Abstract
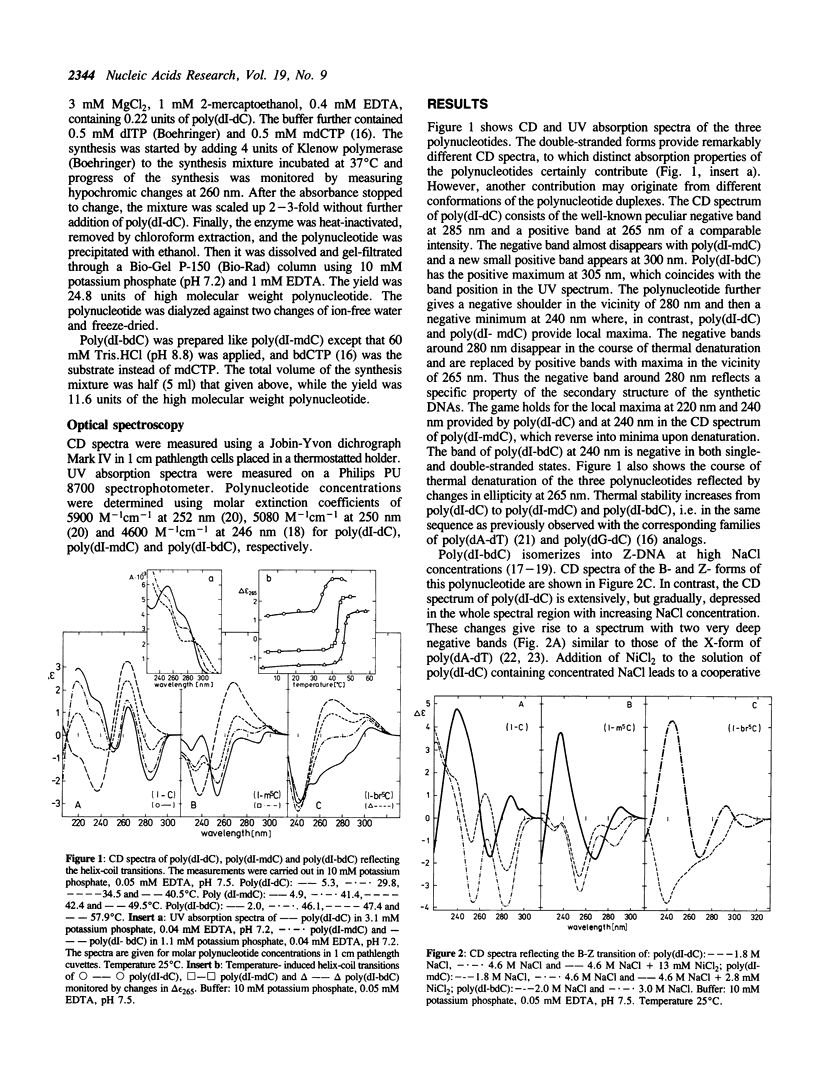
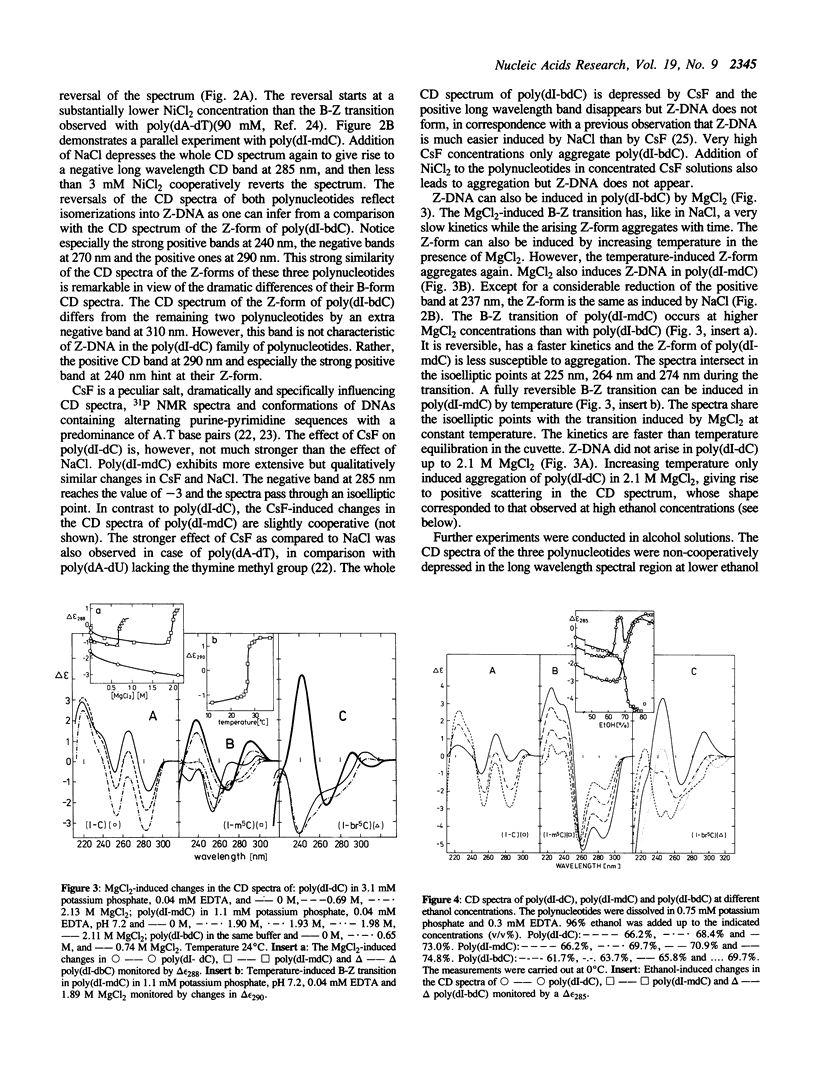
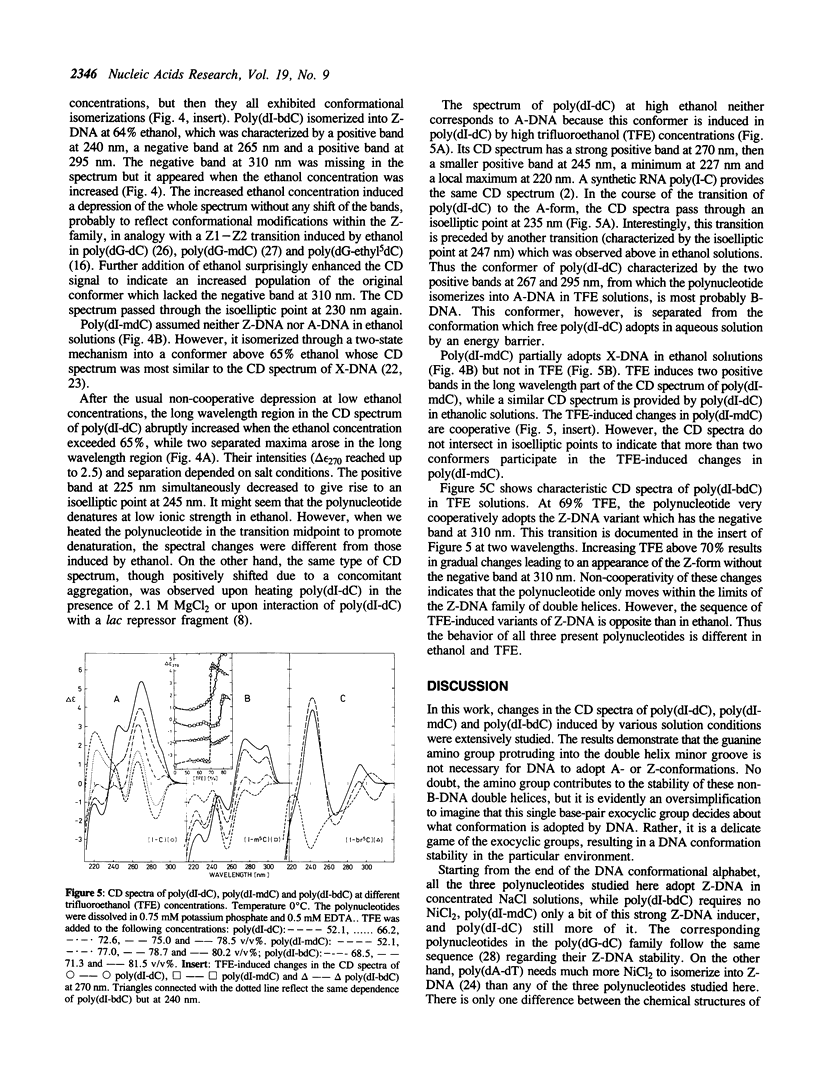
It is shown, using circular dichroism spectroscopy, that poly(dI-dC) is capable to isomerize into both Z-DNA and A-DNA in concentrated NaCl + NiCl2 and trifluoroethanol solutions, respectively. This polynucleotide also undergoes a cooperative, two-state transition in ethanol into a structure which most probably is a canonical B-DNA. This implies that the conformation of poly(dI-dC) is unusual in low-salt aqueous solution. The canonical B-DNA is also adopted by poly(dI-methyl5dC) in trifluoroethanol while this polynucleotide adopts Z-DNA not only in NaCl + NiCl2 but also in the presence of MgCl2. Poly(dI-methyl5dC) partially adopts X-DNA in concentrated CsF and mainly ethanolic solutions. Poly(dI-bromo5dC) isomerizes into Z-DNA not only in concentrated NaCl even in the absence of NiCl2 but also in concentrated MgCl2. This polynucleotide transforms between two distinct variants of Z-DNA in ethanol or trifluoroethanol solutions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Hukins D. W., Smith P. J., Watts L. Structural details of double-helix observed for DNAs containing alternating purine and pyrimidine sequences. J Mol Biol. 1974 Sep 15;88(2):523–533. doi: 10.1016/0022-2836(74)90499-9. [DOI] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtayre P., Liquier J., Pizzorni L., Taillandier E. Z form of poly d(A-T).poly d(A-T) in solution studied by CD and UV spectroscopies. J Biomol Struct Dyn. 1987 Aug;5(1):97–104. doi: 10.1080/07391102.1987.10506378. [DOI] [PubMed] [Google Scholar]
- Bresloff J. L., Crothers D. M. Equilibrium studies of ethidium--polynucleotide interactions. Biochemistry. 1981 Jun 9;20(12):3547–3553. doi: 10.1021/bi00515a038. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E. A new model for DNA containing A.T and I.C base pairs. EMBO J. 1982;1(6):663–667. doi: 10.1002/j.1460-2075.1982.tb01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant R. C., Harwood S. J., Wells R. D. The synthesis and characterization of poly d(I-C) poly d(I-C). J Am Chem Soc. 1968 Jul 31;90(16):4474–4476. doi: 10.1021/ja01018a060. [DOI] [PubMed] [Google Scholar]
- Grant R. C., Kodama M., Wells R. D. Enzymatic and physical studies on (dI-dC) n -(dI-dC) n and (dG-dC) n -(dG-dC) n . Biochemistry. 1972 Feb 29;11(5):805–815. doi: 10.1021/bi00755a020. [DOI] [PubMed] [Google Scholar]
- Hall K. B., Maestre M. F. Temperature-dependent reversible transition of poly(dCdG).poly(dCdG) in ethanolic and methanolic solutions. Biopolymers. 1984 Nov;23(11 Pt 1):2127–2139. doi: 10.1002/bip.360231103. [DOI] [PubMed] [Google Scholar]
- Harder M. E., Johnson W. C., Jr Stabilization of the Z' form of poly(dGdC):poly(dGdC) in solution by multivalent ions relates to the ZII form in crystals. Nucleic Acids Res. 1990 Apr 25;18(8):2141–2148. doi: 10.1093/nar/18.8.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann B., Pilet J., Ptak M., Ramstein J., Malfoy B., Leng M. The B reversible Z transition of poly(dI-br5dC).poly(dI-br5dC). A quantitative description of the Z form dynamic structure. Nucleic Acids Res. 1982 May 25;10(10):3261–3277. doi: 10.1093/nar/10.10.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovin T. M., McIntosh L. P., Arndt-Jovin D. J., Zarling D. A., Robert-Nicoud M., van de Sande J. H., Jorgenson K. F., Eckstein F. Left-handed DNA: from synthetic polymers to chromosomes. J Biomol Struct Dyn. 1983 Oct;1(1):21–57. doi: 10.1080/07391102.1983.10507425. [DOI] [PubMed] [Google Scholar]
- Krueger W. C., Prairie M. D. A circular dichroism study of the binding of CC-1065 to B and Z form poly(dl-5BrdC).poly(dl-5BrdC). Chem Biol Interact. 1987;62(3):281–295. doi: 10.1016/0009-2797(87)90028-7. [DOI] [PubMed] [Google Scholar]
- Kypr J., Sági J., Szabolcs A., Ebinger K., Otvös L., Vorlícková M. Two distinct conformers coexist in a synthetic DNA poly(dA-dT).Poly(dA-dT) in low-salt aqueous solution. Gen Physiol Biophys. 1990 Aug;9(4):415–418. [PubMed] [Google Scholar]
- Kypr J., Vorlícková M. Conformations of alternating purine-pyrimidine DNAs in high-CsF solutions and their reversal by dipyrandium, ethidium and high temperature. Biochim Biophys Acta. 1985 Feb 15;838(2):244–251. doi: 10.1016/0304-4165(85)90085-6. [DOI] [PubMed] [Google Scholar]
- Mirau P. A., Kearns D. R. Comparison of the conformation of poly(dI-dC) with poly(dI-dbr5C) and the B and Z forms of poly(dG-dC). One- and two-dimensional NMR studies. Biochemistry. 1984 Nov 6;23(23):5439–5446. doi: 10.1021/bi00318a010. [DOI] [PubMed] [Google Scholar]
- Mitsui Y., Langridge R., Shortle B. E., Cantor C. R., Grant R. C., Kodama M., Wells R. D. Physical and enzymatic studies on poly d(I-C)-poly d(I-C), an unusual double-helical DNA. Nature. 1970 Dec 19;228(5277):1166–1169. doi: 10.1038/2281166a0. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L., Pohl F. M. "Alternating B-DNA" conformation for the oligo(dG-dC) duplex in high-salt solution. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2508–2511. doi: 10.1073/pnas.76.6.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J. Synthetic RNA and DNA duplexes. Premelting, melting and postmelting transitions of alternating inosine-cytosine polynucleotides in solution. Eur J Biochem. 1978 Feb;83(2):453–464. doi: 10.1111/j.1432-1033.1978.tb12111.x. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Ramaswamy N., Bansal M., Gupta G., Sasisekharan V. Left-handed helices for DNA: studies on poly[d(I-C)]. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6109–6113. doi: 10.1073/pnas.79.20.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M. V., Atreyi M., Kumar G. S., Kumar S. Reversal of the long-wavelength CD band of poly(dI-dC).poly(dI-dC) on specific interaction with the 53-58 peptide fragment of the lac repressor. Biopolymers. 1987 Mar;26(3):329–332. doi: 10.1002/bip.360260302. [DOI] [PubMed] [Google Scholar]
- Sági J., Brahms S., Brahms J., Otvös L. Effect of 5-alkyl substitution of uracil on the thermal stability of poly [d(A-r5U)] copolymers. Nucleic Acids Res. 1979 Jun 25;6(8):2839–2848. doi: 10.1093/nar/6.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorlickova M., Sagi J., Szabolcs A., Szemzo A., Otvos L., Kypr J. Poly(amino2dA-dT) isomerizes into the unusual X-DNA double helix at physiological conditions inducing Z-DNA in poly (dG-methyl5dC). J Biomol Struct Dyn. 1988 Dec;6(3):503–510. doi: 10.1080/07391102.1988.10506503. [DOI] [PubMed] [Google Scholar]
- Vorlicková M., Sági J. Divalent cations are not required for the stability of the low-salt Z-DNA conformation in poly(dG-ethyl5dC). J Biomol Struct Dyn. 1989 Oct;7(2):329–334. doi: 10.1080/07391102.1989.10507775. [DOI] [PubMed] [Google Scholar]
- Vorlícková M., Sági J., Szabolcs A., Szemzö A., Otvös L., Kypr J. Conformation of the synthetic DNA poly(amino2dA-dT) duplex in high-salt and aqueous alcohol solutions. Nucleic Acids Res. 1988 Jan 11;16(1):279–289. doi: 10.1093/nar/16.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]



