Abstract
Towards the functional dissection of neuronal circuits, a number of new genetic tools have been developed that enable rapid and reversible manipulation of genetically defined neuronal subtypes in intact mammalian brain circuits. Alongside the breakthrough technology of optogenetics, receptor-ligand pairs provide complementary approaches to modulate neuronal activity using chemical-genetics.
Introduction
In neuroscience, electrical stimulation, lesions, and inactivation of brain areas have allowed functional mapping of discrete regions and nuclei [1–5]. However, to understand how these regions produce meaningful output, analysis has to zoom in to the level of individual cell-types that constitute local circuits. To this end, clever transgenic methods employing cell type-specific promoters have been used for neuronal ablation [6,7], inactivation [8–11], or inhibition of transmitter release [12–15]. Although some of these methods permit regulation on a time scale of days to weeks, they are in principle chronic and preclude precise temporal deconstruction of complex biological processes. In addition, chronic interventions are susceptible to compensatory interference [16]. Alongside the exquisite temporal resolution afforded through optogenetics [17–19], complementary chemical-genetic approaches that permit rapid and reversible manipulation of neuronal function have now advanced from a proof of principle stage to physiological application.
Here, we review recent developments in chemical-genetic tools for manipulating neuronal activity via receptor-ligand pairs and discuss their application in mammalian brain circuits.
Manipulating neuronal activity with ionotropic receptors
The most direct way to pharmacologically regulate the activity of neuronal cell-types is through targeted expression of ligand-gated ionotropic receptors, followed by activation with exogenous ligands (Figure 1a). An example is the transgenic overexpression of high affinity acetylcholine receptors (nAChRs) in dopaminergic neurons, which resulted in hyperdopaminergic behavior upon low-dose administration of nicotine [20]. However, to allow versatile and precise manipulations of neuronal activity, receptor-ligand pairs must be orthogonal. That is, neither the receptor nor its ligand must have endogenous interaction partners. In addition, the receptor must not show activity in the absence of the ligand, which in turn must not be toxic. Common approaches have been to either hijack receptor-ligand pairs from other tissues and species, or to re-engineer endogenous ionotropic receptors.
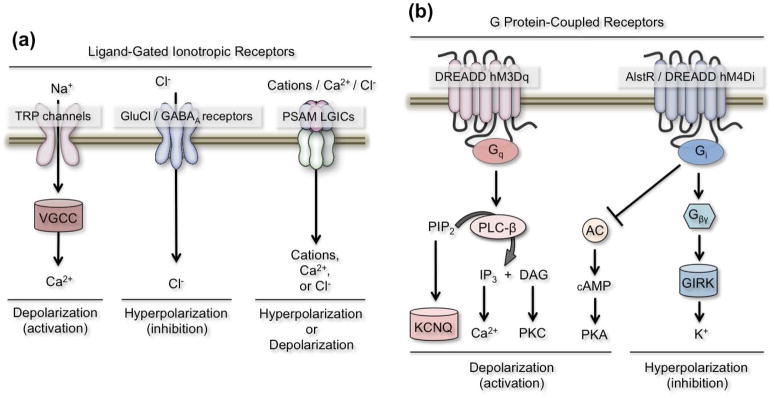
Figure 1. Receptor-ligand systems for rapid modulation of neuronal activity.
(a) Left, ligand-gated influx of free Na+ activates Voltage-gated calcium channels (VGCC), resulting in Ca+2 influx, depolarization, and increased firing. Middle, ligand-gated influx of Cl− results in hyperpolarization and neuronal inhibition. Right, Pharmacologically selective effector molecule (PSEM) gated influx of cations, calcium, or chloride through combination of pharmacologically selective actuator modules (PSAMs) with different ion pore domains to manipulate neuronal activity or inhibition, respectively. (b) Second messenger cascades associated with Gq, and Gi signaling. Left, Gq signaling activates Phospholipase C beta (PLC-β), which hydrolyzes Phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol triphosphate (IP3) and diacylglycerol (DAG). This leads to increased levels of free Ca+2 or Protein kinase C (PKC) activation. Reductions in PIP2 levels may also lead to closure of KCNQ channels causing depolarization and increased neuronal firing. Right, Gi signaling activates inward rectifying potassium channels (GIRKs), resulting in hyperpolarization and inhibition. Independently, activated Gi also inhibits adenylyl cyclase (AC), which promotes cAMP formation and Protein kinase A (PKA) activation.
Neuronal activation with transient receptor potential channels
One of the first successful methods to drive neuronal firing using targeted expression of ionotropic receptors was afforded through the identification of the transient receptor potential cation channel subfamily V member 1 (TRPV1), which is mainly expressed in nociceptive peripheral neurons [21]. When expressed in primary neuronal cultures, TRPV1 drives strong inward currents and membrane depolarization in the presence of vanilloid-like ligands, including the pungent molecule capsaicin [21–23]. Similarly, TRPM8 transduces electrochemical signals in the presence of menthol [22,24]. Although effective for ligand-dependent membrane depolarization, use of TRPV1 for circuit analysis is complicated by baseline effects in the absence of ligand gating, and excitotoxicity in the presence of high agonist concentrations [22,25*]. Nevertheless, in mice conditionally expressing TRPV1, moderate doses of capsaicin have been shown to reversibly induce dose-dependent neuronal firing on a time scale of seconds without overt agonist-independent baseline effects or excitotoxicity [26*]. Furthermore, unilateral activation of striatal neurons in these mice resulted in contra-lateral turning behavior starting within 5 minutes of capsaicin application and lasting for about 10 minutes [26*]. “Caging” ligands for light controllable photo release [22] further enhances the temporal and spatial resolution of this technology. A disadvantage of the TRPV1 approach is that capsaicin activates peripheral pain receptors and does not readily cross the BBB. In vivo experiments will thus benefit from TRPV1 null backgrounds, and the use of other vanilloid-like molecules with better binding kinetics, and BBB permeability to allow systemic ligand administration.
Neuronal silencing with ivermectin-gated chloride channels
For many experimental applications that probe circuit function in vivo, inhibition rather than excitation of neuronal populations is desired. Towards this goal, Lechner et al. have taken advantage of a glutamate-gated chloride channel (GluCl) from C. elegans, which is activated by the anthelmintic drug ivermectin [27–29]. Mammalian neurons expressing the GluCl α and β subunits in vitro showed ivermectin-induced membrane potential hyperpolarization and action potential shunting within seconds, but delayed ligand unbinding and recovery [27]. In vivo intraperitoneal injection of ivermectin evoked turning behavior in mice with unilateral striatal GluCl expression, which peaked 12–48 hours after injection and lasted for days [29]. This silencing method has since been used in mice to investigate the roles of subtypes of GABAergic amygdala neurons in fear conditioning and hypothalamic neurons in aggression [30,31]. Challenges associated with variability in receptor expression, and the need for two subunits [29–31] might be overcome by implementing human α1 glycine receptor subunits engineered to have low glycine, but high ivermectin sensitivity [32]. For this system, however, potential interference with endogenous glycinergic transmission still has to be tested.
Chemically and genetically engineered ligand gated ion channels
Magnus and colleagues have mutated the ligand-binding domain of the α7 nicotinic acetylcholine receptor to be un-responsive to the endogenous ligand acetylcholine, but highly sensitive to a variety of small molecule synthetic ligands which they coined pharmacologically selective effector molecules (PSEMs) [33**]. Fusion of these modified nAChR ligand-binding domains, termed pharmacologically selective actuator modules (PSAMs), with the ion pore domains of different Cys-loop receptors produced receptor channels with different ion selectivities (cations, calcium, or chloride). Chimeras of serotonin 5HT3, or glycine receptor ion pore domains with PSAMs, showed potent activation or silencing in brain slices within seconds to minutes after addition of synthetic ligands. Proof of principle experiments in vivo showed that the chimeric glycine receptor efficiently suppressed Agouti-related-protein-expressing (AgRP) neuron-dependent feeding behavior tens of minutes after intraperitoneal ligand injection. The exact on/off kinetics for each designer receptor will depend on the specific combination of ligand-binding and ion-pore domains, as well as the pharmacokinetic properties of the cognate ligand. Many of these response properties have yet to be determined. However, a notable advantage of this system is the potential to use different ligand and receptor channel combinations to manipulate separate ionic conductances in different neurons or circuits in the same animal [33**].
Allosteric modulation of GABAergic neurotransmission
While most neuronal manipulation techniques influence activity irrespective of network state, the “zolpidem-method” modulates physiological GABAA receptor-mediated transmission via allosteric pharmacology. Mice engineered to harbor a point mutation in the “floxed” GABAA receptor γ2 subunit show unaltered GABAergic transmission, but are insensitive to the allosteric ligands zolpidem and DMCM, which normally enhance and reduce GABA-induced chloride influx through α1–3βγ2 subunit containing receptors (~78% of all GABAA receptors in mammals), respectively [34–36]. Cell type-selective reintroduction of the wild-type γ2 subunit together with Cre recombinase allows cell type-selective subunit swap and reinstatement of drug sensitivity as shown for cerebellar Purkinje cells [37*,38]. In these mice, zolpidem caused enhanced inhibitory postsynaptic currents in Purkinje cells in vitro and motor deficits within minutes after intraperitoneal injection in vivo. The half-life of zolpidem in rodents is about 20 min [39]. An advantage of this system is that the same animal may be used for bidirectional modulation with either zolpidem or the inverse agonist DMCM. Both drugs can be applied systemically and can be acutely antagonized with flumazenil [37*,38]. A disadvantage is the requirement for a genetically engineered zolpidem-insensitive background, which limits its application to mice and rats [40].
Neuronal manipulation using G protein-coupled receptors
The brain expresses a large family of G-protein-coupled receptors (GPCRs), which are activated by a variety of endogenous and pharmacological ligands. Depending on downstream signaling cascades, receptor activation can have a multitude of cellular effects [41]. These include the activation, or inactivation of potassium channels, which in turn lead to reduced or elevated neuronal firing (Figure 1b). The efficacy of GPCR-mediated neuronal silencing was nicely shown by transgenic expression of the Gαi-coupled serotonin receptor (Htr1a) in the amygdala of Htr1a−/− knockout mice. Treatment with the selective agonist 8-hydroxy-2-(di-n-propylamino) tetralin resulted in qualitative changes in conditioned fear responses [42,43]. A clear caveat of this approach was the need for a knockout background. Engineered and heterologously expressed GPCRs address this issue.
Designer GPCRs
As described for engineered ion channels above, one method to create orthogonal GPCR-ligand pairs is to render endogenous receptors insensitive to endogenous ligands, but sensitive to synthetic ones. Systematic mutations of the κ opioid receptor produced RASSLs (receptor activated solely by a synthetic ligands) [44], which were sensitive only to synthetic agonists. RASSLs have since been used in vivo to investigate GPCR signaling in different tissues [45–47]. However, baseline receptor activities and off-target effects of the synthetic ligands precluded their use for precise brain circuit manipulation [48,49]. These teething problems were overcome in a second generation of RASSLs, so called DREADDs (designer receptors exclusively activated by designer drugs) [50].
Engineered from muscarinic acetylcholine receptors (mAChRs), Armbruster and colleagues generated DREADDs with little or no baseline activity that were insensitive to endogenous acetylcholine, but potently activated by the pharmacologically inert molecule clozapine-N-oxide (CNO). Introductions of Y3.33C and A5.46G mutations generated DREADDs that coupled to Gq, Gi, (hM1-5D) or Gs (rM3/β1Ds) signaling pathways without obvious interference with endogenous GPCR-signaling [50,51]. These receptors provided a genetic means for in vivo cell type-selective activation (Gq-coupled hM3Dq) or inhibition (Gi-coupled hM4Di) of neuronal activity, respectively (Figure 1b). Experimentally, pyramidal cells in hippocampal slices of mice transgenically expressing HA-tagged hM3Dq in forebrain showed robust phospholipase C-dependent depolarization and increased firing minutes after CNO application - presumably through closure of KCNQ channels. Apart from reduced locomotion, hM3Dq-expessing mice showed no overt behavioral alterations in the absence of CNO. However, intraperitoneal injection of CNO caused dose- and time-dependent increases in hippocampal network activity and locomotion, with seizures developing at high doses. Effects developed within 15 minutes, peaked ~1 hour post injection, and lasted for ~10 hours. Notably, comparable drug-induced phenotypes were observed after re-injections [52**]. Since then, similar on/off-kinetics have been reported for mCherry-tagged hM3Dq targeted to AgRP neurons of the mouse hypothalamus [53**]. In these animals CNO injections increased feeding behavior within minutes, which lasted for up to 8 hours. Chronic CNO injections caused weight gain, which reversed after CNO withdrawal [53**]. The same authors also expressed the inhibitory DREADD hM4Di in AgRP neurons, where CNO caused hyperpolarization and reduced firing in slices (presumably via GIRK channels), as well as reduced food intake within two hours after intraperitoneal injection [50,53**]. In vivo silencing with hM4Di has also been successfully used to investigate the functions of the striatopallidal or striatonigral pathways in drug sensitization [54*], and the role of serotonergic neurons in respiratory control and thermo regulation [55].
Allatostatin receptor
Another method to drive neuronal hyperpolarization and inhibition of action potential firing has exploited the Drosophila allatostatin receptor (AlstR). Genetic transplantation of the AlstR into mammalian neurons induces Gi-coupled GIRK channel-mediated silencing in the presence of the insect peptide allatostatin (Figure 1b) [56,57]. In ferret cortical slices, allatostatin efficiently reduced membrane potential, input resistance, and action potential frequency within minutes. Similar silencing responses were reported for AlstR-expressing neurons in slices of mouse spinal cord, hippocampus, amygdala, and rat brainstem, as well as cortical neurons in vivo following surface superfusion with allatostatin [58–62]. In vitro, allatostatin-induced effects could be ‘washed-out’ within 15 minutes, but local allatostatin applications below the brain surface in vivo resulted in neuronal inactivation for minutes to hours [62]. Whereas Tan and colleagues found no decrease in silencing efficiency in the continued or repeated presence of allatostatin, Wehr et al. reported partial recovery of activity during allatostatin superfusion, and transient rebounds of hyperexcitability during washout [60,62]. As allatostatin does not cross the BBB, it has to be injected locally. Limited tissue diffusion might account for the reported variability in silencing efficiency and recovery times [62,63]. Nevertheless, this method has proven successful and has been widely used in vitro to study single neuron response properties, and in vivo to delineate the roles of neuronal subtypes in coordinating locomotor rhythms [61,63], respiration [58,64], and encoding fear memories [59].
Conclusions
We have entered an era of experimental neurobiology where imaging, electrophysiological recording, and genetic manipulation technologies are merging [65]. New methods to manipulate neuronal activity through optogenetic and chemical genetic methods now allow interrogation of circuit function from the level of the synapse to behavior. Although all these methods provide the power to probe and map neuronal connectivity with unprecedented resolution, each has its own advantages and disadvantages. For example, optogenetics provides temporal control on a millisecond timescale, which in principle can be used to shape elaborate patterns of activity to investigate details of neuronal coding [66]. However, optogenetics relies on direct access of photons to brain tissue, a methodology that requires brain surgery and is difficult to achieve for prolonged periods of time, or in distributed neuronal populations. Chemical genetics provide an alternative and complementary approach to modulate neuronal activity (see Refs. [53**] and [67*] for comparison of optogenetics and chemical genetics applied to the same cell type). However, chemical genetic methods have their own cast of drawbacks (Table 1). For example, ivermectin-based inhibition through expression of GluCl channels relies on the availability of multiple subunits, and is applicable with timescales of hours to days, whereas allosteric modulation of GABAergic transmission with zolpidem requires expression of wild-type γ2 subunits plus Cre, and is restricted to mice and rats. For GPCR-based methods, efficiency and time-course of the manipulation may vary with the availability of down-stream signaling pathways, and effector molecules in different cell types and developmental stages. In addition, G-protein-signaling will have multiple cellular effects, which may complicate data interpretation in some experimental settings. Regarding reversibility, it should be kept in mind that independent of the method; a neuron might not be the same after a manipulation as before. A great advantage of chemical genetics over optogenetics is the potential for non- or minimally-invasive experimental design through systemic ligand application. This, however, requires transport of the ligand across the BBB, which has not yet been achieved for all systems.
Table 1.
Receptor-ligand systems applied in vivo.
| System (Receptor) | Ligand | Timescale Induction | Timescale Reversal | BBB Permeability | Limitations | References | |
|---|---|---|---|---|---|---|---|
| activation | TRPV1 | Capsaicin | d.a. – secs | d.a. – secs | no | Potential base-line effects, excitotoxicity with high ligand concentrations | [21–23, 25, 26] |
| hM3Dq | CNO | d.a. – secs/mins sys – tens of mins |
sys – hrs | yes | Slow reversal, cellular effects may vary with signalling pathways | [50, 52, 53] | |
| PSAM-5HT3 | PSEMs | d.a. – secs | d.a. – secs | yes | Not yet tested in vivo | [33] | |
|
| |||||||
| inhibition | GABAA | Zolpidem | d.a. – secs/mins sys – mins |
sys – tens of mins | yes | Requires zolpidem-insensitive background, no absolute silencing possible | [34–38] |
| GluCI | Ivermectin | d.a. – secs/min sys – hrs |
d.a. – hrs sys – days |
yes | Slow on-/off-kinetics, ligand may be toxic at higher concentrations | [27–31] | |
| AlstR | allatostatin | d.a. – mins | d.a. – mins/hrs | no | Tissue diffusion of ligand might be limited, effects depend on signalling pathways | [57–64] | |
| hM4Dl | CNO | d.a. – secs/mins sys – hrs |
sys – hrs | yes | Slow reversal, cellular effects may vary with signalling pathways | [50, 53–55] | |
| PSAM-GlyR | PSEMs | d.a. – secs/mins sys – tens of mins |
d.a. secs/mins | yes | Requires further characterization in vivo | [33] | |
D.a., direct tissue application in vitro or in vivo; sys, systemic application in vivo. Note that time scales for induction and reversal are only approximations and may vary with experimental conditions such as route of ligand application, target cell-type, and experimental read-out.
Although neuro-technology is advancing at a breakneck pace, the challenge remains to further define, build upon, and optimize the evolving toolset for investigating brain circuit form and function. New frontiers include the development of synapse-specific manipulation strategies as well as the exploration of molecular receivers for physical signals with easy propagation in brain tissue to combine advantages of current opto- and chemical-genetic techniques. The functional deconstruction of neuronal circuits will help to understand human brain development and disease, and a hope for the future is to advance some of the creative genetic approaches used in the lab towards therapeutic design.
Acknowledgments
We thank T.W. Margrie, T. Newpher, J. Ting, W. Wisden and U.C. Wulff for helpful input and comments on this manuscript. We were supported by MRC grant G0601498, BBSRC grant BB/H001123/1 (P.W.); NINDS grant R00NS064171, NARSAD, and the McNair foundation (B.R.A).
References
- 1.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kesner RP, Gilbert PE, Wallenstein GV. Testing neural network models of memory with behavioral experiments. Curr Opin Neurobiol. 2000;10:260–265. doi: 10.1016/s0959-4388(00)00067-2. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MR, Newsome WT. What electrical microstimulation has revealed about the neural basis of cognition. Curr Opin Neurobiol. 2004;14:169–177. doi: 10.1016/j.conb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Penfield W, Jasper HH. Epilepsy and the Functional Anatomy of the Human Brain. 1. Boston: Little Brown; 1954. [Google Scholar]
- 5.Riedel G, Micheau J, Lam AG, Roloff EL, Martin SJ, Bridge H, de Hoz L, Poeschel B, McCulloch J, Morris RG. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci. 1999;2:898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi K, Morita S, Sawada H, Mizuguchi T, Yamada K, Nagatsu I, Fujita K, Kreitman RJ, Pastan I, Nagatsu T. Immunotoxin-mediated conditional disruption of specific neurons in transgenic mice. Proc Natl Acad Sci U S A. 1995;92:1132–1136. doi: 10.1073/pnas.92.4.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han JH, Kushner SA, Yiu AP, Hsiang HL, Buch T, Waisman A, Bontempi B, Neve RL, Frankland PW, Josselyn SA. Selective erasure of a fear memory. Science. 2009;323:1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- 8.Ehrengruber MU, Doupnik CA, Xu Y, Garvey J, Jasek MC, Lester HA, Davidson N. Activation of heteromeric G protein-gated inward rectifier K+ channels overexpressed by adenovirus gene transfer inhibits the excitability of hippocampal neurons. Proc Natl Acad Sci U S A. 1997;94:7070–7075. doi: 10.1073/pnas.94.13.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu CR, Power J, Barnea G, O’Donnell S, Brown HE, Osborne J, Axel R, Gogos JA. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron. 2004;42:553–566. doi: 10.1016/s0896-6273(04)00224-7. [DOI] [PubMed] [Google Scholar]
- 10.Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, et al. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci. 2009;12:1069–1073. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, Shaham Y. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 2011;14:420–422. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319:1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- 13.Auer S, Sturzebecher AS, Juttner R, Santos-Torres J, Hanack C, Frahm S, Liehl B, Ibanez-Tallon I. Silencing neurotransmission with membrane-tethered toxins. Nat Methods. 2010;7:229–236. doi: 10.1038/nmeth.1425. [DOI] [PubMed] [Google Scholar]
- 14.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Murray AJ, Sauer JF, Riedel G, McClure C, Ansel L, Cheyne L, Bartos M, Wisden W, Wulff P. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci. 2011;14:297–299. doi: 10.1038/nn.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- 17.Kramer RH, Fortin DL, Trauner D. New photochemical tools for controlling neuronal activity. Curr Opin Neurobiol. 2009;19:544–552. doi: 10.1016/j.conb.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miesenbock G. The optogenetic catechism. Science. 2009;326:395–399. doi: 10.1126/science.1174520. [DOI] [PubMed] [Google Scholar]
- 19.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 20.Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney S, Miwa JM, Bupp S, Heintz N, McIntosh JM, Bencherif M, et al. In vivo activation of midbrain dopamine neurons via sensitized, high-affinity alpha 6 nicotinic acetylcholine receptors. Neuron. 2008;60:123–136. doi: 10.1016/j.neuron.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 22.Zemelman BV, Nesnas N, Lee GA, Miesenbock G. Photochemical gating of heterologous ion channels: remote control over genetically designated populations of neurons. Proc Natl Acad Sci U S A. 2003;100:1352–1357. doi: 10.1073/pnas.242738899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 24.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 25*.Crawford DC, Moulder KL, Gereau RWt, Story GM, Mennerick S. Comparative effects of heterologous TRPV1 and TRPM8 expression in rat hippocampal neurons. PLoS One. 2009;4:e8166. doi: 10.1371/journal.pone.0008166. The authors compared baseline and activation effects of TRPV1 and TRPM8 channels expressed in cultured neurons and suggest that TRPM8 might be better suited for in vivo activation of neuronal activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Arenkiel BR, Klein ME, Davison IG, Katz LC, Ehlers MD. Genetic control of neuronal activity in mice conditionally expressing TRPV1. Nat Methods. 2008;5:299–302. doi: 10.1038/nmeth.1190. First study to demonstrate in vivo activation of neuronal firing in mammals using chemical genetics through conditional TRPV1 expression and activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slimko EM, McKinney S, Anderson DJ, Davidson N, Lester HA. Selective electrical silencing of mammalian neurons in vitro by the use of invertebrate ligand-gated chloride channels. J Neurosci. 2002;22:7373–7379. doi: 10.1523/JNEUROSCI.22-17-07373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li P, Slimko EM, Lester HA. Selective elimination of glutamate activation and introduction of fluorescent proteins into a Caenorhabditis elegans chloride channel. FEBS Lett. 2002;528:77–82. doi: 10.1016/s0014-5793(02)03245-3. [DOI] [PubMed] [Google Scholar]
- 29.Lerchner W, Xiao C, Nashmi R, Slimko EM, van Trigt L, Lester HA, Anderson DJ. Reversible silencing of neuronal excitability in behaving mice by a genetically targeted, ivermectin-gated Cl− channel. Neuron. 2007;54:35–49. doi: 10.1016/j.neuron.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 30.Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynagh T, Lynch JW. An improved ivermectin-activated chloride channel receptor for inhibiting electrical activity in defined neuronal populations. J Biol Chem. 2010;285:14890–14897. doi: 10.1074/jbc.M110.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Magnus CJ, Lee PH, Atasoy D, Su HH, Looger LL, Sternson SM. Chemical and genetic engineering of selective ion channel-ligand interactions. Science. 2011;333:1292–1296. doi: 10.1126/science.1206606. Describes a novel approach to activate genetically encoded chimeric ionotropic receptors that respond to synthetic pharmacologically selective effector molecules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cope DW, Wulff P, Oberto A, Aller MI, Capogna M, Ferraguti F, Halbsguth C, Hoeger H, Jolin HE, Jones A, et al. Abolition of zolpidem sensitivity in mice with a point mutation in the GABAA receptor gamma2 subunit. Neuropharmacology. 2004;47:17–34. doi: 10.1016/j.neuropharm.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Ogris W, Poltl A, Hauer B, Ernst M, Oberto A, Wulff P, Hoger H, Wisden W, Sieghart W. Affinity of various benzodiazepine site ligands in mice with a point mutation in the GABA(A) receptor gamma2 subunit. Biochem Pharmacol. 2004;68:1621–1629. doi: 10.1016/j.bcp.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 36.Leppa E, Vekovischeva OY, Linden AM, Wulff P, Oberto A, Wisden W, Korpi ER. Agonistic effects of the beta-carboline DMCM revealed in GABA(A) receptor gamma 2 subunit F77I point-mutated mice. Neuropharmacology. 2005;48:469–478. doi: 10.1016/j.neuropharm.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Wulff P, Goetz T, Leppa E, Linden AM, Renzi M, Swinny JD, Vekovischeva OY, Sieghart W, Somogyi P, Korpi ER, et al. From synapse to behavior: rapid modulation of defined neuronal types with engineered GABAA receptors. Nat Neurosci. 2007;10:923–929. doi: 10.1038/nn1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wisden W, Murray AJ, McClure C, Wulff P. Studying Cerebellar Circuits by Remote Control of Selected Neuronal Types with GABA(A) Receptors. Front Mol Neurosci. 2009;2:29. doi: 10.3389/neuro.02.029.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benavides J, Peny B, Dubois A, Perrault G, Morel E, Zivkovic B, Scatton B. In vivo interaction of zolpidem with central benzodiazepine (BZD) binding sites (as labeled by [3H]Ro 15-1788) in the mouse brain. Preferential affinity of zolpidem for the omega 1 (BZD1) subtype. J Pharmacol Exp Ther. 1988;245:1033–1041. [PubMed] [Google Scholar]
- 40.Cui X, Ji D, Fisher DA, Wu Y, Briner DM, Weinstein EJ. Targeted integration in rat and mouse embryos with zinc-finger nucleases. Nat Biotechnol. 2011;29:64–67. doi: 10.1038/nbt.1731. [DOI] [PubMed] [Google Scholar]
- 41.Spiegel AM, Weinstein LS. Inherited diseases involving g proteins and g protein-coupled receptors. Annu Rev Med. 2004;55:27–39. doi: 10.1146/annurev.med.55.091902.103843. [DOI] [PubMed] [Google Scholar]
- 42.Gozzi A, Jain A, Giovanelli A, Bertollini C, Crestan V, Schwarz AJ, Tsetsenis T, Ragozzino D, Gross CT, Bifone A. A neural switch for active and passive fear. Neuron. 2010;67:656–666. doi: 10.1016/j.neuron.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Tsetsenis T, Ma XH, Lo Iacono L, Beck SG, Gross C. Suppression of conditioning to ambiguous cues by pharmacogenetic inhibition of the dentate gyrus. Nat Neurosci. 2007;10:896–902. doi: 10.1038/nn1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coward P, Wada HG, Falk MS, Chan SD, Meng F, Akil H, Conklin BR. Controlling signaling with a specifically designed Gi-coupled receptor. Proc Natl Acad Sci U S A. 1998;95:352–357. doi: 10.1073/pnas.95.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Redfern CH, Coward P, Degtyarev MY, Lee EK, Kwa AT, Hennighausen L, Bujard H, Fishman GI, Conklin BR. Conditional expression and signaling of a specifically designed Gi-coupled receptor in transgenic mice. Nat Biotechnol. 1999;17:165–169. doi: 10.1038/6165. [DOI] [PubMed] [Google Scholar]
- 46.Sweger EJ, Casper KB, Scearce-Levie K, Conklin BR, McCarthy KD. Development of hydrocephalus in mice expressing the G(i)-coupled GPCR Ro1 RASSL receptor in astrocytes. J Neurosci. 2007;27:2309–2317. doi: 10.1523/JNEUROSCI.4565-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 48.Conklin BR, Hsiao EC, Claeysen S, Dumuis A, Srinivasan S, Forsayeth JR, Guettier JM, Chang WC, Pei Y, McCarthy KD, et al. Engineering GPCR signaling pathways with RASSLs. Nat Methods. 2008;5:673–678. doi: 10.1038/nmeth.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guettier JM, Gautam D, Scarselli M, Ruiz de Azua I, Li JH, Rosemond E, Ma X, Gonzalez FJ, Armbruster BN, Lu H, et al. A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc Natl Acad Sci U S A. 2009;106:19197–19202. doi: 10.1073/pnas.0906593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52**.Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. First application of the excitatory DREAAD hM3Dqin vivo with detailed analysis of time course and dose-dependence of CNO-induced behavioral effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53**.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. The authors used activation via DREAAD hM3Dq and inactivation via DREAAD hM4Di to test the role of hypothalamic AgRP neurons in feeding behavior. For comparison of the different properties of chemical genetics and optogenetics, we recommend reading this paper in parallel with Ref. [67], where hypothalamic AgRP and POMC neurons were activated with channelrhodopsin-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. First published in vivo application of the Gi-coupled inhibitory DREAAD hM4Di. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Birgul N, Weise C, Kreienkamp HJ, Richter D. Reverse physiology in drosophila: identification of a novel allatostatin-like neuropeptide and its cognate receptor structurally related to the mammalian somatostatin/galanin/opioid receptor family. EMBO J. 1999;18:5892–5900. doi: 10.1093/emboj/18.21.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lechner HA, Lein ES, Callaway EM. A genetic method for selective and quickly reversible silencing of Mammalian neurons. J Neurosci. 2002;22:5287–5290. doi: 10.1523/JNEUROSCI.22-13-05287.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marina N, Abdala AP, Trapp S, Li A, Nattie EE, Hewinson J, Smith JC, Paton JF, Gourine AV. Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J Neurosci. 2010;30:12466–12473. doi: 10.1523/JNEUROSCI.3141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci. 2009;12:1438–1443. doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wehr M, Hostick U, Kyweriga M, Tan A, Weible AP, Wu H, Wu W, Callaway EM, Kentros C. Transgenic silencing of neurons in the mammalian brain by expression of the allatostatin receptor (AlstR) J Neurophysiol. 2009;102:2554–2562. doi: 10.1152/jn.00480.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gosgnach S, Lanuza GM, Butt SJ, Saueressig H, Zhang Y, Velasquez T, Riethmacher D, Callaway EM, Kiehn O, Goulding M. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature. 2006;440:215–219. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- 62.Tan EM, Yamaguchi Y, Horwitz GD, Gosgnach S, Lein ES, Goulding M, Albright TD, Callaway EM. Selective and quickly reversible inactivation of mammalian neurons in vivo using the Drosophila allatostatin receptor. Neuron. 2006;51:157–170. doi: 10.1016/j.neuron.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Narayan S, Geiman E, Lanuza GM, Velasquez T, Shanks B, Akay T, Dyck J, Pearson K, Gosgnach S, et al. V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron. 2008;60:84–96. doi: 10.1016/j.neuron.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBotzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci. 2008;11:538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arenkiel BR, Ehlers MD. Molecular genetics and imaging technologies for circuit-based neuroanatomy. Nature. 2009;461:900–907. doi: 10.1038/nature08536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS One. 2007;2:e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67*.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. In this study the authors probed the role of hypothalamic AgRP and POMC neurons in feeding behavior with channelrhodopsin-2. Please see also Ref. [53], which is addressing a related question using chemical genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]