Abstract
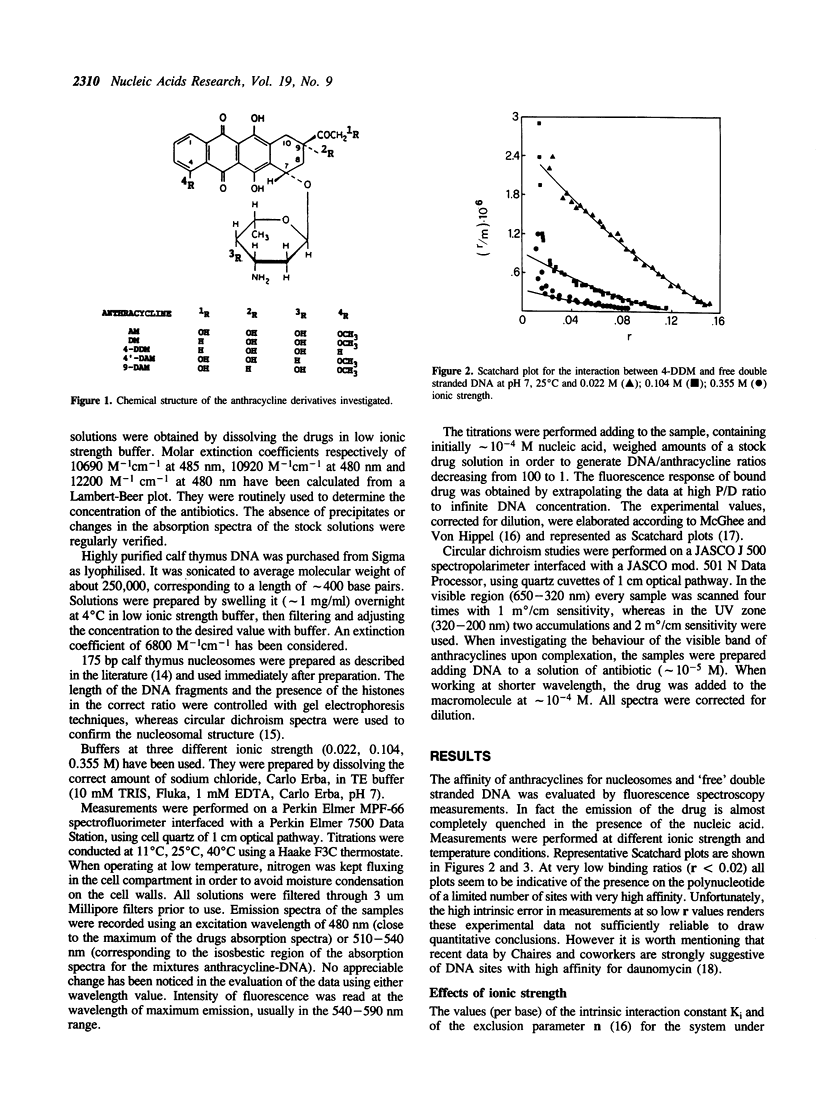
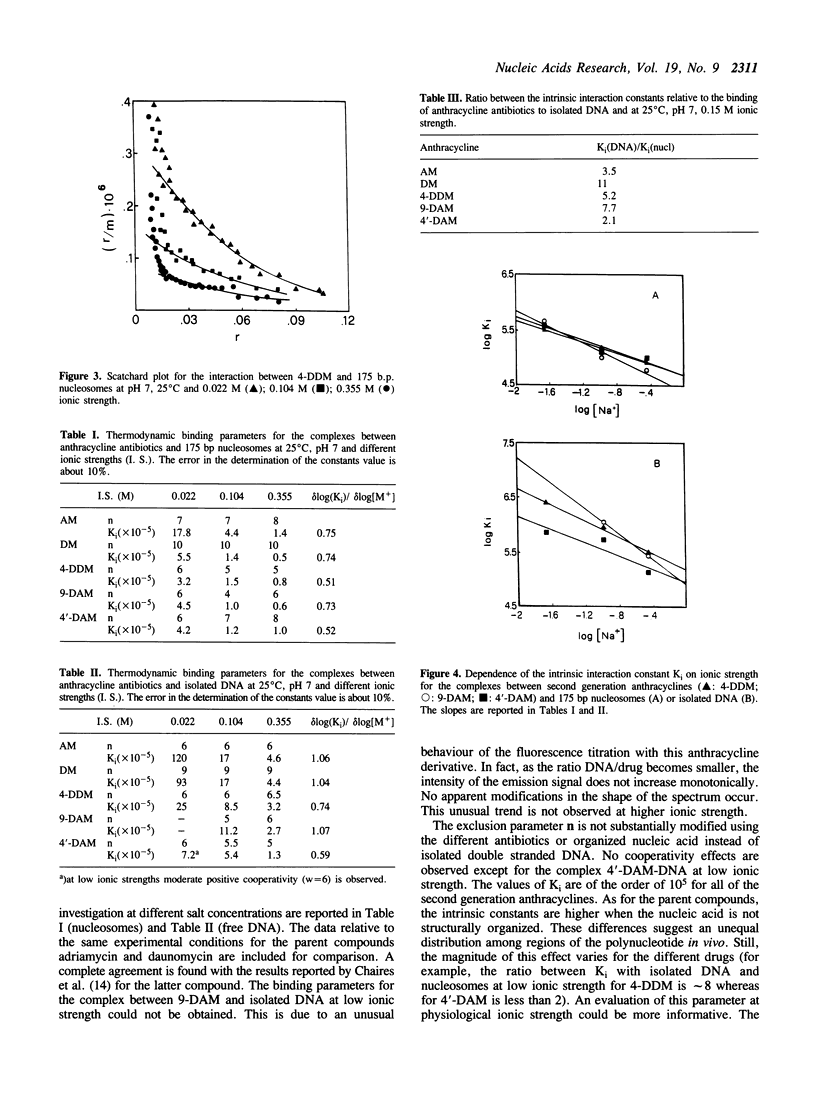
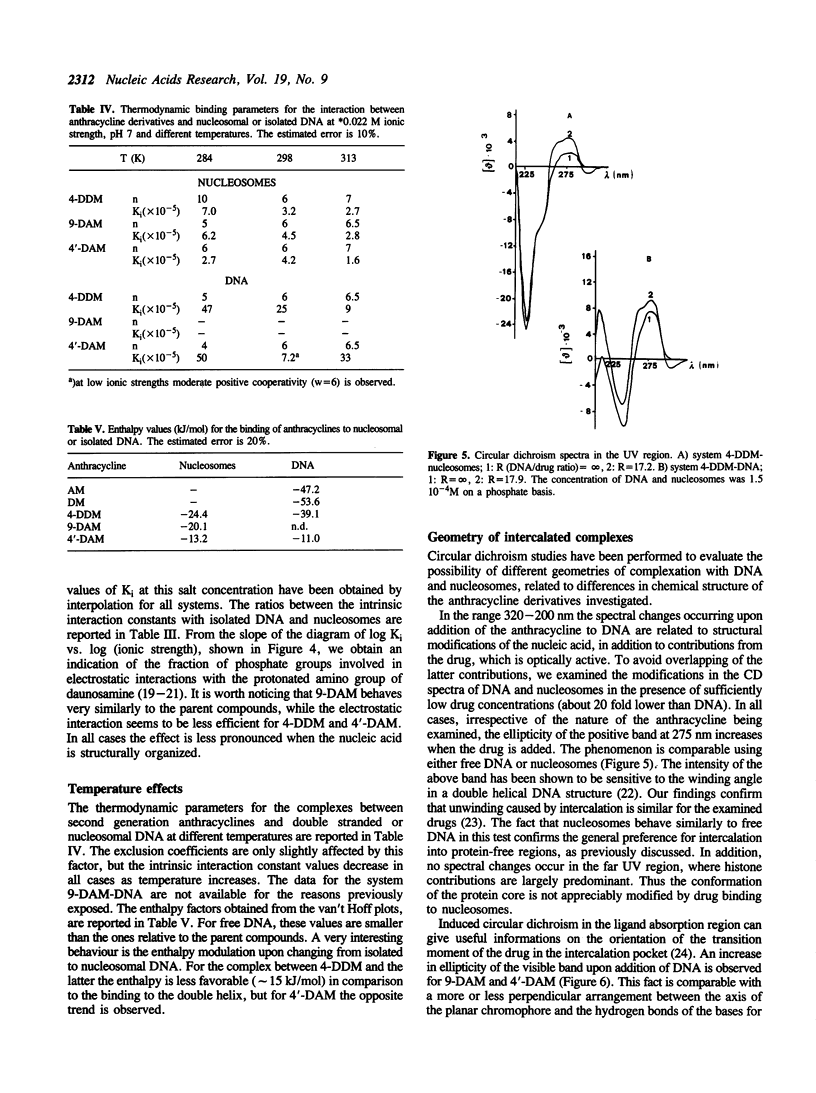
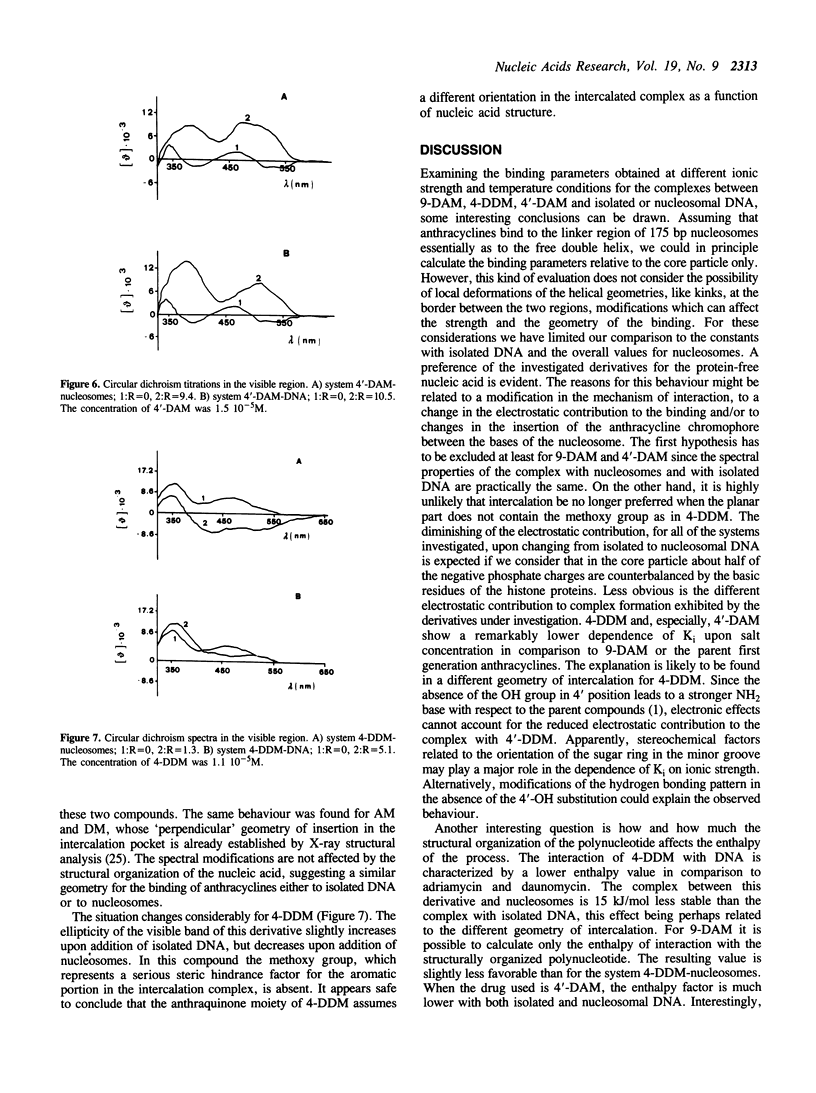
The interaction between three anthracycline antibiotics of second generation (9-deoxydoxorubicin, 9-DAM, 4-demethoxydaunorubicin, 4-DDM, 4'-deoxydoxorubicin, 4'-DAM) and DNA in the nucleosomal structure was investigated using fluorescence and circular dichroism techniques. The thermodynamic parameters of the binding process were obtained at different ionic strength and temperature conditions, thus allowing the calculation of the electrostatic contribution to the free energy and the enthalpy of the process. The same measurements were performed on linear double stranded DNA for comparison. The parent compounds adriamycin and daunomycin were additionally considered. Although the examined drugs greatly vary in biological activity, their binding parameters are only slightly different. Like the parent compounds, 9-DAM, 4-DDM and 4'-DAM exhibit preference for isolated regions of the polynucleotide rather than for nucleosomes. This fact suggests a non-homogeneous distribution of the antibiotics in vivo. The enthalpy values are remarkably lower than the ones characterizing the interaction of adriamycin and daunomycin to DNA. According to CD spectra, all derivatives, but 4-DDM, intercalate into nucleosomal or free DNA in a manner similar to the first generation compounds, namely with the chromophore perpendicular to the hydrogen bonds between the bases. The demethoxy compound, on the other hand, seems to be able to insert its planar moiety in different orientations, which are related to the structure of the nucleic acid being examined. The lack of the methoxy group in the intercalating part of the molecule appears to be responsible for this behaviour. As far as biological activity is concerned, our findings indicate a qualitative correlation between cell cytotoxicity and ability of interaction with nucleosomes at physiological conditions, rather than with free DNA. The modified binding stereochemistry of 4-DDM could play an additive role in modulating the pharmacological effectiveness of the above compounds.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capranico G., Zunino F., Kohn K. W., Pommier Y. Sequence-selective topoisomerase II inhibition by anthracycline derivatives in SV40 DNA: relationship with DNA binding affinity and cytotoxicity. Biochemistry. 1990 Jan 16;29(2):562–569. doi: 10.1021/bi00454a033. [DOI] [PubMed] [Google Scholar]
- Chaires J. B., Dattagupta N., Crothers D. M. Binding of daunomycin to calf thymus nucleosomes. Biochemistry. 1983 Jan 18;22(2):284–292. doi: 10.1021/bi00271a009. [DOI] [PubMed] [Google Scholar]
- Eriksson M., Nordén B., Eriksson S. Anthracycline-DNA interactions studied with linear dichroism and fluorescence spectroscopy. Biochemistry. 1988 Oct 18;27(21):8144–8151. doi: 10.1021/bi00421a024. [DOI] [PubMed] [Google Scholar]
- Fritzsche H., Wähnert U., Chaires J. B., Dattagupta N., Schlessinger F. B., Crothers D. M. Anthracycline antibiotics. Interaction with DNA and nucleosomes and inhibition of DNA synthesis. Biochemistry. 1987 Apr 7;26(7):1996–2000. doi: 10.1021/bi00381a032. [DOI] [PubMed] [Google Scholar]
- Johnson B. B., Dahl K. S., Tinoco I., Jr, Ivanov V. I., Zhurkin V. B. Correlations between deoxyribonucleic acid structural parameters and calculated circular dichroism spectra. Biochemistry. 1981 Jan 6;20(1):73–78. doi: 10.1021/bi00504a013. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B., Sealy R. C., Sinha B. K. An electron spin resonance study of the reduction of peroxides by anthracycline semiquinones. Biochim Biophys Acta. 1984 Jun 29;799(3):270–275. doi: 10.1016/0304-4165(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- Lyng R., Härd T., Norden B. Induced CD of DNA intercalators: electric dipole allowed transitions. Biopolymers. 1987 Aug;26(8):1327–1345. doi: 10.1002/bip.360260809. [DOI] [PubMed] [Google Scholar]
- May P. M., Williams G. K., Williams D. R. Solution chemistry studies of adriamycin--iron complexes present in vivo. Eur J Cancer. 1980 Sep;16(9):1275–1276. doi: 10.1016/0014-2964(80)90189-9. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Montecucco A., Pedrali-Noy G., Spadari S., Zanolin E., Ciarrocchi G. DNA unwinding and inhibition of T4 DNA ligase by anthracyclines. Nucleic Acids Res. 1988 May 11;16(9):3907–3918. doi: 10.1093/nar/16.9.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muindi J. R., Sinha B. K., Gianni L., Myers C. E. Hydroxyl radical production and DNA damage induced by anthracycline-iron complex. FEBS Lett. 1984 Jul 9;172(2):226–230. doi: 10.1016/0014-5793(84)81130-8. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Wang A. H., Ughetto G., van der Marel G., van Boom J. H., Rich A. Molecular structure of an anticancer drug-DNA complex: daunomycin plus d(CpGpTpApCpG). Proc Natl Acad Sci U S A. 1980 Dec;77(12):7204–7208. doi: 10.1073/pnas.77.12.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record M. T., Jr, Anderson C. F., Lohman T. M. Thermodynamic analysis of ion effects on the binding and conformational equilibria of proteins and nucleic acids: the roles of ion association or release, screening, and ion effects on water activity. Q Rev Biophys. 1978 May;11(2):103–178. doi: 10.1017/s003358350000202x. [DOI] [PubMed] [Google Scholar]
- Record M. T., Jr, Lohman M. L., De Haseth P. Ion effects on ligand-nucleic acid interactions. J Mol Biol. 1976 Oct 25;107(2):145–158. doi: 10.1016/s0022-2836(76)80023-x. [DOI] [PubMed] [Google Scholar]
- Rill R. L., Shaw B. R., Van Holde K. E. Isolation and characterization of chromatin subunits. Methods Cell Biol. 1978;18:69–103. doi: 10.1016/s0091-679x(08)60134-x. [DOI] [PubMed] [Google Scholar]
- Siegfried J. A., Kennedy K. A., Sartorelli A. C., Tritton T. R. The role of membranes in the mechanism of action of the antineoplastic agent adriamycin. Spin-labeling studies with chronically hypoxic and drug-resistant tumor cells. J Biol Chem. 1983 Jan 10;258(1):339–343. [PubMed] [Google Scholar]
- Spinelli M., Dabrowiak J. C. Interaction of copper(II) ions with the daunomycin-calf thymus deoxyribonucleic acid complex. Biochemistry. 1982 Nov 9;21(23):5862–5870. doi: 10.1021/bi00266a021. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Yoshida S. Mechanism of the inhibition of calf thymus DNA polymerases alpha and beta by daunomycin and adriamycin. J Biochem. 1980 Mar;87(3):911–918. doi: 10.1093/oxfordjournals.jbchem.a132821. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Ughetto G., Quigley G. J., Rich A. Interactions between an anthracycline antibiotic and DNA: molecular structure of daunomycin complexed to d(CpGpTpApCpG) at 1.2-A resolution. Biochemistry. 1987 Feb 24;26(4):1152–1163. doi: 10.1021/bi00378a025. [DOI] [PubMed] [Google Scholar]
- Zunina F., Gambetta R., Di Marco A. The inhibition in vitro of DNA polymerase and RNA polymerases by daunomycin and adriamycin. Biochem Pharmacol. 1975 Jan 15;24(2):309–311. doi: 10.1016/0006-2952(75)90300-7. [DOI] [PubMed] [Google Scholar]



