or their targets in TGF-β signaling and DN pathogenesis is not fully understood. Given that miRNAs are now identified in biologic fluids such as urine and plasma, there is heightened interest in evaluating them as biomarkers of DN and developing translational approaches to block their actions in vivo.
We previously reported that miR-192 is upregulated in TGF-β–treated mouse MCs and in glomeruli of diabetic mice and contributed to increased accumulation of collagen in MCs via targeting and downregulating E-box repressors such as Zeb1 and Zeb2.12 Along with miR-192, there was also increased expression of several other miRNAs, including miR-200b, miR-200c, miR-216a, and miR-217 in mouse MCs treated with TGF-β and renal glomeruli isolated from diabetic mice.12,15 Several E-boxes were found in the upstream promoter regions of collagen type I α2 (Col1a2), Col4a1, miR-216a/217, and the miR-200 family.15,19 Our data suggested that the downregulation of E-box repressors such as Zeb1/2 by miR-192 can upregulate Col1a2, Col4a1, and these downstream miRNAs as well as subsequent Akt kinase signaling to promote MC hypertrophy.15,19 These data implicate miR-192 as a master regulator of other key renal miRNAs and downstream genes to regulate TGF-β signaling and renal MC fibrosis associated with DN.12,15,17,24 In addition, an in vivo role for miR-192 has been suggested in some nondiabetic mouse models of renal fibrosis,16 and the expression of miR-192 was upregulated in human IgA nephropathy, hypertensive nephrosclerosis, and lupus nephritis.25–27 Taken together, these studies suggest that miR-192 may play a critical role in DN pathogenesis; hence, we hypothesized that miR-192 could be a good therapeutic target.
There is great interest in developing RNA-based therapeutics for blocking disease-associated genes and noncoding RNAs. Chemically modified oligonucleotide (oligo) small interfering RNAs and anti-miRNAs have been used to block specific endogenous genes and miR actions in vivo.28,29 More recently, locked nucleic acid (LNA)–modified oligos have shown great promise in the field of small interfering RNA and miRNA therapeutics28,30–33 with the development of pioneering LNA anti-miR therapies for hyperlipidemia and hepatitis.32,33 However, these reports mainly evaluated liver miRNAs due to much easier inherent accessibility of the liver to injected oligos. In this study, we tested for the first time the efficacy of long-term systemically delivered LNA-modified anti-miR-192 to block kidney disease progression in a mouse model of DN.
Results
LNA–Anti-miR-192 Lowers Endogenous miR-192 Levels in a Mouse Model of Type 1 Diabetes
We recently described the systemic delivery of LNA-modified antisense oligos (LNA–anti-miR-192) targeting renal miR-192 in nondiabetic mice, which showed significant accumulation of the anti-miR in the renal cortical tissues along with specific reduction of miR-192 and downstream signaling molecules in short-term experiments (6 hours and 24 hours postinjection).15 However, the long-term effects of such anti-miRs in the kidney are unknown and, importantly, the renoprotective effects of anti-miR-192 in diabetic mice have not been evaluated. Therefore, we tested the efficacy and therapeutic potential of LNA–anti-miR-192 in type 1 diabetic mice injected with streptozotocin (STZ) at 2, 12, and 17 weeks after LNA–anti-miR-192 treatment. The negative control (NC) oligo was LNA–anti-miR-239b targeting a Caenorhabditis elegans sequence unrelated to mice. In six independent experiments with multiple mice in each, we examined the effects of the anti-miR-192 on early (short term of 2 weeks) and later events of DN (long term of 12 and 17 weeks) as well as functional indices of DN (Figure 1A).
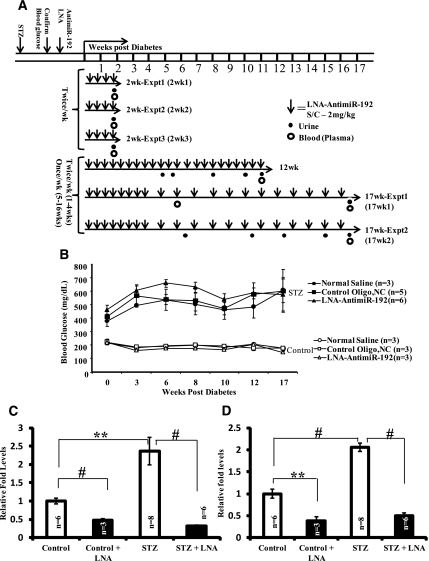
Figure 1.
Experimental design to evaluate efficacy of LNA–anti-miR-192 injections in a mouse model of type 1 diabetes. (A) Diabetes was induced by the 5-day STZ injection protocol in C57BL/6 mice. After hyperglycemia was confirmed, LNA–anti-miR-192 (2 mg/kg) was injected (bold downward arrows) subcutaneously twice a week in the first four experiments. In the remaining two replicate 17-week experiments, mice were injected with LNA–anti-miR-192 twice a week up to 4 weeks and then subsequently (from week 5) once weekly until sacrifice at 17 weeks (experiments 1 and 2). Mice were sacrificed at 2 weeks, 12 weeks, or 17 weeks as shown in the six independent experiments. Closed circles, urine collection; open circles, blood collection. (B) Blood glucose levels in experiment 1 at 17 weeks. Closed symbols, STZ; open symbols, nondiabetic controls. (C) miR-192 levels in renal cortical tissues from mice in experiment 1 at 2 weeks. (D) miR-192 levels in renal glomeruli from mice in experiment 1 at 2 weeks. Data are mean ± SEM. **P<0.01 and #P<0.001, respectively.
LNA–anti-miR-192 (LNA) did not alter nonfasting blood glucose levels significantly in nondiabetic (control) or diabetic mice (STZ) compared with negative controls (NC oligo or normal saline) (Figure 1B). miR-192 expression levels were significantly increased in diabetic mice tissues (approximately 2.5 fold) compared with nondiabetic controls 2 weeks after diabetes onset, and these increases were significantly ameliorated in both the renal cortex and glomeruli by LNA–anti-miR-192 injection in nondiabetic and diabetic mice (Figure 1, C and D). These results demonstrate that LNA–anti-miR-192 can efficiently knockdown endogenous miR-192 levels in diabetic mice.
Efficient and Specific Inhibition of miR-192 in Diabetic Mice Treated with LNA–anti-miR-192
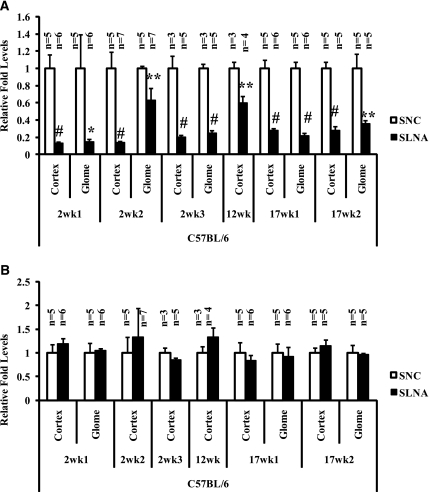
We next examined the efficacy of anti-miR-192 to downregulate endogenous miR-192 in diabetic mice over longer periods. miR-192 levels were significantly suppressed in both renal cortex and glomeruli of STZ-injected diabetic C57BL/6 mice compared with the negative controls in six independent experiments using mice that were diabetic for 2 weeks, 12 weeks, or 17 weeks (Figure 2A). This was specific because the levels of another renal miRNA, miR-194, approximately 100b upstream of miR-192 were not altered (Figure 2B). We also confirmed that miR-192 levels (but not miR-194) were decreased in nondiabetic mice injected with LNA–anti-miR-192 in these experiments (Supplemental Figure 1, A and B). These results demonstrate that LNA–anti-miR-192 (relative to NC) is potent and specific in silencing miR-192 expression levels significantly in diabetic mice, and this is sustained over both a shorter time period (2 weeks) as well as longer time periods (12 and 17 weeks). Similar results were also observed in the DBA/2J mouse strain (Supplemental Figure 2, A and B).
Figure 2.
Levels of miR-192 and miR-194 in the renal cortex and glomeruli of LNA oligos-injected diabetic C57BL/6 mice. (A and B) miR-192 and miR-194 expression levels, respectively, in renal tissues from diabetic mice injected with LNA–anti-miR-192 (LNA) or control mice (NC) (2–17 weeks). White bar, negative control oligo; black bar, LNA–anti-miR-192 treatment (LNA); glome, glomeruli. S-NC, STZ + LNA–anti-miR-239b; S-LNA, STZ + LNA–anti-miR-192. Data are mean ± SEM. *P<0.05, **P<0.01, and #P<0.001, respectively.
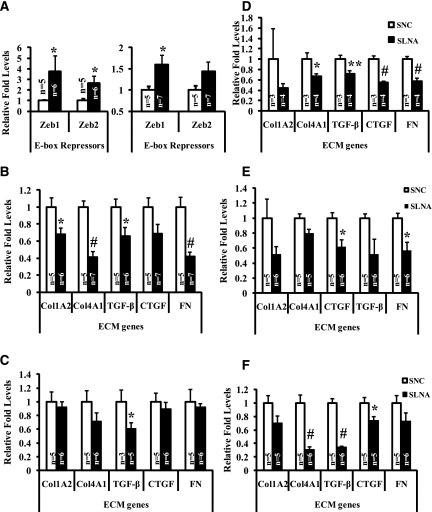
We next examined the effects on miR-192 targets and confirmed a significant increase in the expression levels of Zeb1/Zeb2 (targets of miR-192) in the renal cortex and glomeruli of diabetic mice injected with LNA–anti-miR-192 (S-LNA) compared with diabetic mice injected with negative control oligos (S-NC) (Figure 3A).
Figure 3.
Levels of Zeb1/2 (miR-192 targets) and profibrotic genes in renal cortex and glomeruli of LNA oligos-injected diabetic C57BL/6 mice. (A) Expression levels of E-box repressors (Zeb1/2) in the renal glomeruli (left panel) and cortex (right panel) from mice in experiments one through three at 2 weeks. (B and C) Expression levels of profibrotic genes, Col1A2, Col4A1, connective tissue growth factor, TGF-β, and FN in the renal glomeruli (B) and in the cortex (C) of mice at 2 weeks. Expression levels of profibrotic genes in the (D) renal cortex from mice at 12 weeks, (E) renal glomeruli from mice at 17 weeks, and (F) renal cortex from mice at 17 weeks. White bar, STZ + negative control LNA–anti-miR-239b (S-NC); black bar, STZ + LNA–anti-miR-192 (S-LNA). Data are mean ± SEM. *P<0.05, **P<0.01, and #P<0.001, respectively.
Decreased Renal Expression of Profibrotic Genes in Diabetic Mice Treated with LNA–Anti-miR-192
Real-time quantitative PCR showed that mRNA levels of key ECM-associated profibrotic genes—including those known to be regulated by E-box repressors Zeb1/2 such as collagen I(α2) (Col1A2), collagen IV(α1) (Col4A1), TGF-β, connective tissue growth factor, and FN—were significantly attenuated in diabetic mice injected with LNA–anti-miR-192 not only in the glomeruli and cortex in the short term of 2 weeks (Figure 3, B and C) but also in the cortex in the long term of 12 weeks (Figure 3D) and 17 weeks (glomeruli and cortex) (Figure 3, E and F) compared with NC oligos. Data from nondiabetic controls for ECM gene expression at 2 weeks are shown in Supplemental Figure 3. These gene expression results showed that LNA–anti-miR-192 not only downregulates endogenous miR-192, but also has functional effects by downregulating key profibrotic genes in diabetic mice.
LNA–Anti-miR-192 Treatment Decreases Renal Fibrosis in Diabetic Mice
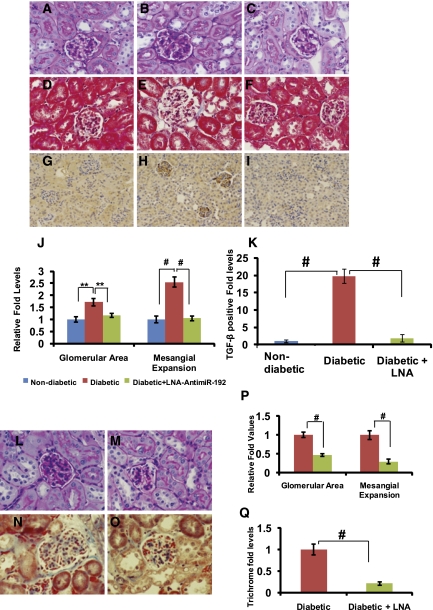
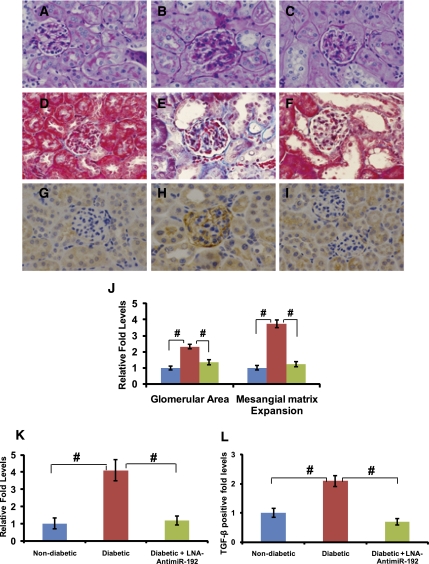
Diabetic renal disease is characterized by glomerulosclerosis and tubulointerstitial fibrosis due to the increased accumulation of ECM proteins.2,3 We observed that both glomerular surface area and mesangial expansion in glomeruli were significantly increased in diabetic renal cortical sections at 2 weeks compared with nondiabetic mice, and these parameters were significantly attenuated in diabetic mice injected with LNA–anti-miR-192 (periodic acid–Schiff [PAS] staining in Figure 4, A–C and J). Masson’s trichrome staining results also confirmed the increased glomerular area in diabetic mice, which was attenuated by LNA–anti-miR-192 treatment (Figure 4, D–F), although there was no increase in trichrome staining itself at this shorter time point (2 weeks after diabetes onset). TGF-β plays an important role in renal fibrosis leading to accelerated DN.6,34 TGF-β levels examined by immunostaining (Figure 4, G–I) were increased especially in glomeruli of the diabetic mice, and this was significantly reduced in diabetic mice treated with LNA–anti-miR-192 (Figure 4, G–I and K).
Figure 4.
Effects of LNA–anti-miR-192 on renal pathology in diabetic mice at 2 weeks and 12 weeks. (A–I) Representative staining in kidney sections at 2 weeks. Representative PAS staining in (A) nondiabetic controls, (B) STZ-induced diabetic mice, and (C) diabetic mice treated with LNA–anti-miR-192. Representative Masson’s trichrome staining in (D) nondiabetic controls, (E) STZ-induced diabetic mice, and (F) diabetic mice treated with LNA–anti-miR-192. Representative TGF-β immunostaining of kidney sections in (G) nondiabetic controls, (H) STZ-induced diabetic mice, and (I) diabetic mice treated with LNA–anti-miR-192. Glomerular area and mesangial expansion index (J) defined by the ratio of mesangial area/glomerular tuft area. (K) Quantification of TGF-β—positive area in the glomeruli. Blue bar, control (n=3); red bar, diabetic (n=5); green bar, diabetic + LNA–anti-miR-192 (n=5). (L–O) Representative kidney sections at 12 weeks. (L) Representative PAS staining of STZ-induced diabetic mice and (M) diabetic mice treated with LNA–anti-miR-192. Representative Masson’s trichrome staining of kidney sections showing glomerular and tubulointerstitial fibrosis in (N) STZ-induced diabetic mice and (O) diabetic mice treated with LNA–anti-miR-192. (P) Glomerular area and mesangial expansion index in PAS-positive sections. (Q) Quantification of Masson’s trichrome positive area in kidney sections. Red bar, diabetic (n=5); green bar, diabetic + LNA–anti-miR-192 (n=4). Data are mean ± SEM. **P<0.01 and #P<0.001, respectively. Original magnification, ×400 in A–I and L–O; ×200 in G–I.
In the 12-week longer-term experiment, renal pathology results for PAS (Figure 4, L and M) and Masson’s trichrome staining (Figure 4, N and O) revealed that the accumulation of matrix proteins within the glomeruli along with interstitial fibrosis in the diabetic mice (Figure 4, L and N) were significantly suppressed in diabetic mice injected with LNA–anti-miR-192 (Figure 4, M, O, P, and Q).
In the longest-term experiments at 17 weeks, staining for PAS (Figure 5B), Masson’s trichrome (Figure 5E), and TGF-β (Figure 5H) were all increased significantly in glomeruli compared with nondiabetic mice (Figure 5, A, D, and G, respectively). Increased PAS staining noted in the glomerular area and mesangial expansion were significantly attenuated in diabetic mice treated with LNA–anti-miR-192 (Figure 5, C and J). Masson’s trichrome usually stains collagens (blue), and the 17-week diabetic sections revealed an increase in glomerular and interstitial collagens and dilated lumen (Figure 5E), which was significantly attenuated in diabetic mice treated with LNA–anti-miR-192 (Figure 5, F and K). In addition, renal glomerular expression of TGF-β was also increased in the diabetic state at 17 weeks (Figure 5H), which was suppressed by LNA–anti-miR-192 (Figure 5, I and L). Overall, these staining data are consistent with the mRNA expression data shown earlier (Figure 3). Taken together, these results demonstrate that miR-192 inhibition by LNA–anti-miR-192 in vivo can significantly attenuate the increases in parameters of glomerular expansion and renal fibrosis in diabetic mice.
Figure 5.
Effects of LNA–anti-miR-192 on renal pathology in 17-week diabetic mice. (A–I) Representative kidney sections (17 weeks). Representative PAS staining in (A) nondiabetic controls, (B) STZ-induced diabetic mice, and (C) diabetic mice treated with LNA–anti-miR-192. Representative Masson’s trichrome staining of kidney sections showing glomerular and tubulointerstitial fibrosis in (D) nondiabetic controls, (E) STZ-induced diabetic mice, and (F) diabetic mice treated with LNA–anti-miR-192. Representative TGF-β immunostaining of kidney sections in (G) nondiabetic controls, (H) STZ-induced diabetic mice, and (I) diabetic mice treated with LNA–anti-miR-192. (J) Glomerular area and mesangial expansion index in PAS-positive sections. (K) Quantification of Masson’s trichrome positive area in kidney sections. (L) Quantification of TGF-β positively stained area in the glomeruli. Blue bar, control (n=3); red bar, diabetic (n=6); green bar, diabetic + LNA–anti-miR-192 (n=5). Data are mean ± SEM. #P<0.001, respectively. Original magnification, ×400 in A through I.
Effects of LNA–Anti-miR-192 on Renal Hypertrophy and Oxidant Stress in Diabetic Mice
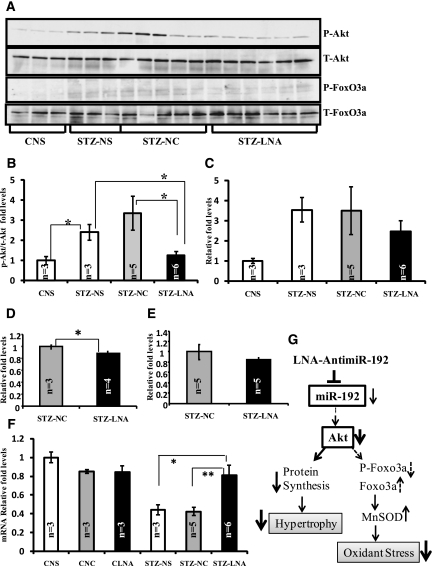
Renal hypertrophy is a key event in the pathogenesis of DN. Evidence shows that miR-192 plays an important role in mesangial hypertrophy by regulating Akt kinase and its downstream targets via a miRNA-based signaling circuit.15,35 Here, we confirmed increased Akt kinase activity (P-Akt) in cortical lysates of diabetic mice compared with nondiabetic mice, and this was significantly reduced in diabetic mice treated with LNA–anti-miR-192 (Figure 6, A and B). Phosphorylation of FoxO3a, a key downstream target of Akt kinase, showed an increased trend in diabetic mice, and this was attenuated, although not significantly, by LNA–anti-miR-192 (Figure 6, A and C). The kidney weight/body weight ratio, a hallmark of renal hypertrophy, was significantly decreased in diabetic mice injected with LNA–anti-miR-192 at 12 weeks (Figure 6D) but did not reach statistical significance at 17 weeks (Figure 6E). These results suggest that renal hypertrophy in diabetic mice may be ameliorated by LNA–anti-miR-192 via inhibition of the Akt signaling pathway.
Figure 6.
Effects of LNA–anti-miR-192 on mesangial hypertrophy and oxidant stress in diabetic mice. (A) Western blots showing relative protein expression levels of P-Akt (phospho-Akt), T-Akt (total-Akt), P-FoxO3a (phospho-FoxO3a), and T-FoxO3a (total-FoxO3a) in 2-week renal cortical tissue lysates. The phospho-Akt blot was stripped and reprobed with total-Akt antibody. The separate phospho-FoxO3A blot was stripped and probed with total FoxO3a antibody. Ratio of (B) P-Akt/T-Akt and (C) P-FoxO3a/T-FoxO3a. Quantitative analyses of fold level change in kidney weight/body weight ratios (hypertrophy) in (D) 12-week and (E) 17-week renal tissues, respectively. (F) mRNA expression levels of antioxidant gene MnSOD in renal glomeruli from 2-week LNA-treated mice. (G) Flow diagram showing the proposed mechanism of action of LNA–anti-miR-192 inreducing renal hypertrophy and oxidant stress in diabetic mice. White bar, normal saline; gray bar, negative control oligos; black bar, LNA–anti-miR-192 oligos; C-NS, control-normal saline; C-NC, control + negative control LNA–anti-miR-239b; C-LNA, control + LNA–anti-miR-192; STZ-NS, STZ + normal saline; STZ-NC, STZ + LNA–anti-miR-239b; STZ-LNA, STZ + LNA–anti-miR-192. Data are mean ± SEM. *P<0.05 and **P<0.01, respectively.
Evidence shows that manganese SOD (MnSOD), an antioxidant protective gene, can also be downregulated by miR-192 through the Akt/FoxO3a signaling pathway.15,35 We observed that MnSOD levels were decreased in diabetic mice compared with nondiabetic mice, and this was reversed in mice treated with LNA–anti-miR-192 (Figure 6F), suggesting that LNA–anti-miR-192 treatment may exert protective effects by also reducing renal oxidant stress in diabetic mice. The proposed mechanism of action of LNA–anti-miR-192 leading to decreased hypertrophy and oxidant stress is shown in Figure 6G.
LNA–Anti-miR-192 Treatment Decreases Proteinuria and Albuminuria in Diabetic Mice
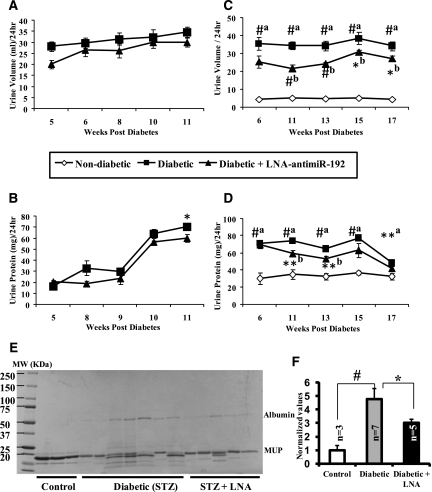
Key hallmarks of DN include polyuria, microalbuminuria, and proteinuria. We evaluated these important parameters in the diabetic mice treated with LNA–anti-miRs in the long-term experiments at 12 weeks and 17 weeks (experiments 1 and 2). Urine volumes in diabetic mice injected with LNA–anti-miR-192 were lower than in control diabetic mice without anti-miR injection in the 12-week experiments (Figure 7A). Urine protein levels were also attenuated in parallel and this reached statistical significance by 11 weeks (Figure 7B). Similar results from the 17-week experiments showed attenuation of the diabetes-induced increase in urine volume in mice treated with LNA–anti-miR-192 (Figure 7C). Urine total protein levels were significantly increased beginning about 6 weeks after diabetes onset and persisted until 17 weeks. This proteinuria was significantly attenuated by LNA–anti-miR-192 beginning around week 11 that persisted until week 13 (Figure 7D).
Figure 7.
LNA–anti-miR-192 treatment and improved renal function in C57BL/6 diabetic mice. (A and C) Changes in mean urine volume (in milliliters) excreted by diabetic mice for a 24-hour period using metabolic cages. (B and D) Changes in protein levels (in milligrams) in the urine of mice treated for 12 weeks and 17 weeks (experiment 2), respectively. Data in A and C are from separate and independently run experiments; data in A and B are from one experiment (12-week LNA); and data in C and D are from the other independent experiment (17-week LNA). For 12 weeks: closed squares, diabetic (n=3); closed triangles, diabetic + LNA–anti-miR-192 (n=4). For experiment 2 at 17 weeks: open diamonds, nondiabetic mice (n=3); closed squares, diabetic mice (n=10); closed triangles, diabetic + LNA–anti-miR-192 (n=5). (E) Coomassie blue staining to detect the urine albumin levels in the diabetic mice at 13 weeks after diabetes onset. (F) Quantitative analysis of albumin levels in urine normalized to mouse major urinary protein (MUP). White bar, control (n=3); gray bar, diabetic (n=7); black bar, diabetic + LNA (n=5). a, STZ versus nondiabetic; b, diabetic versus diabetic + LNA–anti-miR-192. Results expressed as mean ± SEM. *P<0.05, **P<0.01, #P<0.001, respectively.
We next evaluated the effect of LNA–anti-miR-192 on urine albumin levels by using Coomassie blue staining. A significant increase in the intensity of proteins around the albumin molecular weight region was detected in urine samples from diabetic mice compared with nondiabetic mice, and this was significantly attenuated in diabetic mice injected with anti-miR-192 (Figure 7, E and F). Overall, although there was some variation from mouse to mouse in these long-term functional studies, the results from three independent long-term experiments suggest that LNA-based miR-192 inhibitors decrease proteinuria and albuminuria in diabetic mice.
LNA–Anti-miR-192 Did Not Cause a Significant Increase in Toxicity
Evaluation of potential toxicity in the mice injected with anti-miRs is an important step in assessing the overall clinical and therapeutic utility of LNA–anti-miR-192. Results from serum toxicity profiles (Table 1) show that LNA–anti-miR-192 did not cause a significant increase in toxicity even in diabetic animals over both the short-term and long-term periods (2–17 weeks). We also did not observe any mortality in these experiments.
Table 1.
Serum profiling for toxicity in mice injected with LNA–anti-miR-192
| Experimental Group | Number of Mice | Total Protein (g/dl) | Albumin (g/dl) | ALT (U/L) |
|---|---|---|---|---|
| 2-wk LNA | ||||
| C-NS | 7 | 4.54±0.22 | 2.68±0.14 | 34.14±5.86 |
| C-NC | 6 | 4.53±0.23 | 2.7±0.11 | 60.66±12.96 |
| C-LNA | 7 | 4.64±0.32 | 2.7±0.20 | 95.0±29.3 |
| S-NS | 7 | 3.91±0.26 | 2.3±0.17 | 80.57±35.66 |
| S-NC | 9 | 4.22±0.29 | 2.5±0.17 | 58.55±10.99 |
| S-LNA | 12 | 4.5±0.25 | 2.6±0.15 | 87.75±22.99 |
| 17-wk experiment 1 LNA | ||||
| C-NS | 3 | 40±8.88 | ||
| C-NC | 3 | 50±16.46 | ||
| C-LNA | 3 | 32±2.64 | ||
| S-NS | 3 | 44±5.29 | ||
| S-NC | 3 | 55±11.26 | ||
| S-LNA | 5 | 46.5±5.67 | ||
| 17-wk experiment 2 LNA | ||||
| C-NS | 3 | 5.43±0.38 | 3.13±0.23 | 44.33±4.05 |
| C-NC | 3 | 5.46±0.06 | 3.1±0.0 | 33.3±4.09 |
| C-LNA | 3 | 5.9±0.11 | 3.53±0.03 | 32±1.73 |
| S-NS | 3 | 4.83±0.41 | 2.8±0.20 | 47±3.78 |
| S-NC | 3 | 5.23±0.42 | 3.0±0.26 | 72.66±16.38 |
| S-LNA | 5 | 5.0±0.35 | 2.82±0.22 | 108±53.18 |
| Total mice | 88 |
Data are mean ± SEM. C-NS, control + normal saline; C-NC, control + negative control oligo; C-LNA, control + LNA–anti-miR-192; S-NS, STZ + normal saline; S-NC, STZ + negative control oligo; S-LNA, STZ + LNA–anti-miR-192.
Discussion
We and others recently showed that miR-192 is upregulated under diabetic conditions such as TGF-β or high glucose-treated MCs and in glomeruli from diabetic mice.12,14,15,17–19,24 Because miR-192 can also regulate several other renal miRNAs with functions related to DN and promote the expression of fibrotic genes and MC hypertrophy,9,12,15,18,19 we evaluated miR-192 as a target for the treatment of DN. In this study, we evaluated for the first time the efficacy of delivering LNA–modified oligos to the kidneys of diabetic mice with repeated injections over long time periods, and more specifically the therapeutic potential of LNA–anti-miR-192 in mouse models of DN. We confirmed increased levels of miR-192 in diabetic mice, consistent with our earlier results.12,15,17 Furthermore, we found that LNA–anti-miR-192 inhibits endogenous miR-192 levels very efficiently and specifically even in diabetic mice; in parallel, LNA–anti-miR-192 could decrease the levels of profibrotic genes, due at least in part to the increases in expression of the miR-192 targets Zeb1/2. LNA–anti-miR-192 could also attenuate histologic evidence of glomerular expansion and renal fibrosis, as well as confer improvements in kidney functions (urine volume, total protein and albumin levels) in diabetic mice. Because LNA–anti-miR-192 treatment did not affect blood glucose levels in diabetic mice, the renal beneficial effects are more likely due to the inhibition of miR-192 in the kidney.
Because we observed an increase in Zeb1/2 levels and a parallel decrease in profibrotic genes, Col1a2, Col4a1, TGF-β, connective tissue growth factor, and FN in diabetic mice treated with LNA–anti-miR-192, the molecular mechanisms regulated by miR-192 in vivo (decrease of E-box repressors Zeb1/2, loss of repression, and subsequent transcriptional upregulation of profibrotic genes) may be the same as in the in vitro experiments using mouse MCs treated with TGF-β.12,15,17,19 The decrease in TGF-β by the anti-miR-192 is likely due to de-repression at E-boxes in its promoter.
Increased glomerular expansion and renal hypertrophy in the diabetic mice were also significantly decreased with LNA–anti-miR-192 treatment. Akt kinase activation was reduced, whereas levels of the antioxidant gene MnSOD were increased in mice injected with LNA–anti-miR-192. These results further complement previous in vitro findings that miR-192 is a key upstream mediator of mesangial hypertrophy.12,15,19 LNA–anti-miR-192 most likely decreases renal hypertrophy via a dual mechanism wherein decreased Akt levels attenuate protein synthesis in one arm, whereas in the other, the attenuation in P-FoxO3a levels may augment downstream MnSOD to reduce oxidant stress. However, because miR-192 can have other targets,36–39 other mechanisms and pathways related to DN may also be affected by LNA–anti-miR-192, including those in other renal cells such as tubular cells and podocytes.4 Additional studies are needed to evaluate such mechanisms.
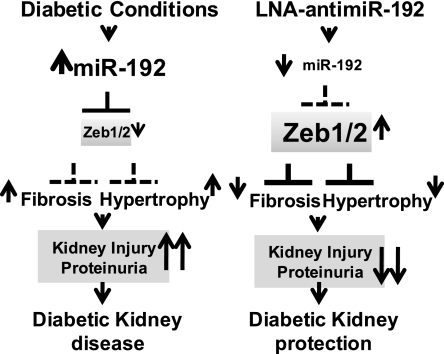
Our results suggest that by downregulating profibrotic genes (TGF-β and collagens), renal fibrosis (ECM staining), hypertrophy (P-Akt), and oxidant stress (increasing MnSOD), LNA–anti-miR-192 can cumulatively decrease kidney injury with improvements in proteinuria and albuminuria in diabetic mice. Furthermore, LNA–anti-miR-192 did not significantly increase toxicity. A scheme for the proposed mechanism of renal protection by LNA–anti-miR-192 in diabetic mice is shown in Figure 8.
Figure 8.
Proposed mechanism of renal protection by LNA–anti-miR-192 in diabetic mice. LNA–anti-miR-192 inhibitor inhibits miR-192 levels, which increase the levels of its targets, Zeb1/2. Increased levels of Zeb1/2 then downregulate several profibrotic genes such as TGF-β, collagens, and other miRs via increased repression at E-boxes leading to decreased fibrosis, hypertrophy, and proteinuria to confer renal protection in diabetic mice.
To our knowledge, this is the first demonstration of the in vivo beneficial effects of LNA–anti-miR against profibrotic gene expression even at 17 weeks after diabetes onset in the STZ mouse model. The decrease in profibrotic genes was more significant in glomeruli than in renal cortical tissues (Figure 3, A–C), possibly due to the presence of other tubular components in the cortical tissues or to potential difference in miR-192 actions in tubular epithelium-like cells19,40,41 versus mesangial or other renal cells. In addition, we observed more profound inhibitory effects on the gene expression at earlier time points (2 weeks) than at later stages of diabetes (17 weeks). This was also evident in the protection against proteinuria, which was significant at 11–13 weeks but not later. Although the exact mechanisms are not fully clear, this could be due to the fact that we decreased the frequency of LNA–anti-miR delivery to once weekly at later points in the 17-week study, or it may be due to other potential compensatory mechanisms initiated by the mice at later time points. In addition, the systemic delivery may potentially affect functions in other organs expressing miR-192. Furthermore, the exact mechanisms underlying the reduction in urine volumes in the diabetic mice treated with LNA–anti-miR-192 are not fully clear.
A report by Krupa et al. showed that the renal levels of miR-192 were decreased in patients with severe DN,40 but miR-192 levels in control healthy volunteers were not provided. It is possible that although patients with severe DN had lower miR-192 levels than those with earlier stages of DN, they might still express higher miR-192 relative to healthy controls. In addition, some studies show that TGF-β can decrease miR-192 expression in proximal tubular cells and other renal cell lines,40,41 whereas other studies show an increase, including our studies (in MCs and diabetic mice glomeruli)12,15,19 as well as those of Chung et al. (in tubular cells),16 Long et al. (in db/db mice glomeruli, high glucose-treated endothelial cells, and podocytes),18 Wang et al. (in STZ mice kidneys),42 Karolina et al. (in human diabetes and a diabetic rat model),43 and Wang et al. (in high glucose-treated human MCs.14 With the miR-200 family members, some studies showed an increase18,19,44 and others showed a decrease.45,46 The reasons for these conflicting results are not clear but could be due to cell-specific effects as indicated earlier, as well as the role of p53 in renal cells. It is not clear if the tubular epithelium-like cell lines commonly used have mutant p53 or altered actions of transcription factors, all of which can affect the expression of basal and induced miRNAs, including the response to TGF-β.47 Our experiments using wild-type mice and cells (with wild-type p53 and other genes) show an increase in miR-192 levels. Furthermore, we did not detect any adverse renal pathology in nondiabetic mice injected with LNA–anti-miR-192 despite significant decreases in miR-192 levels, suggesting that lowering miR-192 levels does not have detrimental effects in the kidney.
Taken together, our current studies reveal for the first time that LNA-based miR inhibitors such as LNA–anti-miR-192 can very efficiently and specifically reduce renal miR-192 levels, profibrotic genes, renal fibrosis, renal hypertrophy, and oxidant stress in diabetic mice over short- and long-term treatment periods. Notably, LNA–anti-miR-192 also improved some kidney functions in diabetic mice by decreasing polyuria, proteinuria, and albuminuria without toxicity. Thus, LNA–anti-miR-192 may be useful to treat or prevent DN. In addition, LNA–anti-miR-192 might also be useful for nondiabetic kidney diseases in which miR-192 levels are significantly increased, such as IgA nephropathy, hypertensive nephrosclerosis, and lupus nephritis.25–27 In summary, these findings are promising for future evaluation of the clinical utility of inhibitors targeting key pathologic renal miRNAs for the treatment of DN.
Concise Methods
Materials
LNA–anti-miR-192 and LNA–anti-miR-239b (NC) were from IDT Inc (Coralville, IA) and Exiqon USA Inc (Woburn, MA).15 RNA-STAT 60 was from Iso-Tex Diagnostics Inc (Friendswood, TX). TRIzol reagent was from Invitrogen (Carlsbad, CA). Gene Amp RNA-PCR and SYBR green real-time PCR kits were from Applied Biosystems (Foster City, CA). miScript cDNA synthesis and real-time PCR kits were from Qiagen (Valencia, CA). The 18SII primers were from Ambion (Austin, TX). Chemiluminescence detection kits for Western blotting were from Pierce (Rockford, IL). STZ was from Sigma-Aldrich (St. Louis, MO).
Animal Studies and DN Model
All animal studies were performed according to approved Institutional Animal Care and Use Committee protocols in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male mice (C57BL/6J or DBA2J strain) aged 9 weeks were purchased from Jackson Laboratories and made diabetic with STZ as previously described.48 Briefly, mice were fasted for 4 hours followed by intraperitoneal STZ injections (50 mg/kg) for 5 consecutive days per the Animal Models of Diabetic Complications Consortium low-dose STZ protocol. Blood glucose levels were monitored 1 week after the last STZ injection using the Alphatrak glucose meter (Abbott Laboratories, Abbott Park, IL). Mice with nonfasting blood glucose levels >400 mg/dl were considered diabetic. Each group had at least three mice per group and at least five to six mice in the STZ groups for potential mortality, although none was noted. Overall, we assessed 69 diabetic mice in the six experiments.
LNA–Anti-miR-192 Delivery to Control and Diabetic Mice
LNA–anti-miR-192 and control LNA–anti-miR-239b (NC) were dissolved in RNase-free sterile PBS to a working concentration of 1 μg/μl before injections and delivered to mice subcutaneously at 40 μg or 40 μl (1 μg/μl) per mouse, which is approximately equivalent to 2 mg/kg. LNA–anti-miR-192 or control injections were given twice weekly (2-week and 12-week groups) and twice weekly for weeks 1–4 and then once a week for the weeks 5–17 after diabetic onset (17weeks, experiments 1 and 2).
Real-Time qPCR
Real-time qPCR was performed on a ABI-7300 machine (Applied Biosystems) using SYBR green master mix (Applied Biosystems). Briefly, 0.3–1 μg of total RNA from the kidney cortex or glomeruli was used to synthesize cDNA by reverse transcription using miScript kits (Qiagen) to detect the miRNAs and genes as described previously.12,15,19 18SII RNA served as the internal control (Ambion). Primer sequences are listed in Supplemental Table 1.
Serum/Plasma Toxicity Analyses
Undiluted serum samples from the mice (80 μl/4 analytes) were sent to the diagnostic laboratory (RADIL, Columbia, MO) for clinical biochemistry panels to evaluate liver toxicity. All clinical parameters were measured on an Olympus AU680 analyzer.
Isolation of Glomeruli from Mouse Kidneys
Kidneys from experimental mice were harvested using sterile techniques, capsules were removed along with adjacent fat, and glomeruli were isolated as described previously.48
Urine Protein and Albumin Assays
Urine was collected from mice using metabolic cages for a 24-hour period, and was then evaluated for the amount of protein (in milligrams) as described previously48 using detergent-compatible protein assay kits (BioRad Inc, Hercules, CA) according to the manufacturer’s instructions.
Urine aliquots were then evaluated for the amount of albumin (micrograms per milliliter) to monitor renal function by running the samples on SDS-PAGE and staining the gels with Coomassie blue. Major urinary protein staining was used as internal control.
Histologic Staining
Tissues were fixed immediately in 10% buffered formalin solution after harvesting and were submitted to the Anatomic Pathology Core at City of Hope Medical Center to obtain paraffin-embedded sections. To evaluate kidney structural function and ECM deposition, sections were processed for hematoxylin and eosin, PAS, Masson trichrome, and silver staining.
Morphometric Analyses
Images were captured using an Olympus DP72 Microscope Digital Camera and were processed with DP2-BSW software. Glomerular area and mesangial expansion index were quantified using Image-Pro Plus 5.1 software (Media Cybernetics Inc, Bethesda, MD).
Statistical Analyses
Data are expressed as mean ± SEM. Paired t tests were used to compare two groups. Statistical significance was detected at the 0.05 level.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors are grateful to the faculty and staff of the Division of the Comparative Medicine at the Beckman Research Institute for assistance.
These studies were supported by Grants R01 DK 081705 and R01 DK 058191 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011050485/-/DCSupplemental.
References
- 1.Declèves AE, Sharma K: New pharmacological treatments for improving renal outcomes in diabetes. Nat Rev Nephrol 6: 371–380, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Steffes MW, Osterby R, Chavers B, Mauer SM: Mesangial expansion as a central mechanism for loss of kidney function in diabetic patients. Diabetes 38: 1077–1081, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Ziyadeh FN: The extracellular matrix in diabetic nephropathy. Am J Kidney Dis 22: 736–744, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Brosius FC, Khoury CC, Buller CL, Chen S: Abnormalities in signaling pathways in diabetic nephropathy. Expert Rev Endocrinol Metab 5: 51–64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA: Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA 90: 1814–1818, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma K, Ziyadeh FN: Hyperglycemia and diabetic kidney disease. The case for transforming growth factor-beta as a key mediator. Diabetes 44: 1139–1146, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P, Stoffel M: A pancreatic islet-specific microRNA regulates insulin secretion. Nature 432: 226–230, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Croce CM, Calin GA: miRNAs, cancer, and stem cell division. Cell 122: 6–7, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Kato M, Arce L, Natarajan R: MicroRNAs and their role in progressive kidney diseases. Clin J Am Soc Nephrol 4: 1255–1266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inui M, Martello G, Piccolo S: MicroRNA control of signal transduction. Nat Rev Mol Cell Biol 11: 252–263, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Sun Y, Koo S, White N, Peralta E, Esau C, Dean NM, Perera RJ: Development of a micro-array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Res 32: e188, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R: MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci USA 104: 3432–3437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian Z, Greene AS, Pietrusz JL, Matus IR, Liang M: MicroRNA-target pairs in the rat kidney identified by microRNA microarray, proteomic, and bioinformatic analysis. Genome Res 18: 404–411, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q, Wang Y, Minto AW, Wang J, Shi Q, Li X, Quigg RJ: MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J 22: 4126–4135, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, Rossi JJ, Natarajan R: TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol 11: 881–889, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung AC, Huang XR, Meng X, Lan HY: miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J Am Soc Nephrol 21: 1317–1325, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato M, Wang L, Putta S, Wang M, Yuan H, Sun G, Lanting L, Todorov I, Rossi JJ, Natarajan R: Post-transcriptional up-regulation of Tsc-22 by Ybx1, a target of miR-216a, mediates TGF-beta-induced collagen expression in kidney cells. J Biol Chem 285: 34004–34015, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long J, Wang Y, Wang W, Chang BH, Danesh FR: MicroRNA-29c is a signature microRNA under high glucose conditions that targets Sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy. J Biol Chem 286: 11837–11848, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato M, Arce L, Wang M, Putta S, Lanting L, Natarajan R: A microRNA circuit mediates transforming growth factor-β1 autoregulation in renal glomerular mesangial cells. Kidney Int 80: 358–368, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harvey SJ, Jarad G, Cunningham J, Goldberg S, Schermer B, Harfe BD, McManus MT, Benzing T, Miner JH: Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. J Am Soc Nephrol 19: 2150–2158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho J, Ng KH, Rosen S, Dostal A, Gregory RI, Kreidberg JA: Podocyte-specific loss of functional microRNAs leads to rapid glomerular and tubular injury. J Am Soc Nephrol 19: 2069–2075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi S, Yu L, Chiu C, Sun Y, Chen J, Khitrov G, Merkenschlager M, Holzman LB, Zhang W, Mundel P, Bottinger EP: Podocyte-selective deletion of dicer induces proteinuria and glomerulosclerosis. J Am Soc Nephrol 19: 2159–2169, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei Q, Bhatt K, He HZ, Mi QS, Haase VH, Dong Z: Targeted deletion of Dicer from proximal tubules protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 21: 756–761, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato M, Natarajan R: MicroRNA cascade in diabetic kidney disease: Big impact initiated by a small RNA. Cell Cycle 8: 3613–3614, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang G, Kwan BC, Lai FM, Choi PC, Chow KM, Li PK, Szeto CC: Intrarenal expression of miRNAs in patients with hypertensive nephrosclerosis. Am J Hypertens 23: 78–84, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Wang G, Kwan BC, Lai FM, Choi PC, Chow KM, Li PK, Szeto CC: Intrarenal expression of microRNAs in patients with IgA nephropathy. Lab Invest 90: 98–103, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Dai Y, Sui W, Lan H, Yan Q, Huang H, Huang Y: Comprehensive analysis of microRNA expression patterns in renal biopsies of lupus nephritis patients. Rheumatol Int 29: 749–754, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Elmén J, Thonberg H, Ljungberg K, Frieden M, Westergaard M, Xu Y, Wahren B, Liang Z, Ørum H, Koch T, Wahlestedt C: Locked nucleic acid (LNA) mediated improvements in siRNA stability and functionality. Nucleic Acids Res 33: 439–447, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M: Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438: 685–689, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Fluiter K, ten Asbroek AL, de Wissel MB, Jakobs ME, Wissenbach M, Olsson H, Olsen O, Oerum H, Baas F: In vivo tumor growth inhibition and biodistribution studies of locked nucleic acid (LNA) antisense oligonucleotides. Nucleic Acids Res 31: 953–962, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mook OR, Baas F, de Wissel MB, Fluiter K: Evaluation of locked nucleic acid-modified small interfering RNA in vitro and in vivo. Mol Cancer Ther 6: 833–843, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Elmén J, Lindow M, Schütz S, Lawrence M, Petri A, Obad S, Lindholm M, Hedtjärn M, Hansen HF, Berger U, Gullans S, Kearney P, Sarnow P, Straarup EM, Kauppinen S: LNA-mediated microRNA silencing in non-human primates. Nature 452: 896–899, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Elmén J, Lindow M, Silahtaroglu A, Bak M, Christensen M, Lind-Thomsen A, Hedtjärn M, Hansen JB, Hansen HF, Straarup EM, McCullagh K, Kearney P, Kauppinen S: Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res 36: 1153–1162, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reeves WB, Andreoli TE: Transforming growth factor beta contributes to progressive diabetic nephropathy. Proc Natl Acad Sci USA 97: 7667–7669, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato M, Yuan H, Xu ZG, Lanting L, Li SL, Wang M, Hu MC, Reddy MA, Natarajan R: Role of the Akt/FoxO3a pathway in TGF-beta1-mediated mesangial cell dysfunction: A novel mechanism related to diabetic kidney disease. J Am Soc Nephrol 17: 3325–3335, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Elvira-Matelot E, Zhou XO, Farman N, Beaurain G, Henrion-Caude A, Hadchouel J, Jeunemaitre X: Regulation of WNK1 expression by miR-192 and aldosterone. J Am Soc Nephrol 21: 1724–1731, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagel R, Clijsters L, Agami R: The miRNA-192/194 cluster regulates the Period gene family and the circadian clock. FEBS J 276: 5447–5455, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Pichiorri F, Suh SS, Rocci A, De Luca L, Taccioli C, Santhanam R, Zhou W, Benson DM, Jr, Hofmainster C, Alder H, Garofalo M, Di Leva G, Volinia S, Lin HJ, Perrotti D, Kuehl M, Aqeilan RI, Palumbo A, Croce CM: Downregulation of p53-inducible microRNAs 192, 194, and 215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma development. Cancer Cell 18: 367–381, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Xie QH, He XX, Chang Y, Sun SZ, Jiang X, Li PY, Lin JS: MiR-192 inhibits nucleotide excision repair by targeting ERCC3 and ERCC4 in HepG2.2.15 cells. Biochem Biophys Res Commun 410: 440–445, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Krupa A, Jenkins R, Luo DD, Lewis A, Phillips A, Fraser D: Loss of MicroRNA-192 promotes fibrogenesis in diabetic nephropathy. J Am Soc Nephrol 21: 438–447, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang B, Herman-Edelstein M, Koh P, Burns W, Jandeleit-Dahm K, Watson A, Saleem M, Goodall GJ, Twigg SM, Cooper ME, Kantharidis P: E-cadherin expression is regulated by miR-192/215 by a mechanism that is independent of the profibrotic effects of transforming growth factor-beta. Diabetes 59: 1794–1802, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang XX, Jiang T, Shen Y, Caldas Y, Miyazaki-Anzai S, Santamaria H, Urbanek C, Solis N, Scherzer P, Lewis L, Gonzalez FJ, Adorini L, Pruzanski M, Kopp JB, Verlander JW, Levi M: Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes 59: 2916–2927, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karolina DS, Armugam A, Tavintharan S, Wong MT, Lim SC, Sum CF, Jeyaseelan K: MicroRNA 144 impairs insulin signaling by inhibiting the expression of insulin receptor substrate 1 in type 2 diabetes mellitus. PLoS One 6: e22839, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oba S, Kumano S, Suzuki E, Nishimatsu H, Takahashi M, Takamori H, Kasuya M, Ogawa Y, Sato K, Kimura K, Homma Y, Hirata Y, Fujita T: miR-200b precursor can ameliorate renal tubulointerstitial fibrosis. PLoS One 5: e13614, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang B, Koh P, Winbanks C, Coughlan MT, McClelland A, Watson A, Jandeleit-Dahm K, Burns WC, Thomas MC, Cooper ME, Kantharidis P: miR-200a prevents renal fibrogenesis through repression of TGF-β2 expression. Diabetes 60: 280–287, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ: The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 10: 593–601, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, Solari A, Bobisse S, Rondina MB, Guzzardo V, Parenti AR, Rosato A, Bicciato S, Balmain A, Piccolo S: A mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell 137: 87–98, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Yuan H, Lanting L, Xu ZG, Li SL, Swiderski P, Putta S, Jonnalagadda M, Kato M, Natarajan R: Effects of cholesterol-tagged small interfering RNAs targeting 12/15-lipoxygenase on parameters of diabetic nephropathy in a mouse model of type 1 diabetes. Am J Physiol Renal Physiol 295: F605–F617, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.