Abstract
Whether reversible ischaemia in patients referred for exercise stress testing and MPI (myocardial perfusion imaging) is associated with changes in circulating cTn (cardiac troponin) levels is controversial. We measured cTnT with a sensitive assay before, immediately after peak exercise and 1.5 and 4.5 h after exercise stress testing in 198 patients referred for MPI. In total, 19 patients were classified as having reversible myocardial ischaemia. cTnT levels were significantly higher in patients with reversible myocardial ischaemia on MPI at baseline, at peak exercise and after 1.5 h, but not at 4.5 h post-exercise. In patients with reversible ischaemia on MPI, cTnT levels did not change significantly after exercise stress testing [11.1 (5.2–14.9) ng/l at baseline compared with 10.5 (7.2–16.3) ng/l at 4.5 h post-exercise, P=0.27; values are medians (interquartile range)]. Conversely, cTnT levels increased significantly during testing in patients without reversible myocardial ischaemia [5.4 (3.0–9.0) ng/l at baseline compared with 7.5 (4.6–12.4) ng/l, P<0.001]. In conclusion, baseline cTnT levels are higher in patients with MPI evidence of reversible myocardial ischaemia than those without reversible ischaemia. However, although cTnT levels increase during exercise stress testing in patients without evidence of reversible ischaemia, this response appears to be blunted in patients with evidence of reversible ischaemia. Mechanisms other than reversible myocardial ischaemia may play a role for acute exercise-induced increases in circulating cTnT levels.
Keywords: angina, cardiac troponin T (cTnT), exercise stress testing, myocardial ischaemia, myocardial perfusion imaging (MPI)
Abbreviations: ACE1 study, Akershus Cardiac Examination 1 study; ACS, acute coronary syndrome; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CI, confidence interval; cTn, cardiac troponin; eGFR, estimated glomerular filtration rate; HR, heart rate; LVEF, left ventricular ejection fraction; MET, metabolic equivalent; MI, myocardial infarction; MPI, myocardial perfusion imaging; OR, odds ratio; SRS, summed rest score
INTRODUCTION
cTns (cardiac troponins) are the preferred biomarkers for diagnosing acute MI (myocardial infarction). In patients with ACS (acute coronary syndrome), increased levels of cTns are associated with increased risk of death and re-admissions, and identify patients who benefit from early, intensified treatment [1–3]. In patients with stable CAD (coronary artery disease), cTnT measured by a highly sensitive assay is detectable in the large majority of patients [4]. Interestingly, even levels below the 99th percentile of a healthy reference population provide prognostic information, and predict risk of mortality and development of HF (heart failure), but not MI [4]. The pathobiological mechanisms underlying cTn release in patients without ACS are, however, unclear, but reversible myocardial ischaemia may represent one mechanism. Two previous studies have assessed the profile of circulating troponins during and after exercise testing and the relationship to myocardial ischaemia, but with diverging results. In one study, no change in circulating cTnT was observed [5], whereas in another study cTnI concentrations measured by a highly sensitive assay increased in proportion to the severity of myocardial ischaemia [6]. Whether these discrepant results can be ascribed to biological differences between cTnI and cTnT or differences in study population composition are unclear.
In the present prospective study, we hypothesized that cTnT levels measured with a sensitive assay would increase more in patients with evidence of reversible ischaemia on MPI (myocardial perfusion imaging) than those without reversible ischaemia, and that, in patients without reversible ischaemia, baseline levels of cTnT would be higher in patients with a history of CAD than those without such a history. In addition, we evaluated the potential incremental diagnostic value of measuring cTnT at baseline and in the recovery phase for diagnosing reversible ischaemia in patients with suspected CAD referred to exercise stress testing and MPI.
MATERIALS AND METHODS
Patients
In total, 200 patients with chest pain and suspected CAD referred for MPI at the Akershus University Hospital were included in the ACE1 study (Akershus Cardiac Examination 1 study). Inclusion was consecutive into three predefined risk strata. Patients were classified according to the probability of reversible myocardial ischaemia (0–100%) by a cardiologist before testing. We included 50 patients with low pre-test probability (<33%), 100 patients with intermediate pre-test probability (33–67%) and 50 patients considered to be at high risk (>67% pre-test probability) of reversible myocardial ischaemia. Exclusion criteria were, besides contraindications to exercise testing of various causes according to guidelines [7], age <18 and>80 years and body weight>120 kg. Two patients were excluded due to disseminated malignant disease diagnosed shortly after the time of inclusion; thus the final cohort comprised 198 patients. Information about co-morbidity, medication and history of CAD were collected from hospital records.
The ACE1 study was carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association, and was approved by the Regional Ethics Committee. Written informed consent was obtained from all patients prior to study commencement.
Exercise stress test and MPI protocol
A stress–rest imaging protocol with a maximal bicycle test was used for MPI. The ramp protocol of the stress test started at 50 watt and increased by 10 watt/min. The criterion for an adequate stress test was>85% of the expected maximal HR [heart rate; 220−age (years)]. Symptoms, HR, BP (blood pressure) and a 12-lead ECG were recorded before the test, midway through each stage and during recovery. The stress test was terminated if there was physical exhaustion, severe chest pain or other symptoms of ACS,>2 mm horizontal or downsloping ST-segment depression, ≥20 mmHg fall in systolic BP or sustained ventricular arrhythmias. Duration of the stress test, workload (Watts) achieved, peak HR and peak BP were recorded. METs (metabolic equivalents) were calculated using the equation:
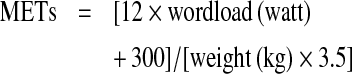 |
Results of the exercise stress test were determined by a cardiologist and categorized as positive, intermediate or negative based on symptoms and ECG alterations.
We administered 250–350 MBq (depending on body weight) of 99mTc (technetium-99m)-tetrofosmin at peak stress and the patient continued to exercise for at least 60 s after the injection. Stress imaging was performed 45 min after tracer administration. At 3–4 h later, 750–900 MBq of 99mTc-tetrofosmin was injected at rest and imaging was performed approximately 45 min after tracer administration. SPECT (single-photon emission computed tomography)-MPI was acquired using a two-headed gamma camera (DST-XL; GE Healthcare Technologies) equipped with a low-energy, high-resolution collimator. Data were reconstructed into short-axis, vertical long-axis and horizontal long-axis slices, according to the individually determined anatomic cardiac long axes. A 17-segment myocardial model was used for semi-quantitative analysis, with a visual perfusion rating of 0–4 for each segment, and the summed stress score, SRS (summed rest score) and summed difference score were calculated. Patients with an SRS score ≥4 were considered to have significant fixed perfusion defect on MPI. Automatically determined semi-quantitative analysis of myocardial perfusion using the commercially available software QPS (Quantitative Perfusion Spect) was used as a supplement. The visual perfusion rating was performed by a specialist in nuclear medicine blinded to troponin data. LVEF (left ventricular ejection fraction) was calculated from the gated rest study using QGS (Quantitative Gated Spect) software.
Blood sampling and laboratory variables
An intravenous line was inserted in an antecubital vein and blood samples were obtained before (baseline), immediately after and 1.5 and 4.5 h after stress testing. Blood samples were processed within 60 min after collection, and stored at −80°C until analysis. All laboratory tests were performed by personnel blinded to clinical information at the Clinical Chemistry laboratory at Akershus University Hospital. cTnT was analysed using a highly sensitive assay (Elecsys Troponin T hs STAT assay; Roche Diagnostics). The limit of blank of this assay is 3 ng/l, the assay 10% coefficient of variance threshold is 13 ng/l, and the 99th percentile value in healthy subjects is 14 ng/l [8]. Serum creatinine was analysed by a standard method, and the eGFR (estimated glomerular filtration rate) was estimated using the Cockcroft–Gault formula [9].
Statistical analysis
Categorical variables are presented as proportions, normally distributed continuous variables as means and S.D. and non-normally distributed continuous variables as medians and interquartile range (Q1–Q3). A Kolmogorov–Smirnov test was used to determine continuous variables were normally distributed. Baseline characteristics were compared using χ2 test for categorical variables, Student's t test for normally distributed variables, and Mann–Whitney U test for non-normally distributed variables. cTnT values <3.0 ng/l were assigned a value of 3.0 ng/l. To avoid that this assignment blunted the variations of cTnT in patient with low concentrations, we performed a subgroup analysis in which patients with cTnT <3 ng/l at baseline were excluded. The median differences between baseline and post-stress test troponin levels were compared using the Friedman test. The Wilcoxon signed rank test was used to compare values between time points. Spearman rank correlation analysis was performed to assess associations between risk factors or indices of exercise capacity and changes in cTnT level. To evaluate whether cTnT measurements at baseline provided incremental information for diagnosing myocardial ischaemia, we assessed associations between baseline cTnT (log-transformed) and reversible ischaemia in univariate and multivariate logistic regression models. We adjusted for the covariates age, sex, BMI (body mass index), eGFR, LVEF, history of hypertension, history of CAD, history of diabetes mellitus and results on exercise stress test. A P<0.05 was considered statistically significant except for the Wilcoxon signed rank test where we applied a Bonferroni corrected P value (P=0.05/3) due to multiple testing. Statistical analyses were performed using SPSS for Windows version 18.0.
RESULTS
Baseline characteristics
Characteristics of patients at baseline according to the presence or absence of reversible myocardial ischaemia on MPI are shown in Table 1. In total, 19 (10%) patients were classified as having reversible myocardial ischaemia. The group of patients classified as being without reversible ischaemia included both patients without evidence of ischaemia (n=156) and patients with non-reversible (fixed) perfusion defects (n=23) on MPI. Overall, the mean age was 60±11 years and 51% were female. The patients with reversible myocardial ischaemia were more likely to be male, to have a history of CAD and to have lower LVEF. However, age, eGFR and BMI, as well as cardiovascular risk factors such as hypertension, diabetes mellitus and current smoking, did not differ between patient groups. At stress testing, the patients with reversible myocardial ischaemia had a median (interquartile range) summed difference score of 4 (3–5), whereas in patients without reversible ischaemia the median (interquartile range) summed difference score was 0 (0–1) (Table 2). Patients with reversible myocardial ischaemia were more likely to have a positive stress test (ECG) and to have lower peak HR.
Table 1. Baseline characteristics of the patients (n=198).
PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease.
| Characteristic | Total (n=198) | Positive MPI (n=19) | Negative MPI (n=179) | P value |
|---|---|---|---|---|
| Troponin T (ng/l) | 6.2 (3.0–10.0) | 11.1 (5.2–14.9) | 5.4 (3.0–9.2) | 0.007 |
| Troponin T <3 ng/l (n) | 53 (27%) | 4 (21%) | 49 (27%) | 0.79 |
| Troponin T ≥14 ng/l (n) | 27 (14%) | 7 (37%) | 20 (11%) | 0.002 |
| Demographics | ||||
| Female | 100 (51%) | 3 (16%) | 97 (54%) | 0.001 |
| Age (years) | 60±11 | 63±12 | 59±11 | 0.12 |
| Risk factors | ||||
| BMI (kg/m2) | 27±4 | 28±3 | 27±4 | 0.13 |
| Hypertension (n) | 73 (37%) | 11 (58%) | 62 (35%) | 0.052 |
| Diabetes mellitus (n) | 25 (13%) | 4 (21%) | 22 (12%) | 0.29 |
| Current smoking (n) | 55 (28%) | 4 (21%) | 51 (29%) | 0.60 |
| Systolic BP (mmHg) | 127±22 | 128±26 | 127±21 | 0.78 |
| Diastolic BP (mmHg) | 85±16 | 84±15 | 86±16 | 0.69 |
| eGFR (ml/min) | 97 (81–119) | 97 (76–142) | 99 (82–121) | 0.69 |
| LVEF | 60 (54–69) | 58 (51–64) | 61 (55–69) | 0.047 |
| Pre-test probability (%) | 60 (38–70) | 60 (60–80) | 50 (30–60) | 0.008 |
| Previous CAD (n) | ||||
| History of CAD | 77 (39%) | 16 (84%) | 61 (34%) | <0.001 |
| Documented MI | 43 (22%) | 10 (53%) | 33 (19%) | 0.001 |
| Positive angiography | 68 (34%) | 16 (84%) | 52 (30%) | <0.001 |
| History of PCI | 55 (30%) | 12 (63%) | 43 (24%) | <0.001 |
| History of CABG | 20 (10%) | 5 (26%) | 15 (9%) | 0.031 |
| History of COPD/asthma | 25 (13%) | 1 (5%) | 24 (13%) | 0.48 |
| Medication (n) | ||||
| Platelet aggregation inhibitor | 118 (60%) | 17 (89%) | 101 (57%) | 0.006 |
| Lipid-lowering drug | 111 (56%) | 18 (95%) | 93 (52%) | <0.001 |
| β-Blocker | 92 (47%) | 14 (74%) | 78 (44%) | 0.013 |
| Diuretics | 39 (20%) | 8 (42%) | 31 (17%) | 0.010 |
Table 2. Results from the exercise stress test.
| Total (n=198) | Positive MPI (n=19) | Negative MPI (n=179) | P value | |
|---|---|---|---|---|
| Stress test performance | ||||
| Positive stress test (ECG) | 61 (31%) | 10 (53%) | 51 (29%) | 0.030 |
| Baseline heart rate (beats/min) | 79 (69–90) | 82 (71–90) | 78 (69–91) | 0.94 |
| Peak heart rate (beats/min) | 153 (144–164) | 139 (134–155) | 155 (146–165) | 0.012 |
| Percentage of maximal expected heart rate | 95±8 | 91±9 | 95±8 | 0.060 |
| Peak systolic BP (mmHg) | 200 (174–211) | 202 (182–208) | 199 (174–212) | 0.73 |
| Peak diastolic BP (mmHg) | 92 (81–106) | 85 (70–110) | 94 (82–106) | 0.21 |
| Duration (min) | 8 (7–10) | 9 (7–10) | 8 (7–11) | 0.36 |
| Maximum strain (watts) | 120 (100–140) | 130 (108–133) | 120 (100–150) | 0.37 |
| Maximum workload (METs) | 7±2 | 6±1 | 7±2 | 0.15 |
| MPI | ||||
| Summed difference score | 0 (0–1) | 4 (3–5) | 0 (0–1) | <0.001 |
cTnT profile
Overall, cTnT, as measured by the highly sensitive assay, was detectable (>3 ng/l) in serum samples from 145 (73%) patients at baseline. The 53 (27%) patients with cTnT levels <3 ng/l at baseline (n=53) were younger than those with higher levels, but the proportion of women did not differ between patients with and without detectable cTnT levels (49 compared with 55%, P=0.51). In total, 16 (8%) patients had no detectable cTnT at any time point. The median (interquartile range) baseline level in the total cohort was 6.2 (3.0–10.0) ng/l, and 27 (14%) patients had values at or above the 99th percentile (14 ng/l) at baseline. After 4.5 h, the median cTnT level in the entire cohort increased to 7.7 (4.9–13.4) ng/l (P<0.001 compared with baseline).
Impact of the presence or absence of reversible myocardial ischaemia
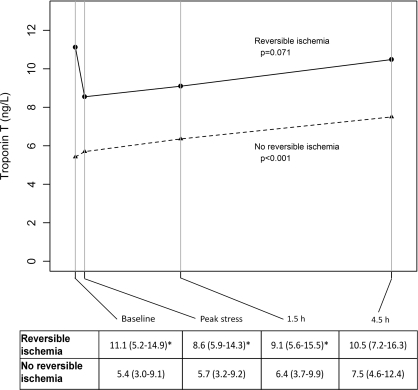
When patients were categorized according to the presence (n=19) or absence (n=179) of reversible myocardial ischaemia, differences in baseline levels and profiles during and after exercise were observed. We found cTnT levels to be significantly higher in patients with reversible myocardial ischaemia compared with those without reversible myocardial ischaemia at baseline, at peak exercise and at 1.5 h, but not at 4.5 h post-exercise (Figure 1). In patients with reversible myocardial ischaemia, cTnT levels declined from baseline [11.1 (5.2–14.9) ng/l] to peak exercise [8.6 (5.9–14.3) ng/l, P=0.23], although this was not statistically significant. Changes from baseline to 1.5 h [8.6 (5.9–14.3) ng/l, P=0.62] or from baseline to 4.5 h [10.5 (7.2–16.3) ng/l, P=0.27] after exercise stress testing were also not statistically significant. Conversely, in patients without reversible myocardial ischaemia, cTnT levels increased from baseline [5.4 (3.0–9.0) ng/l] to 4.5 h after exercise [7.5 (4.6–12.4) ng/l, P<0.001]. Excluding the 53 patients with cTnT <3 ng/l at baseline did not substantially alter the results.
Figure 1. cTnT levels at different time points stratified by MPI results.
The Table below shows the median (interquartile range) cTnT levels at the four different time points. P values in the Figure refer to changes in biomarker levels across time points for each group. *P<0.05 for the comparison with the no reversible ischaemia group.
Impact of the presence or absence of history of CAD
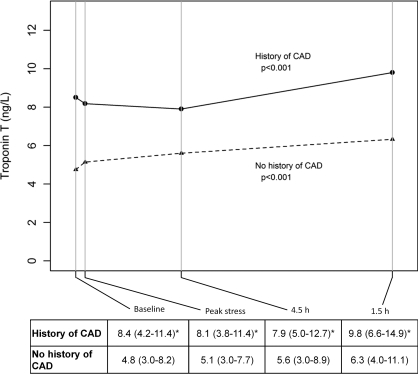
In patients without reversible ischaemia, levels of cTnT were higher in patients with a history of CAD (n=61) compared with no history of CAD (n=118) at all four time points (Figure 2). Levels changed significantly from baseline [8.4 (4.2–11.4) ng/l] to 4.5 h [9.8 (6.6–14.9) ng/l, P<0.001] in patients with a history of CAD, as well as from baseline [4.8 (3.0–8.2) ng/l] to 4.5 h [6.3 (4.0–11.1) ng/l, P<0.001] in patients without a history of CAD. The magnitude of change was not different between the two groups at any time point (Figure 2).
Figure 2. cTnT levels at different time points stratified by history of CAD in patients with negative MPI.
The Table below shows median (interquartile range) cTnT levels at the four different time points in patients without reversible myocardial ischaemia (n=179). P values in the Figure refer to changes in biomarker levels across time points for each group. *P<0.05 for the comparison with the no history of CAD group.
Impact of the presence or absence of fixed perfusion defects on MPI
In patients without reversible ischaemia, levels of cTnT were higher in patients with fixed perfusion defects [n=23; 9.3 (4.1–13.9) ng/l] compared with those without perfusion defects [n=156; 5.3 (3.0–8.7) ng/l] at baseline (P=0.008), at peak exercise [9.2 (4.7–14.4) ng/l compared with 5.5 (3.1–8.9) ng/l, P=0.009], and at 1.5 h [8.3 (5.1–13.6) ng/l compared with 6.0 (3.3–9.7) ng/l, P=0.025] but not at 4.5 h [9.8 (5.5–16.7) ng/l compared with 7.5 (4.4–12.2) ng/l, P=0.27] after stress. Levels did not change significantly from baseline [9.3 (4.1–13.9) ng/l] to peak stress [9.2 (4.7–14.4) ng/l, P=0.84], to 1.5 h [8.3 (5.1–13.6) ng/l, P=0.63] or to 4.5 h [9.8 (5.5–16.7) ng/l, P=0.24] in patients with fixed perfusion defects. However, in patients without fixed perfusion defects, cTnT levels changed significantly from baseline [5.3 (3.0–8.7) ng/l] to 1.5 h [6.0 (3.3–9.7) ng/l, P=0.002] and 4.5 h [7.5 (4.4–12.2) ng/l, P<0.001] after stress, but not to peak exercise [5.5 (3.1–8.9) ng/l, P=0.54].
Variables correlated with a change in cTnT
The potential correlation between the change in cTnT from baseline to 4.5 h post-exercise and cardiovascular risk factors, including age, sex, eGFR, LVEF, history of CAD, hypertension and diabetes mellitus, as well as the MPI results, having a positive exercise stress test (ECG) and indices of exercise capacity, including maximum HR achieved, percentage of maximal predicted HR, maximum work load (watts and METs) and exercise duration, were assessed with correlation analysis. With the exceptions of a weak correlation with the percentage of maximal predicted HR achieved (Spearman ρ=0.16, P=0.028) and a weak negative correlation with estimated METs (ρ=−0.16, P=0.025), no significant correlations were observed.
Diagnostic value of cTnT to predict reversible myocardial ischaemia
In univariate analysis, baseline log-transformed cTnT levels were associated with having reversible myocardial ischaemia {OR (odds ratio), 2.57 [95% CI (confidence interval) 1.34–4.93); P=0.005}. After adjusting for age, gender, BMI, eGFR, LVEF, history of CAD, diabetes mellitus, hypertension and having a positive exercise stress test, the association was no longer significant [OR, 1.48 (95% CI, 0.52–4.22); P=0.46] (Table 3). Having a history of CAD and a positive exercise stress test were the only variables associated with reversible myocardial ischaemia in multivariate analysis.
Table 3. Predictors of reversible ischaemia on MPI.
DM, diabetes mellitus; HT, hypertension.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Parameter | OR (95% CI) | P value | OR (95% CI) | P value |
| Troponin T | 2.57 (1.34–4.93) | 0.005 | 1.48 (0.52–4.22) | 0.46 |
| Age | 1.04 (0.99–1.08) | 0.12 | 1.07 (0.96–1.18) | 0.22 |
| Gender (male) | 6.31 (1.78–22.41) | 0.00 | 3.15 (0.64–15.63) | 0.16 |
| BMI | 1.09 (0.97–1.23) | 0.14 | 1.11 (0.93–1.33) | 0.23 |
| eGFR | 1.01 (0.99–1.02) | 0.38 | 1.03 (0.99–1.06) | 0.11 |
| LVEF | 0.97 (0.93–1.01) | 0.12 | 1.00 (0.94–1.06) | 0.88 |
| History of CAD | 10.32 (2.89–36.78) | <0.001 | 16.15 (2.91–89.73) | 0.001 |
| History of DM | 1.89 (0.58–6.21) | 0.29 | 0.91 (0.20–4.26) | 0.91 |
| History of HT | 2.53 (0.97–6.61) | 0.06 | 3.37 (0.87–13.11) | 0.08 |
| Positive stress test (ECG) | 2.79 (1.07–7.26) | 0.04 | 7.49 (1.63–34.41) | 0.01 |
DISCUSSION
The main findings of the present study are that: (i) cTnT levels are higher at baseline in patients with reversible myocardial ischaemia than in patients without reversible myocardial ischaemia, and (ii) cTnT levels increase after exercise stress testing in patients without reversible myocardial ischaemia, whereas this response is blunted in patients with MPI evidence of reversible myocardial ischaemia. Moreover, in patients without reversible myocardial ischaemia, cTnT levels increase during and after exercise stress testing regardless of the presence or absence of a history of CAD, and although baseline levels are higher in those with a history of CAD, the magnitude of the cTnT increase is similar between groups. Furthermore, similar to patients with reversible perfusion defects, cTnT levels did not change in patients with fixed perfusion defects on MPI. Finally, baseline levels of cTnT were higher in patients classified as having reversible myocardial ischaemia during testing, but this association was attenuated in multivariate models that included standard clinical variables.
Whether reversible myocardial ischaemia is a stimulus for troponin release to the circulation is controversial. Cardiac troponins I and T are predominantly bound to the contractile apparatus in cardiomyocytes, but a minor fraction of these molecules are found freely soluble in the cytosol [10]. Recently, six different mechanisms to explain how this fraction of troponins may be released to the circulation in the absence of MI were proposed [11]: (i) myocyte necrosis, (ii) apoptosis, (iii) normal myocyte cell turnover, (iv) cellular release of proteolytic troponin degradation products, (v) increased cellular wall permeability and (vi) formation and release of membranous blebs. Theoretically, the latter three mechanisms may be stimulated by reversible myocardial ischaemia. Observations from previous clinical studies have, however, yielded diverging results. Kurz et al. [5] found no change in cTnT levels measured by a sensitive assay after stress testing, regardless of the presence or absence of reversible coronary ischaemia on MPI. Conversely, Sabatine et al. [6] reported that cTnI measured with an ultrasensitive assay increased in proportion to the degree of reversible myocardial ischaemia 4 h after exercise stress testing. Our current findings extend information from these prior studies by demonstrating that baseline levels of cTnT are higher in patients with reversible myocardial ischaemia than in those without, whereas exercise stress testing is associated with a significant increase in cTnT in the absence, but not in the presence, of reversible myocardial ischaemia. The latter observation is in direct contrast with our original study hypothesis, but in accordance with very recent data from a study in which patients with and without angiographically significant CAD underwent rapid atrial pacing and coronary sinus sampling for assessment of cTnT and lactate [12]. In that study, coronary sinus levels of cTnT were higher at baseline in patients with CAD than in patients without significant CAD, but the relative increase during and after atrial pacing appeared to be largest in patients without significant CAD.
Although baseline levels of cTnT were associated with reversible myocardial ischaemia in a univariate logistic regression model, this association was attenuated and no longer significant in multivariate analyses. Thus, it seems unlikely that baseline cTnT measurements will be of any practical clinical utility in the setting of exercise stress testing for diagnosing reversible myocardial ischaemia. As no increase in cTnT in proportion to reversible myocardial ischaemia was observed in our study, it is also unlikely that measurements in the recovery phase after stress testing will be of any value in clinical practice. The discrepancy with the findings of Sabatine et al. [6], who observed an increase in cTnI in proportion to the extent of myocardial ischaemia, raises the question whether there is a difference between the two proteins in terms of release from the cytosol. Free troponin I (molecular mass of 22.5 kDa) is a smaller molecule than the free cTnT (39 kDa), which could relate to a more dynamic release of cTnI into the circulation. Alternative explanations for the divergent results between our study and the study of Sabatine et al. [6] include differences in patient characteristics, including the proportion of patients with positive MPI, and different analytical characteristics of the two troponin assays. The larger proportion of patients with troponin levels below the detection limit in our study compared with the study of Sabatine et al.[6] may also have influenced results, although we find similar results after excluding the patients with undetectable cTnT levels. Of note, as reported previously [13], there was a higher prevalence of undetectable cTnT levels among young subjects compared with elderly subjects, while we did not see any difference due to gender.
Strengths and limitations
The strengths of the present study include the prospective design and well-characterized patient population, serial blood sampling and the use of a highly sensitive cTnT assay well suited to detect changes in circulating cTnT levels. Limitations include 27% of the cohort having undetectable levels of cTnT at baseline and 8% having undetectable cTnT levels at any time point, which precludes the detection of minor changes below 3 ng/l. Thus an even more sensitive assay may have been able to detect differences that were not picked up by the current assay. Other limitations are the relatively modest number of patients with reversible myocardial ischaemia and the imperfection of MPI as reference standard for detecting myocardial ischaemia.
Conclusions
In patients referred to MPI with exercise stress testing, cTnT increases from baseline to 4.5 h after stress testing in the absence of reversible myocardial ischaemia, but not in patients with evidence of reversible myocardial ischaemia, implying that mechanisms other than reversible myocardial ischaemia may play a role in the increase. Our findings indicate a need for further clinical studies that include a larger number of patients and a standardized protocol of sampling, in order to clarify the time course of cTnI and cTnT levels after exercise stress in patients with and without CAD.
AUTHOR CONTRIBUTION
Ragnhild Røysland collected the baseline parameters from the patient records, participated in the data analysis, wrote the first draft and participated in the presubmission review of the paper. Gunnhild Kravdal participated in the execution of the study, analysed the MPI data and critically reviewed the paper. Arne Didrik Høiseth analysed the exercise stress ECGs and critically reviewed the paper. Ståle Nygård participated in the data analysis and critically reviewed the paper. Pirouz Badr participated in the execution of the study and critically reviewed the paper. Tor-Arne Hagve participated in the laboratory analysis and critically reviewed the paper. Torbjørn Omland and Helge Røsjø designed the ACE1 study and participated in the execution of the study, data analysis and contributed to the writing and revision of the paper. All authors have seen and approved the final version.
ACKNOWLEDGEMENTS
We thank the contributors to the ACE1 study at the Akershus University Hospital.
FUNDING
This work was supported the South-Eastern Norway Regional Health Authority, the Family Blix' Foundation and Akershus University Hospital.
References
- 1.James S. K., Lindback J., Tilly J., Siegbahn A., Venge P., Armstrong P., Califf R., Simoons M. L., Wallentin L., Lindahl B. Troponin-T and N-terminal pro-B-type natriuretic peptide predict mortality benefit from coronary revascularization in acute coronary syndromes: a GUSTO-IV substudy. J. Am. Coll. Cardiol. 2006;48:1146–1154. doi: 10.1016/j.jacc.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 2.Lindahl B., Toss H., Siegbahn A., Venge P., Wallentin L. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during instability in coronary artery disease. N. Engl. J. Med. 2000;343:1139–1147. doi: 10.1056/NEJM200010193431602. [DOI] [PubMed] [Google Scholar]
- 3.Morrow D. A., Cannon C. P., Jesse R. L., Newby L. K., Ravkilde J., Storrow A. B., Wu A. H., Christenson R. H. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115:e356–e375. doi: 10.1161/CIRCULATIONAHA.107.182882. [DOI] [PubMed] [Google Scholar]
- 4.Omland T., de Lemos J. A., Sabatine M. S., Christophi C. A., Rice M. M., Jablonski K. A., Tjora S., Domanski M. J., Gersh B. J., Rouleau J. L., et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N. Engl. J. Med. 2009;361:2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurz K., Giannitsis E., Zehelein J., Katus H. A. Highly sensitive cardiac troponin T values remain constant after brief exercise- or pharmacologic-induced reversible myocardial ischemia. Clin. Chem. 2008;54:1234–1238. doi: 10.1373/clinchem.2007.097865. [DOI] [PubMed] [Google Scholar]
- 6.Sabatine M. S., Morrow D. A., de Lemos J. A., Jarolim P., Braunwald E. Detection of acute changes in circulating troponin in the setting of transient stress test-induced myocardial ischaemia using an ultrasensitive assay: results from TIMI 35. Eur. Heart J. 2009;30:162–169. doi: 10.1093/eurheartj/ehn504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hesse B., Tagil K., Cuocolo A., Anagnostopoulos C., Bardies M., Bax J., Bengel F., Busemann Sokole E., Davies G., Dondi M., et al. EANM/ESC procedural guidelines for myocardial perfusion imaging in nuclear cardiology. Eur. J. Nucl. Med. Mol. Imaging. 2005;32:855–897. doi: 10.1007/s00259-005-1779-y. [DOI] [PubMed] [Google Scholar]
- 8.Giannitsis E., Kurz K., Hallermayer K., Jarausch J., Jaffe A. S., Katus H. A. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin. Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 9.Cockcroft D. W., Gault M. H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 10.Bleier J., Vorderwinkler K. P., Falkensammer J., Mair P., Dapunt O., Puschendorf B., Mair J. Different intracellular compartmentations of cardiac troponins and myosin heavy chains: a causal connection to their different early release after myocardial damage. Clin. Chem. 1998;44:1912–1918. [PubMed] [Google Scholar]
- 11.White H. D. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J. Am. Coll. Cardiol. 2011;57:2406–2408. doi: 10.1016/j.jacc.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 12.Turer A. T., Addo T. A., Martin J. L., Sabatine M. S., Lewis G. D., Gerszten R. E., Keeley E. C., Cigarroa J. E., Lange R. A., Hillis L. D., de Lemos J. A. Myocardial ischemia induced by rapid atrial pacing causes troponin T release detectable by a highly sensitive assay: insights from a coronary sinus sampling study. J. Am. Coll. Cardiol. 2011;57:2398–2405. doi: 10.1016/j.jacc.2010.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clerico A., Giannoni A. Will high-sensitive troponin immunoassays lead to more clarity or confusion in clinical practice? Clin. Sci. 2010;119:203–205. doi: 10.1042/CS20100234. [DOI] [PubMed] [Google Scholar]