Abstract
Maintenance of hematopoietic stem cells (HSCs) pool depends on fine balance between self-renewal and differentiation of HSCs. HSCs normally reside within the bone marrow niche of an adult mammal. The embryonic development of HSCs is a complex process that involves the migration of developing HSCs in multiple anatomical sites. Throughout the process, developing HSCs receive internal (transcriptional program) and external (HSC niche) signals, which direct them to maintain balance between self-renewal and differentiation, also to generate a pool of HSCs. In physiological condition HSCs differentiate into all mature cell types present in the blood. However, in pathological condition they may differentiate into non-hematological cells according to the need of the body. It was shown that HSCs can transdifferentiate into cell types that do not belong to the hematopoietic system suggests a complete paradigm shift of the hierarchical hematopoietic tree. This review describes the developmental origins and regulation of HSCs focusing on developmental signals that induce the adult hematopoietic stem cell program, as these informations are very critical for manipulating conditions for expansion of HSCs in ex vivo condition. This review also states clinical application and related patents using HSC.
Keywords: Hematopoietic stem cells, embryonic development, regulation, self-renewal, differentiation, transdifferentiation, clinical application, patents
INTRODUCTION
The regeneration of blood cells for whole life of any individuals depend on the ability to self-renew and to differentiate towards multiple lineages of hematopoietic stem cells (HSC) [1]. During embryogenesis, original pool of HSC is developed from a complex process that involves several anatomical sites such as yolk sac, the aorta-gonad-mesonephros (AGM), placenta and fetal liver, however at birth HSCs colonize to the bone marrow (BM). A steady state condition is established during postnatal life, in which a balance between self-renewal and differentiation maintains HSC pool. The specialized microenvironment (niche) of the bone marrow made this possible, where the multipotency of HSCs is conserved through asymmetric cell divisions, while their progeny are directed towards multi-lineage differentiation [2]. Increasing evidences in the field of HSC biology, HSC development, BM niche and homoeostatic regulation advances the efficiency in clinical application. Advancement of research will help in manipulation of HSC in ex vivo or in vitro conditions. HSC transplantation is common practice now-a-days in various malignant and non-malignant hematological disorders. Moreover, immense amount of basic and clinical research is focusing on application of HSCs as a regenerative or adjuvant therapy in various non-hematological conditions such as neurological disorders (Parkinson disease), ischemic conditions (stroke, myocardial ischemia). In this review, we focus on the developmental aspect of HSCs and how microenvironment affects on self-renewal and differentiation processes. Additionally numerous patents related to HSC biology and their clinical implications are also discussed.
ONTOGENY OF HEMATOPOIETIC STEM CELLS
Fetal hematopoiesis is a complex coherent process where multiple factors and different anatomical sites are intertwined with each other and mediate specialized signals. In this complex process, blood cells are generated for immediate embryonic development, growth, and at the same time a stockpile of undifferentiated HSCs are established even though the bone marrow and its specialized niches have not yet developed. The anatomy of the embryo changes during organogenesis. As a result, the shift in site of hematopoiesis is evident from one location to another. Different inductive signals from these compartments of fetal hematopoiesis support these two important processes. Multiple fetal hematopoietic sites are common features in many non-vertebrate and vertebrate animals, such as flies, amphibians, fish, birds, rodents and humans [3, 4]. HSC development is well characterized in the mice, and serves as a model for human hematopoiesis [5-7].
EMERGENCE OF HEMATOPOIETIC STEM CELLS
Close to a century ago first blood cells in vertebrate animal was found in the yolk sac with the simultaneous development of vasculature [8]. The first wave of blood production is called primitive hematopoiesis, occurs in the mammalian yolk sac. At this stage, erythroid cells express embryonic globin proteins. Primitive hematopoiesis support embryonic growth by the production of red blood cells that facilitate tissue oxygenation. The primitive hematopoietic system was found to be transient and rapidly replaced by definitive hematopoiesis. Transplantation of cells isolated from various regions of mouse conceptus at embryonic day (E) 8-E12 into irradiated adult mice have shown that long-term, multilineage HSC population appeared at E10.5 in the AGM region of the embryo, specifically in the vitelline and umbilical arteries [9-11]. Ex-plant culture demonstrated that these adult repopulating HSCs (which are equipotent of adult bone marrow HSCs) are autonomously generated in the AGM [9]. Also it was found that those cells are located in the ventral side of the dorsal aorta [12-14]. HSCs were also found in other tissues, such as placenta and liver [9, 10, 15, 16]. De novo generation of hematopoietic cells in fetal liver, yolk sac and placenta was controversial for long time. It was shown that liver does not produce hematopoietic cells de novo rather it is colonized during late E9, whereas these cells were generated in other tissues [17, 18]. The possibility of the yolk sac and placenta as de novo generators of HSCs is argued by the involvement of embryonic circulation. Data suggest that circulation is established approximately E8.25-E8.5 [19] and it is likely that this circulation distribute HSCs throughout the conceptus. However, quantitative analysis of HSCs indicate that the placenta [15, 16] and yolk sac [20] may contribute to HSC pool in the liver. It was found that there were more number of HSCs in the fetal liver than in the AGM alone [20], which suggests the possible contribution of yolk sac, placenta and AGM together to fetal liver population [15]. However, it is possible that the liver may help in expansion of HSC population [21]. So, two main distinct unrelated classes of functional hematopoietic cells are generated in mouse conceptus. Primitive erythrocytes evolved at E7.5 and definitive adult repopulating HSCs generated at E10.5.
Other classes of progenitor cells such as myeloid progenitors and lymphoid-myeloid progenitors are also generated in the mouse conceptus between E7.5 and E10.5. Experiments demonstrated that the presence of myeloid progenitors in the yolk sac and para-aortic splanchnopleura (pSp; prospective AGM region) even before the circulation is established at E8.25 [22-24]. The lymphoid-myeloid progenitors were found to be multipotent [25] and were also located in the E8 pSp-AGM of the embryo before the circulation is established [24]. However, Yolk sac explants do not contain such cells until the circulation is established, which suggests that cells with lymphoid-myeloid potential are generated de novo in the pSp-AGM. Even similar observation was found in explant culture of human yolk sac and pSp-AGM tissues [26]. Hence, there are several broad classes of hematopoietic cells are found in the mammalian conceptus as defined by activity in in vitro clonogenic or transplantation assays and these cells are generated independent of each other and in distinct anatomical sites Fig. (1).
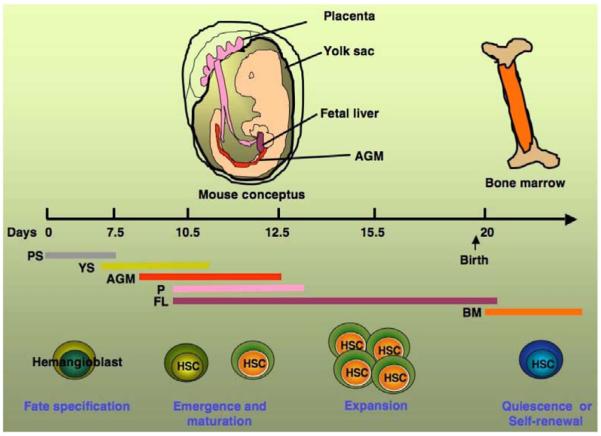
Fig. (1).
Development of hematopoietic stem cells in the different regions of mouse conceptus. Hematopoietic stem cells (HSC) development starts at primitive streak (PS) after fate specification from hemangioblast. HSCs undergo several stages of maturation, expansion, in the yolk sac (YS), aorta-gonad mesonephros (AGM) region, placenta (P), and fetal liver (FL). Subsequently, after birth HSC reside within the bone marrow (BM) in a preferred quiescent state.
DEVELOPMENTAL ORIGIN OF HSC
Developmental origin of HSCs is less unanimous than where they were found during development. The century-old theory proposes that primitive erythrocytes and endothelial cells were closely associated physically and they have a common mesodermal precursor called hemangioblast [27]. Later on in vitro differentiation of mouse embryonic stem cells proved the existence of hemangioblast [28, 29]. Analyses of early stage mouse conceptus have shown that hemagioblast express both the mesodermal marker brachyury and fetal liver kinase 1 (Flk1) in the posterior region of the primitive streak [30]. These hemangioblasts migrate to the yolk sac and committed to endothelial and hematopoietic progenitors and many of them contribute to the formation of each blood island [31, 32]. Thus, during mammalian embryonic development, the earliest population of mesodermal cells emerging from the primitive streak and transform into endothelial and hematopoietic fate before formation of blood island and give rise to primitive red blood cells and some of the vasculature in the yolk sac.
On the other hand, a younger theory proposes that haematopoietic stem cells (HSCs) originate from a subset of early endothelial cells known as hemogenic endothelium. Based on morphology it has been proposed that as the AGM forms, hemogenic endothelial cells in the ventral wall of the aorta, which bud off HSCs. Experiments show that the transcription factor Runx1 is necessary for formation of blood from hemogenic endothelium but not from yolk sac hemangioblasts [13, 33]. The relationship between haemangioblasts and hemogenic endothelium has never been resolved until recently. Current in vitro observations suggest that haematopoietic progenitor cells arise from haemangioblasts through a haemogenic endothelial intermediate [34]. It is also found that haemogenic endothelial cells can be generated in vitro from ES cells and naturally present in the mouse embryos [35]. Finally, it was shown that, Runx1 expression with in endothelium is essential for the formation of HSCs and their progenitors over a period of roughly 3 days during mouse embryonic development (E8.25-11.5) [36]. These observations strongly suggest that HSC emerge directly from hemogenic endothelial cells. Moreover, it was also found that most fetal liver cells and adult bone-marrow cells originate from the hemogenic endothelium [37]. Several other previous studies in mouse also suggest that the direct precursors of HSCs are hemogenic endothelial cells [13, 14, 38]. Recent study also suggests HSCs emerge in large vessels in the placenta [39].
FACTORS REGULATING HSC DEVELOPMENT
Development of hematopoietic cells is programmed by sets of transcriptional factors and is influenced by morphogens and signaling molecules originating from the adjacent germ cell layer and tissues. De novo generation, variation in numbers of HSCs in different anatomical territories such as yolk sac, placenta, AGM suggest that genetic program, complex regulatory networks, downstream targets, interacting molecules and developmental timing are overlapped among these anatomical sites and leading to hematopoietic specification [40]. It is also likely that hematopoietic specification may due interplay between specific transcription factors at different stages of development as transcriptional factors work in concerts. Interactions between endoderm and mesoderm are necessary for the formation of primitive erythroblast in chick embryo [41-43] as culturing any one of them alone hinder the development. The importance of endodermal signaling for primitive hematopoiesis also evident in mouse conceptus studies [44, 45]. Several signaling molecules, such as VEGF, bFGF and TGF-1 have the ability to substitute the endodermal signal [46]. Hedgehog signaling is essential for primitive erythropoiesis [45]. In dorsal aorta, hedgehog stimulates blood cell formation through a complex signaling cascade that includes the down stream effectors such as VEGF, Notch, GATA-2 and Runx1 [47]. VEGF, bFGF, TGF- and BMP4 are considered as ventralizing factors and involved in hematopoiesis [48-50] where as dorsalizing factors (EGF, TGF−) antagonize hematopoietic induction [46]. The role of BMP4 signaling in hematopoietic cell formation and induction of HSCs is also evident from embryonic stem cell-differentiation cultures [51] and from explant cultures of AGM [52]and other related tissues [53]. Ventralizing factors also may control the expression of transcription factors such as SCL and GATA-1, essential for hematopoiesis. Notch 1 signaling is very important for the survival of conceptus and AGM hematopoiesis [54] and it is also evident that Notch family and its ligands are expressed in endothelial cells lining the dorsal aorta [55]. The role of transcription factors such as GATA-2, Runx1and PU.1 in primitive and adult hematopoiesis is very important, and complex interactions of these factors determine stage and site specific hematopoiesis. GATA-2 is expressed in the aortic endothelium [56] and mice lacking GATA-2 suffer from impaired primitive erythropoiesis and other committed progenitors, and die at E10.5 [57]. Runx1, key transcription factor for definitive hematopoiesis is expressed ventrally in the mesenchyme, endothelium and hematopoietic clusters of the dorsal aorta [13, 33]. Prostaglandin E2 also affects hematopoiesis in the zebrafish and mouse AGM [58]. Thus, an understanding of how the ‘master regulators’ are being controlled and are ‘fine tuned’ in terms of their amounts in different hematopoietic subpopulations and sites will provide insight into the genetic network that governs hematopoietic emergence in the conceptus.
DIFFERENCES BETWEEN FETAL AND ADULT HSCS
Fetal hematopoiesis occurs in multiple sites including yolk sac, AGM, and the fetal liver, where as adult hematopoiesis occurs primarily in bone marrow. Fetal and adult HSCs express different surface markers and exert unique characteristics. It has been reported that HSCs acquire quiescent adult phenotype from a proliferating fetal phenotype after 4 weeks of birth [59]. Different transcriptional regulators such as Gfi-1 [60], Tel/Etv6 [61], and Bmi-1 [62] maintain quiescence of adult HSCs. In contrast, Sox17 is tightly restricted to the fetal and neonatal HSCs and is required for their maintenance and proliferation, however, Sox17 is not required for adult HSC maintenance, proliferation, or mobilization [63]. Thus, this complex genetic regulation along with niche regulation support expansion of HSC in the fetal liver, whereas most adult HSCs are quiescent [64]. In human, fetal liver provide a niche to HSCs by regulating Wnt signaling, whereas bone marrow niche is tightly regulated by Notch signaling pathway [65]. However, there is no clear demonstration of identical role of Wnt signaling both in human and mouse hematopoietic development. Further studies on the regulation of Wnt signaling along with Sox17 in mouse and human fetal hematopoiesis will help in understanding the regulatory mechanisms in development of HSCs across the species. It has also found that HSCs acquire differentiation potentials throughout ontogeny and in the adult bone marrow are very different. Red blood cells derived from HSCs in the yolk sac contains embryonic hemoglobins and nucleated, whereas erythrocytes derived from FL and adult BM are containing only adult hemoglobin and nonnucleated [66-68]. Various aspects of the fetal and adult T [69-71] and B cell developmental programs are also different [72]. It has also been shown that IL-7 is required for adult B cell development, but it is dispensable during fetal hematopoiesis [73, 74]. However, the molecular mechanisms are yet to be learned regarding the transition from proliferative fetal HSCs to quiescent adult HSCs.
SELF-RENEWAL VERSUS DIFFERENTIATION
HSCs can differentiate to form mature blood cells and simultaneously can replicate themselves to maintain self-renewal. Transplanting a single HSC from the bone marrow into a lethally irradiated animal can assess self-renewal of HSC and as a result the entire immune system can be rescued. Self-renewal occurs in a cell-autonomous manner but is governed by highly orchestrated integration of environmental signals originate from the stem cell niche [75, 76]. It is evident that asymmetric cell division is responsible for functional heterogeneity of HSC population [77]. Various cytokines [78], and proteins [79] support the asymmetric mode of HSC division. Self-renewal is regulated by many molecular signals in a distinct manner in different tissues.
Various signals such as developmental regulators or certain oncogenes activate self-renewal process. It was found that Myc, Notch and leukaemic fusion proteins stimulate self-renewal of HSCs [80, 81]. Thus, it is likely that signaling through multiple pathways is responsible for triggering a set of cellular events associated with self-renewal. Some of the embryonic pathways mediated by BMPs, fibroblast growth factors, Delta-like, Wnt proteins, PGE2, also play important role in adult HSC self-renewal. However, Conditional gene knockout experiments have shown that developmental factors such as Wnt proteins and Notch are not usually involved in HSC maintenance but are involved in stress-induced situations or during regeneration [82, 83]. But other essential factors for HSC production during embryogenesis, such as SCL and Runx1 are not required for adult HSC self-renewal [84]. Cell cycle regulation is another mechanism, which also contribute to self-renewal of HSC. It has been found that chromatin-associated factor Bmi1 regulates the transcription of the cell-cycle regulator Ink4A [85, 86] and contribute to self-renewal of HSC. Self-renewal also can be stimulated by addition of factors like Wnt3A [87], angiopoietin-like factors [88]or PGE2 [58], which can be used for in vitro or in vivo manipulation of HSC self-renewal pathways. Self-renewal is also regulated by Hox gene expression. It is evident that overexpression of Hoxb4 [89] or Hoxa9 [90] in mouse bone marrow cells leads to increased self-renewal of HSCs. The switch between self-renewal and differentiation is governed by competition between transcription-factor complexes [91]. The inactivation of a variety of transcription factors, such GATA2 [92], Gfi1 [93], Myc [94] and Smad4 [95] has been shown to inhibit HSC self-renewal. Thus, self-renewal signaling pathways are conserved and the orchestrated integration between transcriptional factors provide stem cell characteristics.
DIFFERENTIATION OF HSC
HSCs are multipotent cells and have a capacity to differentiate into progenitors of all blood cells such as, erythrocytes, granulocytes, monocytes, platelets, and all subtypes of lymphocytes. Multipotent nature of HSC is evident by a reconstitution of normal polyclonal hematopoiesis in the recipient after successful hematopoietic stem cell transplantation (HSCT). Recently, a series of exciting reports have demonstrated the possibility that HSC may transdifferentiate into cell types of unrelated tissues. Reports have shown that cells found in BM are capable of giving rise to endothelial precursors [96], brain microglia and macroglia [97, 98, 99], hepatic cells [100, 101], skeletal muscle [102-104] and cardiac muscle [103, 105] cells. However, transdifferentiation potential of HSCs remains to be demonstrated. The ability of the HSCs to transdifferentiate into cell types other than hematopoietic system indicates a complete paradigm shift of the hierarchial hematopoietic tree. The differentiation of HSCs into muscle can be logical considering the fact that blood cells and muscle cells belong to the same germ layer, the mesoderm [106]. This process also may be bidirectional. Thus, plasticity can be referred as crossing barriers within the same germ layer. In contrast, HSCs differentiating into neural cells would indicate crossing of the embryonic germ layer barrier (mesoderm to ectoderm) and thus referred to as transdifferentiation. In this section, we have emphasized the clinical studies, which demonstrate plasticity or transdifferentiation of HSC into non-hematological cells in humans who underwent allogeneic HSCT Fig. (2).
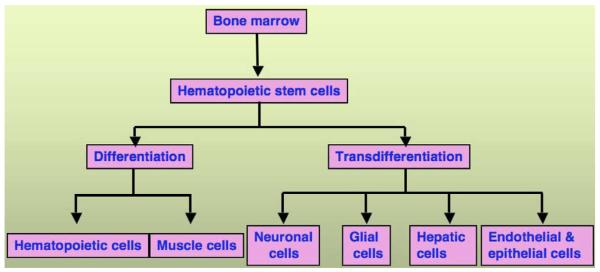
Fig. (2).
Plasticity of bone marrow-derived hematopoietic stem cells. Potential contribution of hematopoietic stem cells obtained from bone marrow in to different tissue system by differentiation or transdifferentiation.
HEMATOPOIETIC DIFFERENTIATION OF HSCS
Continuous blood cell production throughout the lifetime of an individual is ensured by differentiation of HSCs to all the blood cells of the hematopoietic system. As the life-span of many hematopoietic cells is short, HSCs maintain a delicate balance between differentiation and self-renewal in order to fulfill an enormous need of daily blood cell supply of 1 × 1011 to maintain homeostasis [107]. During the course of differentiation, as an HSC commits to become a mature functional cell of a particular lineage, it first loses its self-renewal capacity and loses lineage potential in a step by step manner. The mammalian blood system composed of different mature cell types such as, red blood cells (erythrocyte), platelets, monocytes, macrophages, granulocytes, mast cells, T and B lymphocytes, natural killer cells (NK), and dendritic cells (DC). HSCs give rise to the multi potent progenitors (MPPs), which have reduced self-renewal ability and maintaining full-lineage differentiation potential [108, 109]. It is evident that MPP is a heterogeneous population [109, 110]. MPPs then give rise to two oligopotent (which differentiates into several but not all lineages of a tissue/organ) progenitors, common myeloid progenitor (CMP) [111] and common lymphoid progenitor (CLP) [112-114]. Further downstream, CMPs give rise to megakaryocyte-erythroid progenitors (MEPs) committed to the formation of erythroid and megakaryocytic progeny and also granulocyte-macrophage progenitors (GMPs), able to generate granulocytic, macrophage, and eosinophil progenitors [115, 116]. However, mast-cell progenitors are segregated in the CMP populations but they do not pass through the GMP stage [117]. DC can be derived either from CMP or CLP [118, 119].
However, there is increasing consensus for revision of the classical model of hematopoietic differentiation of HSC in both mouse and human on the basis of recent findings [120-126]. In mouse, the classical model suggests the initial lineage strictly divide into separate common myeloiderythroid and lymphoid lineages. But recent studies suggest that multipotential progenitors (MPPs) initially differentiate into lymphoid primed multipotential progenitors (LMPPs) with lymphoid and granulocyte-macrophage but no megakaryocyte-erythroid potential [120-122, 127]. More committed myeloid and lymphoid progenitors remain towards the downstream [111, 116]. Recent persuasive data also suggest that human hematopoiesis does not follow a rigid model of myeloid-lymphoid segregation as evident in mouse. Human multi lymphoid progenitors (MLPs) were identified as Thy-1neg–loCD45RA+ cells in the immature CD34+CD38− compartment of both cord blood and bone marrow that also contains Thy-1+CD45RA− HSCs and Thy-1−CD45RA− candidate MPPs [124]. It has also found that these isolated CD34+CD38− Thy-1neg–loCD45RA+ cells gave rise to all lymphoid cell types, as well as monocytes, macrophages and dendritic cells, suggesting that these myeloid lineages arise in early lymphoid lineage specification [126]. In mammalian hematopoietic system this complex multi-tiered process allows generation of terminally differentiated cells and at the same time maintains regulation of HSC homeostasis. Hematopoietic differentiation is regulated by particular combination of transcription factors in a specific manner. It was found that overexpression of RARa leads to monocyte formation at the expense of granulopoiesis [128, 129]. In another example, when GATA-1 is over expressed CLPs converted to megakaryocyte and erythroid precursors [130] but underexpression of PU.1 led to excess granulocyte formation at the expense of monocyte formation [131].Thus, small imbalance in a transcription factor complex can determine differentiation and fate of HSCs.
NON-HEMATOPOIETIC DIFFERENTIATION OF HSC
Transdifferentiation potential of HSC in the central nervous system in preclinical models has been reported in several literatures. It was found that after transplantation of bone marrow cells into normal and ischemic brain, BM cells could differentiate into neurons and astrocytes [132-134]. At the same time it was also reported that after intravenous administration of BM cells into terminally irradiated rats, neuronal cells derived from injected BM origin found in the brain [97, 98]. Similar transdifferentiation patterns of HSC also found in human. It was reported that after transplantation of male bone marrow into females resulted to presence of transgender neurons (1% of all neurons), astrocytes and microglia (1-2% of all glial cells) in the female autopsy brain after 6 years. Donor derived neurogenesis was evaluated by immunohistochemistry, fluorescence in situ hybridization (FISH) and tissue analyses. These assays demonstrated the presence of only one X chromosome in the host brain tissue samples excluding the possibility of cell fusion, suggesting HSCs can transdifferentiate into neurons, astrocytes and microglia [135]. In separate study in human, similar conclusion was made where 3 out of 4 required criteria to demonstrate plasticity of adult stem cells were fulfilled [136]. These criteria are as follows: 1) prospective isolation and transplantation of donor population without culture manipulation, and transplanted stem cells should give rise to robust and sustained regeneration of the target tissues; 2) the differentiated cells should not only exhibit desired morphological and molecular phenotype, but also should be functionally active; 3) transdifferentiated stem cells should have normal chromosome contents; finally, 4) transplanted cells should be homogeneous and the presence of contaminated cells should be systematically ruled out [137]. The female patients in this study had received either bone marrow or stem cell (CD34+ enriched) transplants from their brother. FISH analysis and other assays exclude the possibility of cell fusion and fetomaternal microchimerism. However, the number of labeled cells was 10-fold lower than the rodent studies [97, 98]. There are also reports, which contradict these transdifferentiation phenomenon of HSCs where other groups have shown that Purkinje neurons from the cerebellum can fuse with bone marrow-derived cells in both mouse and human beings [138-140].
Highly purified HSCs are able to differentiate into colonies of hepatocytes when transplanted into fumarylacetoacetate hydrolase−/− (FAH−/−) mice [141]. After HSC transplantation, liver function was restored in FAH−/− mice and at the same time these transplanted mice also showed hematopoietic reconstitution. Later on it was shown that cell fusion could be a mechanism that explains part of the results of transdifferentiation of HSCs into liver cells [142]. Although some clinical studies have shown the presence of hepatocytes in the transplanted patients were of donor bone marrow origin after HSCT [143, 144]. In attempt to repair myocardial infarction, lots of efforts have been made to demonstrate the regenerative efficiency of different populations of hematopoietic stem and progenitors cells obtained from bone marrow or umbilical cord blood indicated their ability to differentiate into cardiomyocytes. It was shown that after transplantation of purified population of HSC in to myocardial infarcted mouse, donor cells differentiated into myocytes and vascular structures and also ameliorating the function of the infarcted heart [105]. In humans, several studies indicate improvement in cardiac function when autologous bone marrow or peripheral stem cell is administered directly into damaged myocardium. But these studies did not explain whether improved outcomes were result from generation of HSC derived myocytes or were secondary effects or due to paracrine signaling [145-149]. Apart from the major organ regeneration potential, it was also found that HSC could be transdifferentiated into endothelial and epithelial cell lineages after HSCT [150]. Donor specific marrow cell-derived epithelial and endothelial cells were found in the recipient skin biopsy [151, 152] or peripheral and aortic arteries [153]. In vivo study also revealed that the adult valve fibroblasts may be derived from HSCs. Y-chromosome-specific FISH analysis of female to male transplanted mice suggest that the EGFP+ valve cells are the result of HSC-derived cell differentiation and not the fusion of EGFP+ donor cells with host somatic cells [154]. Recently it has also been found that mitral valve leaflets contain endothelial cells with multilineage mesenchymal differentiation potential, including osteogenic differentiation [155], suggesting the trans-differentiation ability of different progenitor cells. In another interesting study, the in vivo plasticity of peritoneal macrophages and their ability to transdifferentiate from a myeloid to mesenchymal phenotype has also been reported [156].
However, there are reports, which provide the partial explanation of these afore-mentioned transdifferentiation observations. They have explained that BM-derived cells fuse in vivo with Purkinje neurons in the brain, hepatocytes in liver and cardiac muscle in the heart [138, 157]. As a result multinucleated cells are formed. This mechanism refuses the transdifferentiation of HSC.
The controversies on plasticity are related to the methodology to detect donor-derived non-hematopoietic cells in various tissues and organs. The proposed criteria as discussed earlier should be fulfilled in order to claim the occurrence of HSC plasticity or transdifferentiation after HSCT [137]. Conceptually, differences between donor and recipient cells can be evaluated by genetic analysis. Cell fusion phenomena can be excluded by performing karyotyping with FISH, which distinguishes between cellular transdifferentiation (diploid, donor genotype) and cell fusion (tetraploid or greater, mixed donor and recipient genotypes). In situ hybridization method is frequently employed to determine the sex of individuals. However, this technique is only suitable in case of sex mismatched transplantation in human. The DNA-based small tandem repeat (STR) is other popular method to detect donor derived HSC chimerism of HSC transplantation. This method only confirms the presence of donor cells but the cell type of the donor cells could not be identified. Thus, there may be undetected mixed non-hematopoietic chimerism during the HSC transplantation. Furthermore, these undetected non-hematopoietic cells of donor origin may differentiate into the desired cell types suggesting the false positive transdifferentiation phenomenon.
CLINICAL APPLICATIONS OF HSC
In physiological condition HSCs give rise to all mature blood cells through the process of hematopoiesis also maintains the balance of blood cells by balancing between quiescent and actively cycling state. However, in response to stress during certain pathophysiological conditions these rare cell populations differentiate according to the need of tissues. HSCs are used in autologous or allogeneic transplantations for the treatment of patients with diverse hematopoietic disorders to reconstitute the hematopoietic cell lineages [158]. As allograft contains mature immune cells, it can respond to host specific antigens and mediate graft-versus-host disease (GVHD) after HSC transplantations. Thus, different approaches have been taken to reduce or neutralize this reaction such as myeloablative therapy or ionizing radiation [159]. There are several sources of HSC, such as bone marrow, mobilized peripheral blood (MPB) or umbilical cord blood (UCB). In clinical practice, BM-derived HSCs from patients or healthy donors may be collected from BM aspirate or by apheresis after their mobilization to the peripheral blood by administering mobilizing agents such as granulocyte macrophage colony stimulating factor, granulocyte colony-stimulating factor, or by using synthetic chemical compounds like AMD 3100 [160, 161]. The increased number of mobilized HSCs in peripheral blood samples can be stored and used in transplant therapies. Recently, immunomagnetic isolation of CD34+ immature HSCs from BM or peripheral blood samples were performed. Hence, BM or MPB HSC-containing samples or isolated HSC preparations may be used as autografts or allografts to the recipients. In addition, bank-stored UCB derived HSCs are also popular in allogeneic or autologous transplantation in certain clinical practice, as these cells induce less intense alloreactive response [162, 163]. There are several challenges involved in HSC transplantation in regular clinical practice obtained from these sources, such as increased risk of infection and delayed engraftment in post transplanted patients. It has been observed that recovery of white blood cell were occurred around 20 days after conventional BM transplant, whereas, 28 days in umbilical cord blood transplant. The number of HSCs obtained from UCB is also not sufficient for routine clinical application. Among several attempts, one important approach is the transplantation of two umbilical cord blood specimens to enhance HSC engraftment as it boosted total HSC number but recovery of neutrophil could not be improved [164]. Thus, several ex vivo HSC expansion protocols are underway to fulfill this unmet need [165]. Despite of these deficiencies, HSCs are used in autologous or allogeneic transplantations for the treatment of malignancies, such as lymphoma and leukaemia as well as for autoimmune diseases and other blood-related disorders [158]. The combination of HSC transplants with high-dose of chemotherapy or ionizing radiation has found to be an alternative successful therapeutic strategy [166-168]. Several approaches have been adopted to improve the quality of life and to increase the life expectancy of patients with hematological malignancies. Different methods of HSC therapy are found to be beneficial in leukemic patients [169-171].
HSC transplantation induce donor-specific tolerance to solid organs [172] and also useful for the treatment of severe autoimmune diseases (ADs) [173, 174]. HSC transplant improves the immune response of patients, and thereby helps to repair damaged tissues in diverse pathological conditions and at the same time prevents infectious diseases after the transplantation of tissues. In AD condition, therapeutic benefit of syngeneic or autologous HCT can be achieved without replacing the host hematopoietic system. In different clinical studies it has been found that some patients with AD show long-lived clinical remissions, whereas others relapsed after initial benefit as a result of autologous HCT. Transplant-related mortality ranged from 0% to 12.5% [173, 174]. On the contrary, evidences of allogeneic HCT therapy to treat AD are more uniform and successful [175, 176]. The beneficial results from allogeneic HCT studies suggest that donor cells effectively modify recipient immune responses [177]. The list of patents regarding hematopoietic stem cell therapy in various disease states is provided in the Table 1.
Table 1.
List of Selected Patents related to HSC Development and Clinical Application
| Patent No./ Issued Year |
Title of the Patent and Brief Description | Inventions |
|---|---|---|
| 1. US 6455678 [178] (09/24/2002) |
Human hematopoietic stem and progenitor cell antigen: Methods for the enrichment and charac- terization of human hematopoietic progenitor and stem cells. Antigen AC133 can be used for the identification and/or separation of HSCs. |
Identification of HSC |
| 2. US 5283354 [179] (02/01/1994) |
Nucleic acids encoding hematopoietic stem cells receptors Flk-1:Isolated mammalian nucleic acid molecules encoding receptor protein tyrosine kinases expressed in primitive hematopoietic cells and not expressed in mature hematopoietic cells. |
Identification of HSC |
| 3. US 7718379 [180] (05/18/2010) |
Identifying hematopoietic stem cells based on cell surface markers: Method of identifying a HSC or progeny using the marker of carbohydrate sequence (glucuronic acid or N-acetylglucosamine) |
Identification of HSC |
| 4. US 7850960 [181] (12/14/2010) |
Methods for regulation of stem cells: Method for increasing the successful activity of stem and progenitor cells like HSC and other types of stem cells. |
Regulation of HSC self- renewal and differentia- tion |
| 5. US 5523286 [182] (06/04/1996) |
Stroma-derived proteoglycan containing composition, which promotes differentiation and main- tains the self-renewal capacity of long-term bone marrow culture initiating cells. |
Regulation of HSC self- renewal and differentia- tion |
| 6. US 6887704 [183] (05/03/2005) |
Methods of controlling proliferation and differentiation of stem and progenitor cells. | Regulation of HSC self- renewal and differentia- tion |
| 7. US 5905041 [184] (05/18/1999) |
Process for preparing and cultivating hematopoietic progenitor cells: The in vitro production of non-immortalized hematopoietic progenitor cells of the erythroid lineage. |
Regulation of HSC self- renewal and differentia- tion |
| 8. US 5851984 [185] (12/22/1998) |
Method of enhancing proliferation or differentiation of HSC using Wnt polypeptides. | Regulation of HSC pro- liferation and differentia- tion |
| 9.WO2010052580 [186] (08/19/2010) |
HSC self-renewal: The invention is related to methods to culture hematopoietic stem cells (HSC) in vitro. The methods describe about maintaining/expanding stem cells in undifferentiated state. Stem cells were cultured with small molecule that activates signaling pathways activated by Ras association (RalGDS/AF-6) domain family 8 and cells were used for treating malignancy. |
HSC self-renewal |
| 10. WO2010017551 [187] (11/2/2010) |
Method for promoting hematopoietic stem cell self renewal and expansion using MIR-125A. In- creased expression of mir-125A increased HSC self-renewal by 6-30 folds, which can be used to expand HSCs ex vivo and in vivo. |
Regulation of HSC self- renewal |
| 11. WO04085616 [188] (07/10/2004) |
Methods for regulating self-renewal of stem cells (mouse and human) by modulating the function and/or activity of target factors, includes AML1, C/EBPa, and/or PU.1 either individually, or in combinations. |
Regulation of HSC self- renewal |
| 12. US 20090285786 [189] (11/19/2009) |
Method to modulate hematopoietic stem cell growth: Methods for modulating hematopoietic stem cells using HCS modulators, which are agents that either increase HSC numbers or decrease HSC numbers as desired by a particular indication. |
HSC growth |
| 13. US 6613565 [190] (09/02/2003) |
Use of delta-like protein to inhibit differentiation of stem cells: Fetal liver microenvironment was mimicked to show the importance HSC microenvironment. |
Regulation of HSC niche |
| 14. US 6841386 [191] (01/11/2005) |
Modulation of primary stem cell differentiation using an insulin-like growth factor binding pro- tein: The regulation of HSC differentiation or self renewal by specific peptides. |
Regulation of HSC dif- ferentiation |
| 15. US 6136952 [192] (10/24/2000) |
Human Jagged polypeptide, encoding nucleic acids and method of use: Isolated polypeptide, which can inhibit HSC differentiation. |
Regulation of HSC dif- ferentiation |
| 16. US 7744927 [193] (06/29/2010) |
Methods of promoting hematopoietic and mesenchymal cell proliferation and differentiation: Describes hematopoietic and mesenchymal lineage specific cell proliferation and differentiation in presence of angiotensinogen, angiotensin 1 and other angiotensin analogues. |
Regulation of HSC pro- liferation and differentia- tion |
| 17. US 5677139 [194] (10/14/1997) |
In vitro differentiation of CD34+ progenitor cells into lymphocytes: Method for production of T cell population. |
HSC differentiation |
| 18. US 5830760 [195] (11/03/1998) |
Creating novel hematopoietic cell lines by expressing altered retinoic acid receptors: To establish continuous SCF dependent lympho-hematopoietic progenitor cell lines capable of differentiating into erythroid, myeloid and B lymphocytic and other blood cell lineages. |
Genetic regulation of differentiation |
| 19. US 6159461 [196] (12/12/2000) |
Use of c-kit ligand with TNF-a and hematopoietic factors for the expansion and differentiation of hematopoietic cells: Method describes composition of factors useful for expansion and differentia- tion of HSC. |
Regulation of HSC pro- liferation and differentia- tion |
| 20. US 5214133 [197] (05/25/1993) |
SCL: a hematopoietic growth and differentiation factor: Invention identified the role of SCL in HSC differentiation into specific lineages. |
Genetic regulation of differentiation |
| 21.US 5837507 [198] (11/17/1998) |
HOX-induced enhancement of in vivo and in vitro proliferative capacity and gene therapeutic methods: Method demonstrates the role of HOXB4 in HSC self-renewal and proliferation. |
Genetic regulation of HSC self-renewal |
| 22.US 6093531 [199] (07/25/2000) |
Generation of hematopoietic cells from multipotent neural stem cells: Method describing genera- tion of hematopoietic system by stimulating multipotent neural stem cell |
Trans-differentiation |
| 23. US 6872812 [200] (03/29/2005) |
Hepp, a novel gene with a role in hematopoietic and neural development: Invention is directed to isolated Hepp gene and protein, which has a role in mammalian hematopoiesis. |
Genetic regulation of hematopoietic develop- ment |
| 24.US 5750397 [201] (05/12/1998) |
Human hematopoietic stem cell: Method describes isolation of homogenous human hematopoietic stem cells and determines of their potential. |
Identification of human HSC |
| 25. US20060140912 [202] (06/29/2006) |
Methods for enhancing engraftment of purified hematopoietic stem cells in allogeneic recipients: Provides a method of achieving a higher rate of allogeneic hematopoietic stem cell engraftment by either matching the major histocompatibility complex class I K locus between donors and recipi- ents or identifying how class I K on HSC interact with FC (CD8/33Kd receptor complex). |
Allogeneic HSC trans- plantation |
| 26. US20070098693 [203] (05/03/2007) |
Methods for enhancing engraftment of purified hematopoietic stem cells in allogeneic recipients: CD8~7TCR~ bone marrow cells facilitate engraftment of hemapoietic stem cells. The invention defines a direct functional role for p-preDC in HSC engraftment and will have a significant impact on strategies to design effective cell-based therapies for transplantation |
Allogeneic HSC trans- plantation |
| 27. US 5192553 [204] (03/09/1993) |
Isolation and preservation of fetal and neonatal hematopoietic stem and progenitor cells of the blood and methods of therapeutic use: Invention describes the therapeutic use of HSC obtained from preserved neonatal and fetal blood. |
HSC transplantation |
| 28. US 5800539 [205] (09/01/1998) |
Method of allogeneic hematopoietic stem cell transplantation without graft failure or graft vs. host disease: Method of transplanting hematopoietic system reconstituting cells from a donor into an allogeneic recipient. |
HSC transplantation |
| 29. US 6143292 [206] (11/07/2000) |
Allogeneic cell therapy for cancer following allogeneic stem cell transplantation: Methods de- scribe procedure for treatment of cancer patient who has undergone a cancer therapy along with allogeneic stem cell transplantation. |
HSC transplantation |
| 30. US20050129665 [207] 06/16 /2005 |
Isolated lineage negative hematopoietic stem cells and methods of treatment therewith: Isolated, mammalian, adult bone marrow-derived, lineage negative hematopoietic stem cell populations contain endothelial progenitor cells (EPCs) capable of rescuing retinal blood vessels and neuronal networks in the eye. |
HSC transplantation |
| 31. US 7645447 [208] (01/12/2010) |
Treating retinal degeneration caused by retinal vein occlusion or retinal ischemia: Method of treat- ing retinal degeneration by peripheral blood hematopoietic stem cell along with granulocyte- colony stimulating factor. |
HSC transplantation |
CONCLUSION AND FUTURE DEVELOPMENT
After decades of research on developmental stages and biology of HSC, improvement in understanding of stem cell niche has been achieved and current investigations are underway for better understanding of this important aspect of HSC biology. Thus, studying the microenvironment of HSC during embryogenesis and in adult life has an immense therapeutic potential. There are inherent differences between embryonic and adult HSC as shown by their predisposition to undergo expansion or quiescence. This is due to the presence of distinct regulatory mechanisms, which dictate either de novo generation of HSC and expansion during embryogenesis or support the quiescence of HSCs during steady state hematopoiesis in the adult. However, there are many common signals may be used in embryonic and adult HSC niches. Along with the niche signaling, differential expression of transcription factors also coordinate during HSC development and adult hematopoiesis. For clinical purpose, these inherent differences should be exploited to identify a suitable subtype of HSCs, which can retain self-renewal ability during therapeutic manipulation. By dissecting the HSC niches in various anatomical locations during each stage of HSC development, specific cellular and molecular component can be identified. And this knowledge can be translated as a tool to culture and manipulate HSCs in vitro or ex vivo. The primary difficulty in HSC transplantation is to maintain self-renewal and multipotency during ex vivo culture. Thus, better understanding of these signaling molecules, proteins those maintain self-renewal during HSC development will help to develop better culture system. As a result, by mimicking the appropriate HSC niche, it will be possible to perform ex vivo expansion of HSCs obtained from umbilical cord blood or other sources for successful and efficient clinical transplantation. These advances will then vertically improve HSC-based therapies for malignant and non-malignant hematopoietic disorders and immunodeficiencies.
ACKNOWLEDGEMENTS
This work was supported in part by National Institutes of Health grants, K01 AR054114 (NIAMS), SBIR R44 HL092706-01 (NHLBI), R21 CA143787 (NCI) and The Ohio State University start-up fund. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
REFERENCES
- [1].Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–68. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- [2].Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- [3].Ciau-Uitz A, Walmsley M, Patient R. Distinct origins of adult and embryonic blood in Xenopus. Cell. 2000;102:787–96. doi: 10.1016/s0092-8674(00)00067-2. [DOI] [PubMed] [Google Scholar]
- [4].Traver D, Zon LI. Walking the walk: migration and other common themes in blood and vascular development. Cell. 2002;108:731–4. doi: 10.1016/s0092-8674(02)00686-4. [DOI] [PubMed] [Google Scholar]
- [5].Tavian M, Peault B. Embryonic development of the human hematopoietic system. Int J Dev Biol. 2005;49:243–50. doi: 10.1387/ijdb.041957mt. [DOI] [PubMed] [Google Scholar]
- [6].Tavian M, Peault B. Analysis of hematopoietic development during human embryonic ontogenesis. Methods Mol Med. 2005;105:413–24. doi: 10.1385/1-59259-826-9:413. [DOI] [PubMed] [Google Scholar]
- [7].Tavian M, Peault B. The changing cellular environments of hematopoiesis in human development in utero. Exp Hematol. 2005;33:1062–9. doi: 10.1016/j.exphem.2005.06.025. [DOI] [PubMed] [Google Scholar]
- [8].Sabin F. Studies on the origin of blood vessel and red blood corpuscles as seen in the living blastoderm of chicks during the second day of incubation. Contrib Embryol. 1920;9:214. [Google Scholar]
- [9].Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- [10].Muller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1:291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- [11].de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. Embo J. 2000;19:2465–74. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Taoudi S, Medvinsky A. Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proc Natl Acad Sci U S A. 2007;104:9399–403. doi: 10.1073/pnas.0700984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].North TE, de Bruijn MF, Stacy T, Talebian L, Lind E, Robin C, et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–72. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- [14].de Bruijn MF, Ma X, Robin C, Ottersbach K, Sanchez MJ, Dzierzak E. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity. 2002;16:673–83. doi: 10.1016/s1074-7613(02)00313-8. [DOI] [PubMed] [Google Scholar]
- [15].Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8:365–75. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- [16].Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell. 2005;8:377–87. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- [17].Johnson GR, Moore MA. Role of stem cell migration in initiation of mouse foetal liver haemopoiesis. Nature. 1975;258:726–8. doi: 10.1038/258726a0. [DOI] [PubMed] [Google Scholar]
- [18].Houssaint E. Differentiation of the mouse hepatic primordium. II. Extrinsic origin of the haemopoietic cell line. Cell Differ. 1981;10:243–52. doi: 10.1016/0045-6039(81)90007-5. [DOI] [PubMed] [Google Scholar]
- [19].Downs KM, Gifford S, Blahnik M, Gardner RL. Vascularization in the murine allantois occurs by vasculogenesis without accompanying erythropoiesis. Development. 1998;125:4507–20. doi: 10.1242/dev.125.22.4507. [DOI] [PubMed] [Google Scholar]
- [20].Kumaravelu P, Hook L, Morrison AM, Ure J, Zhao S, Zuyev S, et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129:4891–9. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- [21].Takeuchi M, Sekiguchi T, Hara T, Kinoshita T, Miyajima A. Cultivation of aorta-gonad-mesonephros-derived hematopoietic stem cells in the fetal liver microenvironment amplifies long-term repopulating activity and enhances engraftment to the bone marrow. Blood. 2002;99:1190–6. doi: 10.1182/blood.v99.4.1190. [DOI] [PubMed] [Google Scholar]
- [22].Ferkowicz MJ, Starr M, Xie X, Li W, Johnson SA, Shelley WC, et al. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development. 2003;130:4393–403. doi: 10.1242/dev.00632. [DOI] [PubMed] [Google Scholar]
- [23].Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–84. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- [24].Cumano A, Dieterlen-Lievre F, Godin I. Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell. 1996;86:907–16. doi: 10.1016/s0092-8674(00)80166-x. [DOI] [PubMed] [Google Scholar]
- [25].Cumano A, Ferraz JC, Klaine M, Di Santo JP, Godin I. Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity. 2001;15:477–85. doi: 10.1016/s1074-7613(01)00190-x. [DOI] [PubMed] [Google Scholar]
- [26].Tavian M, Robin C, Coulombel L, Peault B. The human embryo, but not its yolk sac, generates lympho-myeloid stem cells: mapping multipotent hematopoietic cell fate in intraembryonic mesoderm. Immunity. 2001;15:487–95. doi: 10.1016/s1074-7613(01)00193-5. [DOI] [PubMed] [Google Scholar]
- [27].Murray P. The development in vitro of the blood of the early chick embryo. Proc Royal Soc london. 1932;11:497–521. [Google Scholar]
- [28].Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–32. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- [29].Fehling HJ, Lacaud G, Kubo A, Kennedy M, Robertson S, Keller G, et al. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–27. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- [30].Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–30. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- [31].Ferkowicz MJ, Yoder MC. Blood island formation: longstanding observations and modern interpretations. Exp Hematol. 2005;33:1041–7. doi: 10.1016/j.exphem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- [32].Ueno H, Weissman IL. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell. 2006;11:519–33. doi: 10.1016/j.devcel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- [33].North T, Gu TL, Stacy T, Wang Q, Howard L, Binder M, et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126:2563–75. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- [34].Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–5. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- [36].Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–91. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3:625–36. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bertrand JY, Giroux S, Golub R, Klaine M, Jalil A, Boucontet L, et al. Characterization of purified intraembryonic hematopoietic stem cells as a tool to define their site of origin. Proc Natl Acad Sci U S A. 2005;102:134–9. doi: 10.1073/pnas.0402270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, et al. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008;2:252–63. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Robin C, Ottersbach K, Durand C, Peeters M, Vanes L, Tybulewicz V, et al. An unexpected role for IL-3 in the embryonic development of hematopoietic stem cells. Dev Cell. 2006;11:171–80. doi: 10.1016/j.devcel.2006.07.002. [DOI] [PubMed] [Google Scholar]
- [41].Miura Y, Wilt FH. Tissue interaction and the formation of the first erythroblasts of the chick embryo. Dev Biol. 1969;19:201–11. doi: 10.1016/0012-1606(69)90055-4. [DOI] [PubMed] [Google Scholar]
- [42].Pardanaud L, Dieterlen-Lievre F. Emergence of endothelial and hemopoietic cells in the avian embryo. Anat Embryol (Berl) 1993;187:107–14. doi: 10.1007/BF00171741. [DOI] [PubMed] [Google Scholar]
- [43].Wilt FH. Erythropoiesis In The Chick Embryo: The Role Of Endoderm. Science. 1965;147:1588–90. doi: 10.1126/science.147.3665.1588. [DOI] [PubMed] [Google Scholar]
- [44].Belaoussoff M, Farrington SM, Baron MH. Hematopoietic induction and respecification of A-P identity by visceral endoderm signaling in the mouse embryo. Development. 1998;125:5009–18. doi: 10.1242/dev.125.24.5009. [DOI] [PubMed] [Google Scholar]
- [45].Dyer MA, Farrington SM, Mohn D, Munday JR, Baron MH. Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development. 2001;128:1717–30. doi: 10.1242/dev.128.10.1717. [DOI] [PubMed] [Google Scholar]
- [46].Pardanaud L, Dieterlen-Lievre F. Manipulation of the angiopoietic/hemangiopoietic commitment in the avian embryo. Development. 1999;126:617–27. doi: 10.1242/dev.126.4.617. [DOI] [PubMed] [Google Scholar]
- [47].Gering M, Patient R. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev Cell. 2005;8:389–400. doi: 10.1016/j.devcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- [48].Faloon P, Arentson E, Kazarov A, Deng CX, Porcher C, Orkin S, et al. Basic fibroblast growth factor positively regulates hematopoietic development. Development. 2000;127:1931–41. doi: 10.1242/dev.127.9.1931. [DOI] [PubMed] [Google Scholar]
- [49].Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–6. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- [50].Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–16. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- [51].Johansson BM, Wiles MV. Evidence for involvement of activin A and bone morphogenetic protein 4 in mammalian mesoderm and hematopoietic development. Mol Cell Biol. 1995;15:141–51. doi: 10.1128/mcb.15.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Durand C, Robin C, Bollerot K, Baron MH, Ottersbach K, Dzierzak E. Embryonic stromal clones reveal developmental regulators of definitive hematopoietic stem cells. Proc Natl Acad Sci U S A. 2007;104:20838–43. doi: 10.1073/pnas.0706923105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kanatsu M, Nishikawa SI. In vitro analysis of epiblast tissue potency for hematopoietic cell differentiation. Development. 1996;122:823–30. doi: 10.1242/dev.122.3.823. [DOI] [PubMed] [Google Scholar]
- [54].Kumano K, Chiba S, Kunisato A, Sata M, Saito T, Nakagami-Yamaguchi E, et al. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. 2003;18:699–711. doi: 10.1016/s1074-7613(03)00117-1. [DOI] [PubMed] [Google Scholar]
- [55].Robert-Moreno A, Espinosa L, de la Pompa JL, Bigas A. RBPjkappa-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development. 2005;132:1117–26. doi: 10.1242/dev.01660. [DOI] [PubMed] [Google Scholar]
- [56].Minegishi N, Ohta J, Yamagiwa H, Suzuki N, Kawauchi S, Zhou Y, et al. The mouse GATA-2 gene is expressed in the para-aortic splanchnopleura and aorta-gonads and mesonephros region. Blood. 1999;93:4196–207. [PubMed] [Google Scholar]
- [57].Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M, et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–6. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- [58].North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–11. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bowie MB, McKnight KD, Kent DG, McCaffrey L, Hoodless PA, Eaves CJ. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J Clin Invest. 2006;116:2808–16. doi: 10.1172/JCI28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hock H, Hamblen MJ, Rooke HM, Schindler JW, Saleque S, Fujiwara Y, et al. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature. 2004;431:1002–7. doi: 10.1038/nature02994. [DOI] [PubMed] [Google Scholar]
- [61].Hock H, Meade E, Medeiros S, Schindler JW, Valk PJ, Fujiwara Y, et al. Tel/Etv6 is an essential and selective regulator of adult hematopoietic stem cell survival. Genes Dev. 2004;18:2336–41. doi: 10.1101/gad.1239604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–5. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- [63].Kim I, Saunders TL, Morrison SJ. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell. 2007;130:470–83. doi: 10.1016/j.cell.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Mikkola HK, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–44. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- [65].Martin MA, Bhatia M. Analysis of the human fetal liver hematopoietic microenvironment. Stem Cells Dev. 2005;14:493–504. doi: 10.1089/scd.2005.14.493. [DOI] [PubMed] [Google Scholar]
- [66].Stamatoyannopoulos G. Control of globin gene expression during development and erythroid differentiation. Exp Hematol. 2005;33:259–71. doi: 10.1016/j.exphem.2004.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Brotherton TW, Chui DH, Gauldie J, Patterson M. Hemoglobin ontogeny during normal mouse fetal development. Proc Natl Acad Sci U S A. 1979;76:2853–7. doi: 10.1073/pnas.76.6.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].McGrath KE, Palis J. Hematopoiesis in the yolk sac: more than meets the eye. Exp Hematol. 2005;33:1021–8. doi: 10.1016/j.exphem.2005.06.012. [DOI] [PubMed] [Google Scholar]
- [69].Ikuta K, Kina T, MacNeil I, Uchida N, Peault B, Chien YH, et al. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990;62:863–74. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- [70].Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–85. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- [71].Ikuta K, Uchida N, Friedman J, Weissman IL. Lymphocyte development from stem cells. Annu Rev Immunol. 1992;10:759–83. doi: 10.1146/annurev.iy.10.040192.003551. [DOI] [PubMed] [Google Scholar]
- [72].Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- [73].Hardy RR, Hayakawa K. A developmental switch in B lymphopoiesis. Proc Natl Acad Sci U S A. 1991;88:11550–4. doi: 10.1073/pnas.88.24.11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(−/)- mice. J Exp Med. 2001;194:1141–50. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–7. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- [76].Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- [77].Brummendorf TH, Dragowska W, Zijlmans J, Thornbury G, Lansdorp PM. Asymmetric cell divisions sustain long-term hematopoiesis from single-sorted human fetal liver cells. J Exp Med. 1998;188:1117–24. doi: 10.1084/jem.188.6.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Takano H, Ema H, Sudo K, Nakauchi H. Asymmetric division and lineage commitment at the level of hematopoietic stem cells: inference from differentiation in daughter cell and granddaughter cell pairs. J Exp Med. 2004;199:295–302. doi: 10.1084/jem.20030929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Beckmann J, Scheitza S, Wernet P, Fischer JC, Giebel B. Asymmetric cell division within the human hematopoietic stem and progenitor cell compartment: identification of asymmetrically segregating proteins. Blood. 2007;109:5494–501. doi: 10.1182/blood-2006-11-055921. [DOI] [PubMed] [Google Scholar]
- [80].Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–22. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- [81].Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6:1278–81. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- [82].Mancini SJ, Mantei N, Dumortier A, Suter U, MacDonald HR, Radtke F. Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood. 2005;105:2340–2. doi: 10.1182/blood-2004-08-3207. [DOI] [PubMed] [Google Scholar]
- [83].Congdon KL, Voermans C, Ferguson EC, DiMascio LN, Uqoezwa M, Zhao C, et al. Activation of Wnt signaling in hematopoietic regeneration. Stem Cells. 2008;26:1202–10. doi: 10.1634/stemcells.2007-0768. [DOI] [PubMed] [Google Scholar]
- [84].Mikkola HK, Klintman J, Yang H, Hock H, Schlaeger TM, Fujiwara Y, et al. Haematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukaemia SCL/tal-1 gene. Nature. 2003;421:547–51. doi: 10.1038/nature01345. [DOI] [PubMed] [Google Scholar]
- [85].Kotake Y, Cao R, Viatour P, Sage J, Zhang Y, Xiong Y. pRB family proteins are required for H3K27 trimethylation and Polycomb repression complexes binding to and silencing p16INK4alpha tumor suppressor gene. Genes Dev. 2007;21:49–54. doi: 10.1101/gad.1499407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Bracken AP, Kleine-Kohlbrecher D, Dietrich N, Pasini D, Gargiulo G, Beekman C, et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21:525–30. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–14. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- [88].Zhang CC, Kaba M, Ge G, Xie K, Tong W, Hug C, et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12:240–5. doi: 10.1038/nm1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Sauvageau G, Thorsteinsdottir U, Eaves CJ, Lawrence HJ, Largman C, Lansdorp PM, et al. Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev. 1995;9:1753–65. doi: 10.1101/gad.9.14.1753. [DOI] [PubMed] [Google Scholar]
- [90].Thorsteinsdottir U, Mamo A, Kroon E, Jerome L, Bijl J, Lawrence HJ, et al. Overexpression of the myeloid leukemia-associated Hoxa9 gene in bone marrow cells induces stem cell expansion. Blood. 2002;99:121–9. doi: 10.1182/blood.v99.1.121. [DOI] [PubMed] [Google Scholar]
- [91].Nerlov C, Graf T. PU.1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev. 1998;12:2403–12. doi: 10.1101/gad.12.15.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Rodrigues NP, Janzen V, Forkert R, Dombkowski DM, Boyd AS, Orkin SH, et al. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood. 2005;106:477–84. doi: 10.1182/blood-2004-08-2989. [DOI] [PubMed] [Google Scholar]
- [93].Zeng H, Yucel R, Kosan C, Klein-Hitpass L, Moroy T. Transcription factor Gfi1 regulates self-renewal and engraftment of hematopoietic stem cells. Embo J. 2004;23:4116–25. doi: 10.1038/sj.emboj.7600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wilson A, Murphy MJ, Oskarsson T, Kaloulis K, Bettess MD, Oser GM, et al. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 2004;18:2747–63. doi: 10.1101/gad.313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Karlsson G, Blank U, Moody JL, Ehinger M, Singbrant S, Deng CX, et al. Smad4 is critical for self-renewal of hematopoietic stem cells. J Exp Med. 2007;204:467–74. doi: 10.1084/jem.20060465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- [97].Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–82. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- [98].Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–9. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- [99].Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci U S A. 1997;94:4080–5. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, et al. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. doi: 10.1038/35018642. [DOI] [PubMed] [Google Scholar]
- [101].Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–70. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- [102].Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–30. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- [103].Bittner RE, Schofer C, Weipoltshammer K, Ivanova S, Streubel B, Hauser E, et al. Recruitment of bone-marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anat Embryol (Berl) 1999;199:391–6. doi: 10.1007/s004290050237. [DOI] [PubMed] [Google Scholar]
- [104].Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–4. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- [105].Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Transplanted adult bone marrow cells repair myocardial infarcts in mice. Ann N Y Acad Sci. 2001;938:221–9. doi: 10.1111/j.1749-6632.2001.tb03592.x. discussion 9-30. [DOI] [PubMed] [Google Scholar]
- [106].Peault B, Oberlin E, Tavian M. Emergence of hematopoietic stem cells in the human embryo. C R Biol. 2002;325:1021–6. doi: 10.1016/s1631-0691(02)01514-7. [DOI] [PubMed] [Google Scholar]
- [107].Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci U S A. 1994;91:9857–60. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–73. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- [109].Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A. 2001;98:14541–6. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–5. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- [111].Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–7. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- [112].Serwold T, Ehrlich LI, Weissman IL. Reductive isolation from bone marrow and blood implicates common lymphoid progenitors as the major source of thymopoiesis. Blood. 2009;113:807–15. doi: 10.1182/blood-2008-08-173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Karsunky H, Inlay MA, Serwold T, Bhattacharya D, Weissman IL. Flk2+ common lymphoid progenitors possess equivalent differentiation potential for the B and T lineages. Blood. 2008;111:5562–70. doi: 10.1182/blood-2007-11-126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–72. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- [115].Na Nakorn T, Traver D, Weissman IL, Akashi K. Myeloerythroid-restricted progenitors are sufficient to confer radioprotection and provide the majority of day 8 CFU-S. J Clin Invest. 2002;109:1579–85. doi: 10.1172/JCI15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Pronk CJ, Rossi DJ, Mansson R, Attema JL, Norddahl GL, Chan CK, et al. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell. 2007;1:428–42. doi: 10.1016/j.stem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- [117].Chen CC, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ. Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci U S A. 2005;102:11408–13. doi: 10.1073/pnas.0504197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Traver D, Akashi K, Manz M, Merad M, Miyamoto T, Engleman EG, et al. Development of CD8alpha-positive dendritic cells from a common myeloid progenitor. Science. 2000;290:2152–4. doi: 10.1126/science.290.5499.2152. [DOI] [PubMed] [Google Scholar]
- [119].Manz MG, Traver D, Miyamoto T, Weissman IL, Akashi K. Dendritic cell potentials of early lymphoid and myeloid progenitors. Blood. 2001;97:3333–41. doi: 10.1182/blood.v97.11.3333. [DOI] [PubMed] [Google Scholar]
- [120].Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- [121].Arinobu Y, Mizuno S, Chong Y, Shigematsu H, Iino T, Iwasaki H, et al. Reciprocal activation of GATA-1 and PU.1 marks initial specification of hematopoietic stem cells into myeloerythroid and myelolymphoid lineages. Cell Stem Cell. 2007;1:416–27. doi: 10.1016/j.stem.2007.07.004. [DOI] [PubMed] [Google Scholar]
- [122].Lai AY, Kondo M. Asymmetrical lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. J Exp Med. 2006;203:1867–73. doi: 10.1084/jem.20060697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Hao QL, Zhu J, Price MA, Payne KJ, Barsky LW, Crooks GM. Identification of a novel, human multilymphoid progenitor in cord blood. Blood. 2001;97:3683–90. doi: 10.1182/blood.v97.12.3683. [DOI] [PubMed] [Google Scholar]
- [124].Hoebeke I, De Smedt M, Stolz F, Pike-Overzet K, Staal FJ, Plum J, et al. T-, B- and NK-lymphoid, but not myeloid cells arise from human CD34(+)CD38(-)CD7(+) common lymphoid progenitors expressing lymphoid-specific genes. Leukemia. 2007;21:311–9. doi: 10.1038/sj.leu.2404488. [DOI] [PubMed] [Google Scholar]
- [125].Six EM, Bonhomme D, Monteiro M, Beldjord K, Jurkowska M, Cordier-Garcia C, et al. A human postnatal lymphoid progenitor capable of circulating and seeding the thymus. J Exp Med. 2007;204:3085–93. doi: 10.1084/jem.20071003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Doulatov S, Notta F, Eppert K, Nguyen LT, Ohashi PS, Dick JE. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat Immunol. 11:585–93. doi: 10.1038/ni.1889. [DOI] [PubMed] [Google Scholar]
- [127].Yoshida T, Ng SY, Zuniga-Pflucker JC, Georgopoulos K. Early hematopoietic lineage restrictions directed by Ikaros. Nat Immunol. 2006;7:382–91. doi: 10.1038/ni1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Kastner P, Lawrence HJ, Waltzinger C, Ghyselinck NB, Chambon P, Chan S. Positive and negative regulation of granulopoiesis by endogenous RARalpha. Blood. 2001;97:1314–20. doi: 10.1182/blood.v97.5.1314. [DOI] [PubMed] [Google Scholar]
- [129].Taschner S, Koesters C, Platzer B, Jorgl A, Ellmeier W, Benesch T, et al. Down-regulation of RXRalpha expression is essential for neutrophil development from granulocyte/monocyte progenitors. Blood. 2007;109:971–9. doi: 10.1182/blood-2006-04-020552. [DOI] [PubMed] [Google Scholar]
- [130].Iwasaki H, Mizuno S, Wells RA, Cantor AB, Watanabe S, Akashi K. GATA-1 converts lymphoid and myelomonocytic progenitors into the megakaryocyte/erythrocyte lineages. Immunity. 2003;19:451–62. doi: 10.1016/s1074-7613(03)00242-5. [DOI] [PubMed] [Google Scholar]
- [131].Dahl R, Walsh JC, Lancki D, Laslo P, Iyer SR, Singh H, et al. Regulation of macrophage and neutrophil cell fates by the PU.1:C/EBPalpha ratio and granulocyte colony-stimulating factor. Nat Immunol. 2003;4:1029–36. doi: 10.1038/ni973. [DOI] [PubMed] [Google Scholar]
- [132].Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–7. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- [133].Eglitis MA, Dawson D, Park KW, Mouradian MM. Targeting of marrow-derived astrocytes to the ischemic brain. Neuroreport. 1999;10:1289–92. doi: 10.1097/00001756-199904260-00025. [DOI] [PubMed] [Google Scholar]
- [134].Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–70. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- [135].Cogle CR, Yachnis AT, Laywell ED, Zander DS, Wingard JR, Steindler DA, et al. Bone marrow transdifferentiation in brain after transplantation: a retrospective study. Lancet. 2004;363:1432–7. doi: 10.1016/S0140-6736(04)16102-3. [DOI] [PubMed] [Google Scholar]
- [136].Crain BJ, Tran SD, Mezey E. Transplanted human bone marrow cells generate new brain cells. J Neurol Sci. 2005;233:121–3. doi: 10.1016/j.jns.2005.03.017. [DOI] [PubMed] [Google Scholar]
- [137].Anderson DJ, Gage FH, Weissman IL. Can stem cells cross lineage boundaries? Nat Med. 2001;7:393–5. doi: 10.1038/86439. [DOI] [PubMed] [Google Scholar]
- [138].Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–73. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- [139].Weimann JM, Charlton CA, Brazelton TR, Hackman RC, Blau HM. Contribution of transplanted bone marrow cells to Purkinje neurons in human adult brains. Proc Natl Acad Sci U S A. 2003;100:2088–93. doi: 10.1073/pnas.0337659100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Weimann JM, Johansson CB, Trejo A, Blau HM. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat Cell Biol. 2003;5:959–66. doi: 10.1038/ncb1053. [DOI] [PubMed] [Google Scholar]
- [141].Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–34. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- [142].Grompe M. The role of bone marrow stem cells in liver regeneration. Semin Liver Dis. 2003;23:363–72. doi: 10.1055/s-2004-815560. [DOI] [PubMed] [Google Scholar]
- [143].Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, et al. Liver from bone marrow in humans. Hepatology. 2000;32:11–6. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- [144].Korbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, et al. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med. 2002;346:738–46. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]
- [145].Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361:45–6. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- [146].Tse HF, Kwong YL, Chan JK, Lo G, Ho CL, Lau CP. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet. 2003;361:47–9. doi: 10.1016/S0140-6736(03)12111-3. [DOI] [PubMed] [Google Scholar]
- [147].Mathur A, Martin JF. Stem cells and repair of the heart. Lancet. 2004;364:183–92. doi: 10.1016/S0140-6736(04)16632-4. [DOI] [PubMed] [Google Scholar]
- [148].Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–8. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- [149].Griesel C, Heuft HG, Herrmann D, Franke A, Ladas D, Stiehler N, et al. Good manufacturing practice-compliant validation and preparation of BM cells for the therapy of acute myocardial infarction. Cytotherapy. 2007;9:35–43. doi: 10.1080/14653240601052734. [DOI] [PubMed] [Google Scholar]
- [150].Borue X, Lee S, Grove J, Herzog EL, Harris R, Diflo T, et al. Bone marrow-derived cells contribute to epithelial engraftment during wound healing. Am J Pathol. 2004;165:1767–72. doi: 10.1016/S0002-9440(10)63431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Jiang S, Walker L, Afentoulis M, Anderson DA, Jauron-Mills L, Corless CL, et al. Transplanted human bone marrow contributes to vascular endothelium. Proc Natl Acad Sci U S A. 2004;101:16891–6. doi: 10.1073/pnas.0404398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Murata H, Janin A, Leboeuf C, Soulier J, Gluckman E, Meignin V, et al. Donor-derived cells and human graft-versus-host disease of the skin. Blood. 2007;109:2663–5. doi: 10.1182/blood-2006-07-033902. [DOI] [PubMed] [Google Scholar]
- [153].Suzuki T, Nishida M, Futami S, Fukino K, Amaki T, Aizawa K, et al. Neoendothelialization after peripheral blood stem cell transplantation in humans: a case report of a Tokaimura nuclear accident victim. Cardiovasc Res. 2003;58:487–92. doi: 10.1016/s0008-6363(02)00780-0. [DOI] [PubMed] [Google Scholar]
- [154].Visconti RP, Ebihara Y, LaRue AC, Fleming PA, McQuinn TC, Masuya M, et al. An in vivo analysis of hematopoietic stem cell potential: hematopoietic origin of cardiac valve interstitial cells. Circ Res. 2006;98:690–6. doi: 10.1161/01.RES.0000207384.81818.d4. [DOI] [PubMed] [Google Scholar]
- [155].Wylie-Sears J, Aikawa E, Levine RA, Yang JH, Bischoff J. Mitral valve endothelial cells with osteogenic differentiation potential. Arterioscler Thromb Vasc Biol. 31:598–607. doi: 10.1161/ATVBAHA.110.216184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Mooney JE, Rolfe BE, Osborne GW, Sester DP, van Rooijen N, Campbell GR, et al. Cellular plasticity of inflammatory myeloid cells in the peritoneal foreign body response. Am J Pathol. 176:369–80. doi: 10.2353/ajpath.2010.090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Medvinsky A, Smith A. Stem cells: Fusion brings down barriers. Nature. 2003;422:823–5. doi: 10.1038/422823a. [DOI] [PubMed] [Google Scholar]
- [158].Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169:338–46. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Ringden O, Remberger M, Svenberg P, Svahn BM, Dahllof G, Gustafsson B, et al. Fludarabine-based disease-specific conditioning or conventional myeloablative conditioning in hematopoietic stem cell transplantation for treatment of non-malignant diseases. Bone Marrow Transplant. 2007;39:383–8. doi: 10.1038/sj.bmt.1705602. [DOI] [PubMed] [Google Scholar]
- [160].Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- [161].De Clercq E. Potential clinical applications of the CXCR4 antagonist bicyclam AMD3100. Mini Rev Med Chem. 2005;5:805–24. doi: 10.2174/1389557054867075. [DOI] [PubMed] [Google Scholar]
- [162].Brunstein CG, Setubal DC, Wagner JE. Expanding the role of umbilical cord blood transplantation. Br J Haematol. 2007;137:20–35. doi: 10.1111/j.1365-2141.2007.06521.x. [DOI] [PubMed] [Google Scholar]
- [163].Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2:155–62. doi: 10.1007/s12015-006-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Ballen KK, Spitzer TR, Yeap BY, McAfee S, Dey BR, Attar E, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13:82–9. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Sorrentino BP. Clinical strategies for expansion of haematopoietic stem cells. Nat Rev Immunol. 2004;4:878–88. doi: 10.1038/nri1487. [DOI] [PubMed] [Google Scholar]
- [166].Hale GA. Autologous hematopoietic stem cell transplantation for pediatric solid tumors. Expert Rev Anticancer Ther. 2005;5:835–46. doi: 10.1586/14737140.5.5.835. [DOI] [PubMed] [Google Scholar]
- [167].George RE, Li S, Medeiros-Nancarrow C, Neuberg D, Marcus K, Shamberger RC, et al. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. J Clin Oncol. 2006;24:2891–6. doi: 10.1200/JCO.2006.05.6986. [DOI] [PubMed] [Google Scholar]
- [168].Frickhofen N, Berdel WE, Opri F, Haas R, Schneeweiss A, Sandherr M, et al. Phase I/II trial of multicycle high-dose chemotherapy with peripheral blood stem cell support for treatment of advanced ovarian cancer. Bone Marrow Transplant. 2006;38:493–9. doi: 10.1038/sj.bmt.1705472. [DOI] [PubMed] [Google Scholar]
- [169].Barfield RC, Hale GA, Burnette K, Behm FG, Knapp K, Eldridge P, et al. Autologous transplantation of CD133 selected hematopoietic progenitor cells for treatment of relapsed acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;48:349–53. doi: 10.1002/pbc.20687. [DOI] [PubMed] [Google Scholar]
- [170].Oyan B, Koc Y, Kansu E. Successful salvage with high-dose sequential chemotherapy coupled with in vivo purging and autologous stem cell transplantation in 2 patients with primary refractory mantle cell lymphoma presenting in the leukemic phase. Int J Hematol. 2005;81:155–8. doi: 10.1532/ijh97.e0303. [DOI] [PubMed] [Google Scholar]
- [171].Krause DS, Lazarides K, von Andrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006;12:1175–80. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- [172].McSweeney PA, Storb R. Mixed chimerism: preclinical studies and clinical applications. Biol Blood Marrow Transplant. 1999;5:192–203. doi: 10.1053/bbmt.1999.v5.pm10465099. [DOI] [PubMed] [Google Scholar]
- [173].Tyndall A, Koike T. High-dose immunoablative therapy with hematopoietic stem cell support in the treatment of severe autoimmune disease: current status and future direction. Intern Med. 2002;41:608–12. doi: 10.2169/internalmedicine.41.608. [DOI] [PubMed] [Google Scholar]
- [174].Burt RK, Slavin S, Burns WH, Marmont AM. Induction of tolerance in autoimmune diseases by hematopoietic stem cell transplantation: getting closer to a cure? Blood. 2002;99:768–84. doi: 10.1182/blood.v99.3.768. [DOI] [PubMed] [Google Scholar]
- [175].Ikehara S. Treatment of autoimmune diseases by hematopoietic stem cell transplantation. Exp Hematol. 2001;29:661–9. doi: 10.1016/s0301-472x(01)00645-2. [DOI] [PubMed] [Google Scholar]
- [176].van Bekkum DW. Experimental basis of hematopoietic stem cell transplantation for treatment of autoimmune diseases. J Leukoc Biol. 2002;72:609–20. [PubMed] [Google Scholar]
- [177].Marmont AM. Stem cell transplantation for severe autoimmune disorders, with special reference to rheumatic diseases. J Rheumatol Suppl. 1997;48:13–8. [PubMed] [Google Scholar]
- [178].Yin AH, Miraglia S, Godfry WG, Buck DW. Human hematopoietic stem and progenitor cell antigen. US 6,455,678. 2002
- [179].Lemischka IR. Nucleic acids encoding hematopoietic stem cells receptors Flk-1. US 5,283,354. 1994
- [180].Nilsson SK, Simmons PJ, Haylock DN. Identifying hematopoietic stem cells based on cell surface markers. US 7,718,379. 2010
- [181].Moon RT, Bhatia M, Trowbridge J. Methods for regulation of stem cells. US 7,850,960. 2010
- [182].McGlave PB, Verfaillie CM. Stroma-derived proteoglycan containing composition which promotes differentiation and maintains the self-renewal capacity of long-term bone-marrow cultureinitiationg cells. US 5,523,286. 1996
- [183].Peled T, Fibach E, Tresves A. Method of controlling proliferation and differentiation stem and progenitor cells. US 6,887,704. 2005
- [184].Beug H, Wessely O, Steinlein P, Deiner E, Von Lindern MM. Process for preparing and cultivating hematopoietic progenitor cells. US 5,905,041. 1999
- [185].Matthews W, Austin TW. Method of enhancing proliferation or differentiation of hematopoietic stem cells using Wnt polypeptide. US 5,851,984. 1998
- [186].Verfaillie C, Schoemans H, Snoeckx R. HSC self-renewal. WO2010052580. 2010
- [187].Scadden D, Guo S, Lu J. Method for MIR-125A in promoting hematopoietic stem cell self renewal and expansion. WO2010017551. 2010
- [188].Klug CA. Regulation of self-renewal in stem cells. WO04085616. 2004
- [189].Zon LI, North TE, Goessling W. Method to modulate hematopoietic stem cell growth. 20090285786. 2009
- [190].Witte L, Pytowski B, Moore KA, Lemischka IR. Use of delta-like protein to inhibit the differentiation of stem cells. US 6,613,565. 2003
- [191].Krause M, Deng H, Liu L. Modulation of primary stem cell differentiation using an insulin-like growth factor binding protein. US 6,841,386. 2005
- [192].Li L, Hood L. Human jagged polypeptide,encoding nucleic acids and methods of use. US 6,136,952. 2000
- [193].Rodgers K, diZerega G. Methods of promoting hematopoietic and mesenchymal cell proliferation and differentiation. US 7,744,927. 2010
- [194].Johnson RP, Rosenzweig M, Scadden DT. In vitro differentiation of CD34+ progenitor cells into T lymphocytes. US 5,677,139. 1997
- [195].Tsai S, Collins SJ. Creating novel hematopoietic cell lines by expressing altered retinoic acid receptors. US 5,830,760. 1998
- [196].Besmer P, Buck J, Moore MAS, Nocka K. Use of c-kit ligand with TNF- and hematopoietic factors for the expansion and differentiation of hematopoietic cells. US 6,159,461. 2000
- [197].Kirsch IR, Begley CG. SCL: a hematopoietic growth and differentiation factor. US 5,214,133. 1993
- [198].Largman C, Lawrence HJ, Humphries RK, Sauvageau G. Hox-induced enhancement of in vivo and in vitro proliferative capacity and gene therapeutic methods. US 5,837,507. 1998
- [199].Bjornson CR, Rietze RL, Reynolds BA, Vescovi AL. Generation of hematopoietic cells from multipotent neural stem cells. US 6,093,531. 2000
- [200].Jurecic R, Nachtman RG. Hepp, a novel gene with a role in hematopoietic and neural development. US 6,872,812. 2005
- [201].Tsukamoto A, Baum CM, Aihara Y, Weissman I. Human hematopoietic stem cell. US 5,750,397. 1998
- [202].Ildstad ST. Methods for enhancing engraftment of purified hematopoietic stem cells in allogeneic recipients. US20060140912. 2006
- [203].Ildstad ST. Methods for enhancing engraftment of purified hematopoietic stem cells in allogeneic recipients. US20070098693. 2007
- [204].Boyse EA, Broxmeyer HE, Douglas GW. Isolation and preservation of fetal and neonatal hematopoietic stem and progenitor cells of the blood and methods of therapeutic use. US 5,192,553. 1993
- [205].Waller EK. Method of allogeneic hematopoietic stem cell transplantation without graft failure or graft vs host disease. US 5,800,539. 1998
- [206].Slavin S. Allogeneic cell therapy for cancer following allogeneic stem cell transplantation. US 6,143,292. 2000
- [207].Friedlander M, Otani A, Silva KD, Moreno S. Isolated lineage negative hematopoietic stem cells and methods of treatment therewith. US20050129665. 2005
- [208].Li H, Lee YJ. Treating retinal degeneration caused by retinal vein occlusion or retinal ischemia. US 7,645,447. 2010