Abstract
Background
Wnt signaling plays an essential role in gastrointestinal epithelial proliferation. Most investigations have focused on developmental and immune responses. Bacterial infection can be chronic and increases the risk of inflammatory bowel disease and colitis-associated cancer. However, we lack studies on how bacteria regulate Wnt proteins and how Wnts modulate the host responses to enteric bacteria. This study investigated the effects of Salmonella and E. coli on Wnt2, one of the Wnt family members, in intestinal epithelia cells.
Methodology/Findings
Using cultured epithelial cells, a Salmonella-colitis mouse model, and a gnotobiotic mouse model, we found that Wnt2 mRNA and protein expression levels were elevated after bacterial infection. Enteric bacteria regulate Wnt2 location in the intestine. Furthermore, we found that elevation of Wnt2 was a strategy for host defense by inhibiting cell apoptosis and inflammatory responses to infection. Using Wnt2 siRNA analysis, we show enhanced inflammatory cytokine IL-8 in epithelial cells. Cells over-expressed Wnt2 had less bacterial-induced IL-8 secretion. AvrA is a bacterial protein that inhibits inflammation by stabilizing beta-catenin, the down-stream target of Wnt. We found that the stabilization of Wnt2 was regulated through ubiquitination. Moreover, the bacterial protein AvrA from Salmonella and E. coli stabilized Wnt2 protein expression in vivo. In an ex-germ-free system, E. coli F18 expressing AvrA increased Wnt2 expression and changed Wnt2 distribution in intestine.
Conclusion
Wnt2 contributes to host protection in response to enteric bacteria. Our findings thus reveal a previously undefined role of Wnt for host-pathogen interaction and inflammation.
Keywords: Wnt, inflammation, gnotobiotic mice, E. coli, Salmonella
Introduction
The glycoprotein ligand Wnt proteins, including Wnt2, control cell fate during embryonic development and homeostasis in adult self-renewing tissues (1). Wnts are known to activate the β-catenin pathway, a regulator of intestinal epithelial proliferation and inflammation (2–7). Recently, several studies have implied the involvement of Wnt-Frizzled signaling in the activation of proinflammatory mediators in inflammatory disorders (8). Patients with inflammatory bowel diseases (IBD) have significantly higher expression of Wnts compared to non-IBD patients (9). Moreover, the transmembrane Wnt receptors, Frizzled (Fz)3 and Fz4, exhibited significantly increased expression, whereas Fz1 and Fz5 exhibited significantly decreased expression in ulcerative colitis patients (9). A recent study indicated a reduced expression of the Wnt-signaling pathway transcription factor TCF-4 in ileal Crohn’s disease (10). However, some of these studies have focused on the responses of monocytes to the inflammatory cytokines in the immune system through Wnt5A or Wnt3A (11–12). Enteric bacteria and intestinal epithelial cells are known key plays in the pathogenesis of IBD (13–14). The effects and molecular mechanisms of the other Wnt proteins in regulating intestinal inflammation remain unknown.
Previous studies have shown that down-stream targets and regulators of the Wnt/β-catenin signaling are involved in bacterial infection. Uropathogenic Escherichia coli attach to the superficial facet cell layer of the bladder epithelium via FimH adhesin. A DNA microarray study found that FimH-mediated attachment suppresses transforming growth factor-beta and Wnt5A signaling to promote subsequent differentiation of basal/intermediate cells (15). Chibby physically interacts with β-catenin to repress its activation of transcription. Chibby knockout mice challenged with Pseudomonas aeruginosa are unable to clear the bacteria from the nasal cavity (16). M. tuberculosis infected macrophages had elevated Fz1/Wnt3A expression (17). Infection of gastric epithelial cells with Helicobacter pylori increases the function of the proximal Wnt signaling components low-density lipoprotein receptor-related protein 6 (18) and Dishevelled (Dvl) in the activation of β-catenin (19–20). Bacteroides fragilis activated the β-catenin pathway in the intestinal epithelia cells (21–22). We reported that Salmonella activates the Wnt/β-catnein pathway to modulate intestinal inflammation, cell proliferation and intestinal stem cell niches (5–6, 23–25).
Recent population-based cohort study demonstrates an increased risk of inflammatory bowel diseases (IBD) in individuals with Salmonella infection (26). Chronic Salmonella colonization in intestine increases the risk of the intestinal fibrosis, inflammation, and liver abscess in mice (18, 27). However, how bacteria directly regulate individual Wnt family members in intestine and how Wnt modulates the host responses to pathogenic bacteria are unknown.
In the current study, we focused on Wnt2 because Wnt 2 is known to directly regulate β-catenin activity, the key player in proliferation and inflammation (2–7). We hypothesized that Wnt2 is involved in host protection in intestine by inhibiting inflammation and apoptosis induced by Salmonella infection. Using in vitro and in vivo models, we found that Salmonella-colonized intestinal epithelial cells had elevated mRNA and protein expression levels for Wnt2. Over-expression of Wnt2 is able to lower IL-8 secretion induced by Salmonella infection. Moreover, we identified a bacterial effector protein AvrA that is able to regulate Wnt2 expression. Furthermore, the physiological relevance of bacterial Wnt2 activation was investigated in vivo. Immunostaining further identified that activated Wnt2 localized to intestinal epithelial cells. Overall, our studies emphasize the importance of Wnt2 in modulating mucosal inflammation in host-bacterial interactions.
Results
Salmonella increase the mRNA expression of Wnt2 and its receptors in host cells
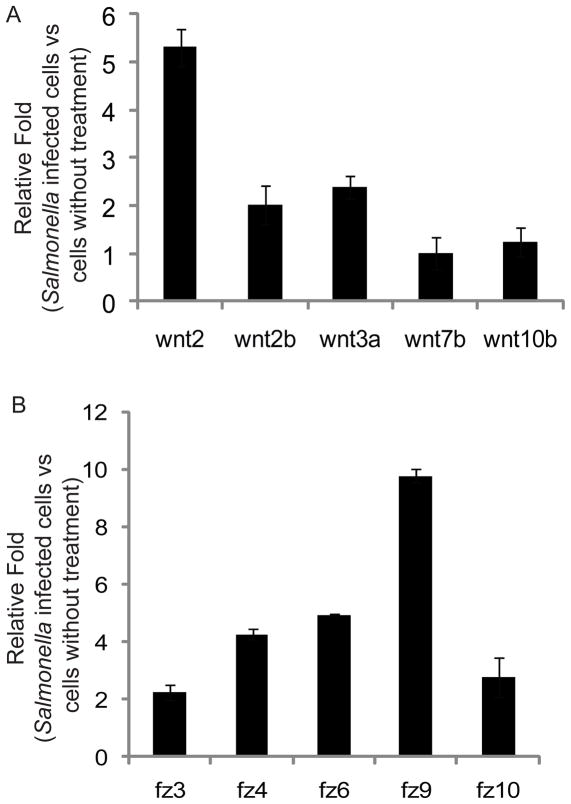
First, we used real-time PCR to investigate Wnt2 mRNA expression in cells before or after Salmonella typhimurium treatment. Figure 1A shows the mRNA expression of Wnt 2 and other Wnt proteins in intestinal epithelial IEC-18 cells colonized with S. typhimurium. We found that Wnt 2 expression levels in Salmonella-colonized cells were significantly higher than that in the control cells, whereas Wnt2b, 3a, 7b and 10b were slightly altered by Salmonella colonization. TNF-α is a proinflammatory cytokine. Therefore, we tested if TNF was able to alter Wnt expression after TNF treatment for only 30 minutes, which is considered the early stage of inflammation (28). Our data indicated that TNF-α did not increase Wnt2 expression (data not shown).
Figure 1.
Salmonella infection increases mRNA expression of Wnt 2 and Fz receptors in host cells in vitro. (A) Relative expression of Wnt family members in bacterial-colonized cells. Rat small intestinal epithelial IEC-18 cells were incubated with and without Salmonella for 30 minutes, then rinsed and incubated in medium containing gentamicin for 1 hour. (B) Relative expression of Fz family members in bacterial-colonized HCT116 cells. Total cell RNAs were extracted for real-time PCR. The relative expression levels were determined by normalizing the ΔCt values against the average ΔCt values of normal cells for specified genes. Data are from a single experiment and are representative of 3 separate experiments.
Fz and Wnt interact with other structural components at the cell surface to initiate complex signal transduction cascades that culminate in the transcriptional regulation of gene expression (29). We investigated the expression of Fz receptors for Wnt2 in cells with S. typhimurium colonization. Wnt receptors, including Fz4 and 9 for Wnt2 (30–31), were increased upon bacterial colonization (Fig. 1B). These data indicated that the transcriptional levels of Wnt2 and its receptors were enhanced by Salmonella infection in intestinal epithelial cells.
Salmonella infection increases the protein expression level of Wnt2 in intestinal epithelial cells
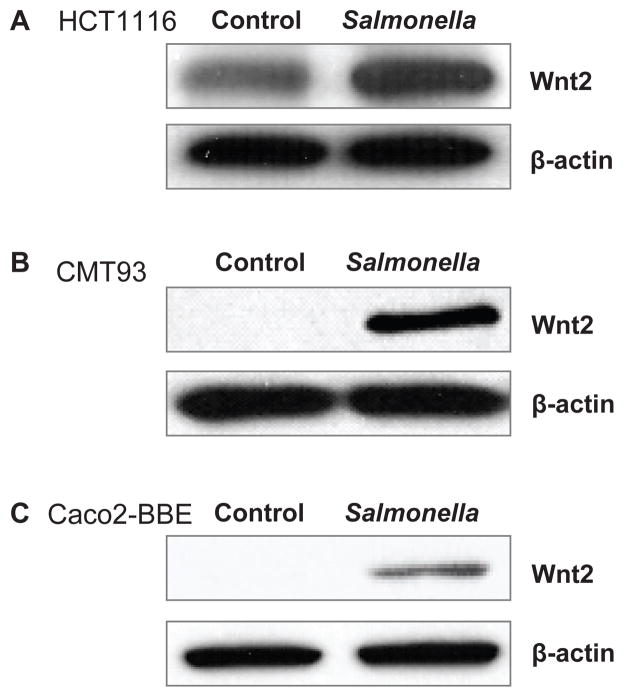
We then examined Wnt2 protein expression after pathogenic S. typhimurium infection in vitro. Using Western blot assays, we found that Wnt2 expression increased after infection with Salmonella SL1344 in human colonic epithelial HCT116 cells (Fig. 2A). We tested this effect in different cell lines. A similar change in Wnt2 expression was also found in mouse intestinal epithelial CMT93 cells (Fig. 2B) and human Caco2-BBE cells (Fig. 2C). Taken together, our in vitro data indicated that Wnt2 protein expression was elevated by pathogenic Salmonella colonization in intestinal epithelial cells.
Figure 2.
Salmonella infection increases the protein expression of Wnt 2 in various intestinal epithelial cell lines. (A) Wnt 2 protein expression in human epithelial HCT 116; (B) mouse intestine epithelial CMT 93 cells; and (C) human colon epithelial Caco2-BBE cells. Cells were treated with S. typhimurium SL1344 for 30 minutes, washed and then incubated in fresh DMEM for 1 h. Total cell lysates were analyzed for Wnt 2 by immunoblotting. Data are from a single experiment and are representative of >3 separate experiments.
Wnt 2 is involved in the regulation of inflammatory pathways in Salmonella-epithelial interactions
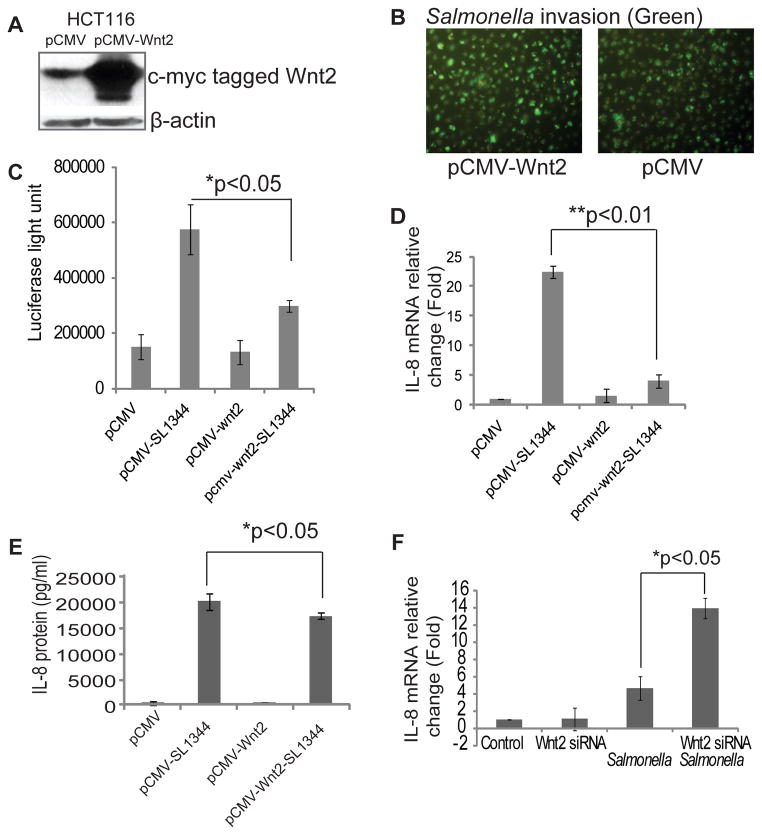
Because our data suggested that Wnt2 is involved in Salmonella colonization in intestinal epithelial cells, we further hypothesized that Wnt2 signaling contributes to inflammatory responses. We transfected a c-myc-tagged Wnt2 plasmid into human epithelail cells and found enhanced Wnt2 protein levels by using an anti-c-myc antibody (Fig. 3A). However, there was no difference in bacterial invasion between normal cells and cells over-expressing Wnt2 (Fig. 3B). In the over-expressed Wnt2 system, we found that the NF-κB activity was lower after Salmonella colonization (Fig. 3C). The mRNA expression of the inflammatory cytokine IL-8 was significantly down-regulated in the Wnt2 over-expressed cells after pathogenic Salmonella colonization (Fig. 3D). Furthermore, the IL-8 secreted into the cell culture media was detected by ELISA. There was a significantly suppressed IL-8 level in cells over-expressing Wnt2 (Fig. 3E).
Figure 3.
Wnt 2 status and host responses to Salmonella in mouse intestinal cells in vitro. (A) Wnt over-expression in HCT116 cells. (B) Wnt 2 expression levels did not contribute to protection from bacterial invasion in human intestinal epithelial HCT116 cells. GFP-Salmonella invasion in cells with different Wnt expression statuses. (C) Wnt 2 overexpression inhibits the transcriptional activity of NF-κB by the luciferase reporter assay. (D) IL-8 mRNA in Wnt 2 over-expressing HCT116 cells after Salmonella colonization. (E) IL-8 protein secretion in cell culture media in Wnt 2 over-expressing HCT116 cells after Salmonella colonization. (F) IL-8 mRNA in cells after using Wnt 2 siRNA to block the Wnt 2 expression. Data are from 3 separate experiments.
To clarify the effect of Wnt2 on the inflammatory response, we further blocked Wnt2 expression by siRNA (Fig. 3F). We found that IL-8 expression was significantly higher in cells with low Wnt2 expression compared to control cells after Salmonella colonization. Hence, these data indicated that Wnt2 expression is able to inhibit Salmonella-induced IL-8 in intestinal epithelial cells.
Over-expressed Wnt2 protects cells from Salmonella-induced apoptosis
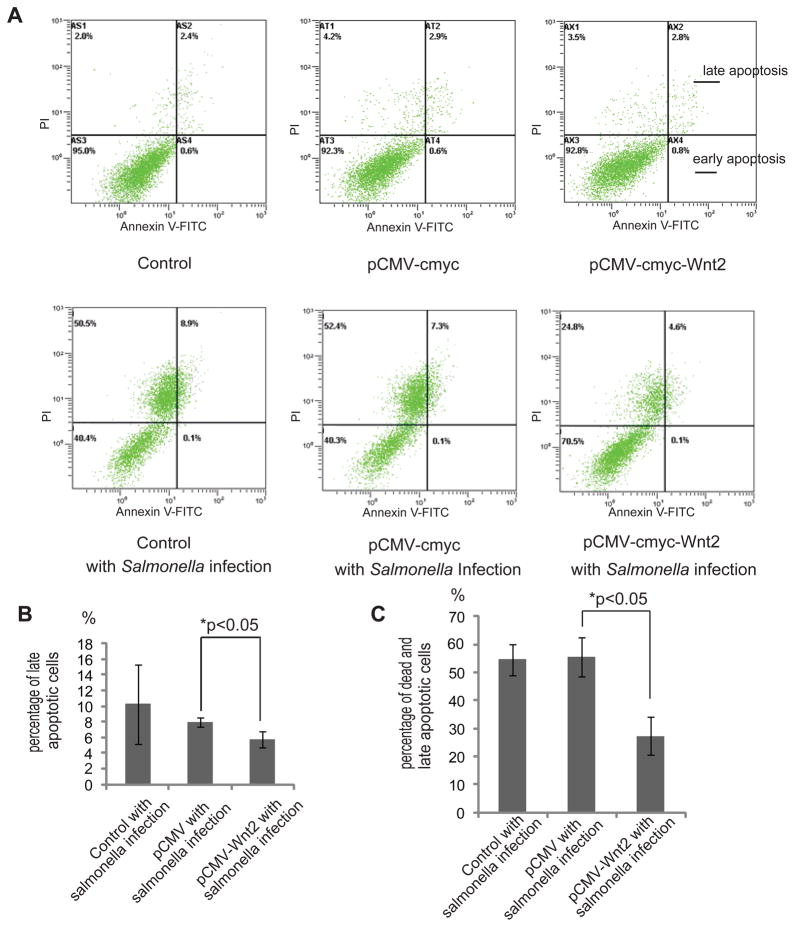
We postulated that over-expression of Wnt2 is a strategy for host defense. Apoptosis is one of the biological effects induced by bacterial infection (32–33). We then determined the percentage of apoptotic cells with different Wnt2 expression statuses. As shown here, Salmonella induced 8.9 % later apoptosis in HCT116 cells and 7.3 % in cells transfected with an empty pCMY-myc plasmid (Fig. 4A, upper right panel of the histogram). In contrast, Wnt2 over-expressing cells had only 4.6 % apoptotic/necrotic cells. The difference of late apoptotic cells was significant between the cells with normal Wnt2 and over-expressed Wnt2 (Fig. 4B). Salmonella induced 58.9% apoptosis/necrosis in HCT116 cells and 59.7% in cells transfected with an empty pCMY-myc plasmid (Figs. 4C left and right panels of the histogram). In contrast, Wnt2 over-expressing cells had only 25.4% apoptotic/necrotic cells (Fig. 4B). Overall, there was a significant decrease of apoptotic/necrotic cells in the Wnt2 over-expression group (Fig. 4C). The lower right panel in Fig. 4A represents early apoptotic cells. We did not find any changes in early apoptotic cells with over-expressed Wnt2. These data indicated that Wnt2 over-expression is able to protect cells from apoptosis and necrosis at late stages.
Figure 4.
Protective role of Wnt 2 in intestinal epithelial apoptosis. (A) Apoptotic cells with or without Wnt over-expression after Salmonella colonization. Apoptosis in Salmonella-colonized cells with or without Wnt 2 over-expression. After 24 hours of treatment with Salmonella, 1 × 106 adherent cells were trypsinized and incubated with FITC-conjugated annexin V and propidium iodide (PI). Cells were analyzed by flow cytometry. The percentages of cells in each group within the gated areas are indicated. In the gated areas, the upper right panel is for the population of later apoptotic cells, and the lower right panel is for early apoptotic cells. Dot plots of early apoptotic cells have increased Annexin VFITC fluorescence only, whereas necrotic and late apoptotic cells have increased annexin V and PI fluorescence. Axes are labeled with arbitrary fluorescence units. Data are representative of three independent experiments that gave similar results. (B) Statistical analysis of late apoptotic percentages. Data are from 3 separate experiments. (C) Statistical analysis of dead and late apoptotic percentages. Data are from 3 separate experiments.
Wnt2 protein degradation is regulated by ubiquitination
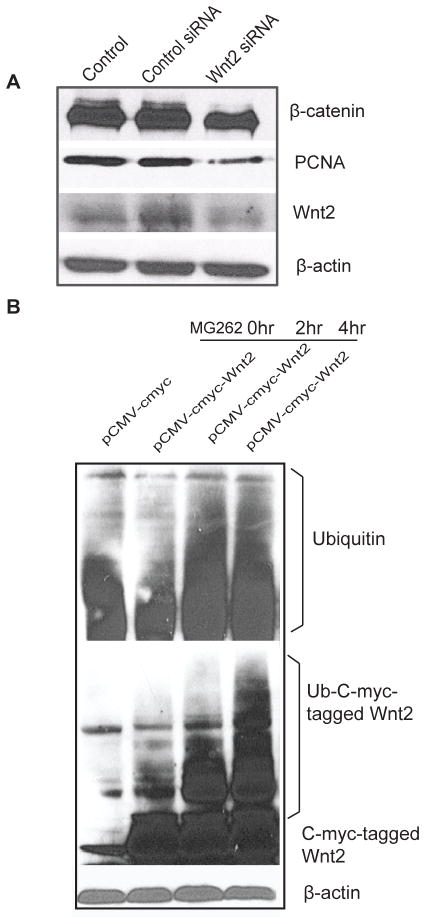
Wnt2 is known to be associated with β-catenin activity and cell proliferation (34–35). We used Wnt2 siRNA to block Wnt2 expression and further tested its effects on the β-catenin pathway in intestinal epithelial cells. We found that β-catenin levels were also low in cells with low levels of Wnt2. Moreover, PCNA, a marker for proliferative cells, was decreased in cells with low Wnt2 levels (Fig. 5A).
Figure 5.
Wnt 2 levels and Wnt 2 down-stream effects in the intestinal epithelial cells. (A) Blocking Wnt 2 decreased β-catenin and PCNA expression. (B) Ubiquitination of Wnt (Ub-Wnt 2) in intestinal epithelial cells treated with MG262 for 4 hours. Please note the ub-Wnt 2 bands above the regular Wnt 2 protein. Data are representative of 3 independent experiments.
We further hypothesized that Wnt2 stabilization is regulated through ubiquitination. To test our hypothesis, we treated cells with MG262, a proteasome inhibitor. We found enhanced Wnt2 ubiquitination in cells after MG262 treatment (Fig. 5B). This result indicated that the stabilization of Wnt2 is regulated through ubiquitination/proteasome. We tested the protein level of Wnt11, the other Wnt family member, with or without MG262 treatment. However, Wnt 11 expression was not changed by MG262 treatment (data not shown).
Salmonella effector AvrA up-regulates Wnt2 expression
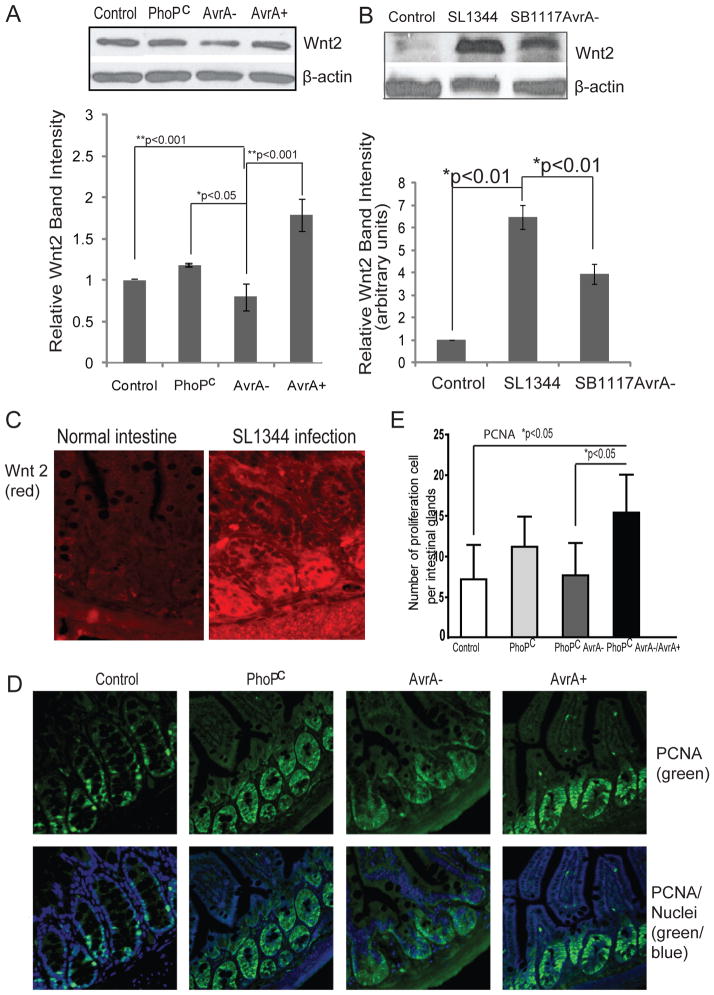
AvrA is a known bacterial effector protein that inhibits inflammation in bacterial-induced intestinal inflammation by stabilizing β-catenin, the down-stream target of Wnt signaling (6, 25). Furthermore, we hypothesized that AvrA is involved in Wnt2 signaling during bacterial colonization. We examined the effects of AvrA on induction of Wnt2 in a human epithelial cell model. PhoPc is a mutant strain derived from Salmonella ATCC14028 and has sufficient AvrA protein expression (25). We used a parental PhoPc AvrA mutant (AvrA−) or the AvrA-complemented strain (PhoPc AvrA−/AvrA+) from previous studies (36). Parental PhoPc strain colonization stabilized Wnt2, whereas cells treated with PhoPcAvrA− bacteria displayed reduced levels of Wnt2. In cells colonized by PhoPc AvrA−/AvrA+, complementary AvrA expression increased Wnt2 protein expression (Fig. 6A). The statistical analysis of Wnt2 intensity further indicated that AvrA expression in host cells significantly stabilized Wnt responses in vitro (Fig. 6A).
Figure 6.
Salmonella AvrA stabilizes Wnt 2 protein expression in mouse intestinal cells in vivo. (A) Wnt 2 protein expression in human epithelial HCT 116. Cells were treated with S. typhimurium PhoPc with AvrA protein expression, PhoPc AvrA− (AvrA gene mutant) or PhoPc AvrA−/AvrA+. Wnt 2 protein expression was determined by Western blot. Relative Wnt 2 band intensity was shown. Data are presented as the mean ± SD. (B) Wnt 2 protein expression level regulated Salmonella AvrA in a mouse model. Wnt protein expression in the small intestine with or without Salmonella infection was determined by Western blot. Relative Wnt 2 band intensity was lower in normal mice compared with the infected mice. Data are presented as the mean ± SD. n= 3 mice per group. (C) Wnt 2 distribution in small intestine with or without Salmonella infection. (D) Proliferative marker PCNA in intestine infected with S. typhimurium PhoPc with AvrA protein expression, PhoPc AvrA− (AvrA gene mutant) or PhoPc AvrA−/AvrA+. Please note the enhanced proliferation in PhoPc and PhoPc AvrA−/AvrA+ infection groups. (E) Proliferation index in intestinal epithelial cells. The number of PCNA-positive cells per three high powered fields was counted. Data are presented as the mean ± SD. n= 3 mice per group.
Bacterial colonization increases Wnt2 in mouse intestine in vivo
To assess the physiological relevance of Wnt2 in Salmonella-induced infection, we investigated the signaling pathway using a Salmonella-colitis mouse model (37–38). Wild-type strain SL1344 expresses AvrA protein, whereas SB1117 is an AvrA− mutant derived from SL1344. We found that Wnt2 protein expression was upregulated by AvrA expression in SL1344 intestine, whereas there was less Wnt2 in intestine infected with the SB1117 AvrA-mutated bacterial strain (Fig. 6B). Overall, Wnt2 protein expression was significantly enhanced in Salmonella SL1344 infected intestine.
We also investigated the distribution of Wnt2 in mouse intestine before and after Salmonella infection (Fig. 6C). We found clear positive staining for Wnt2 in epithelial cells lining the crypt region as well as the villus and surface epithelium. Pathogenic Salmonella infection enhanced Wnt2 staining. Hence, Salmonella infection not only increased Wnt2 expression but also induced relocation of Wnt2 in intestine. Wnt2 activation is associated with intestinal proliferation. Moreover, we found that PCNA staining, a readout for cell proliferation, was enhanced in the PhoPc and the AvrA-complemented strain PhoPc AvrA−/AvrA+ infected intestine (Fig. 6D).
Bacterial protein AvrA enhances Wnt2 expression
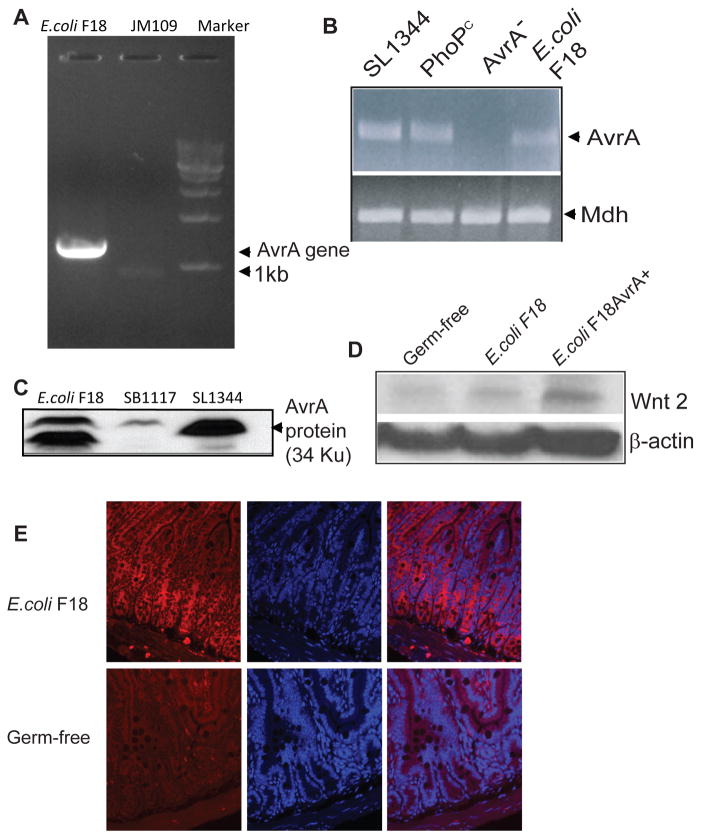
Recently, we found that the AvrA gene existed in a human-isolated commensal Escherichia coli F18 strain (Fig. 7A). The full length of AvrA was amplified in E. coli F18 but not in E. coli JM109. We also identified AvrA mRNA in F18 and various Salmonella strains but not in the Salmonella AvrA− strain (Fig. 7B). AvrA protein expression was found in E. coli strain F18 by Western blot analysis using an anti-AvrA antibody (Fig. 7C).
Figure 7.
E. coli AvrA enhances Wnt 2 protein levels. (A) The full-length AvrA gene is found in E. coli F18 strain. (B). AvrA mRNA expression in both Salmonella and E. coli strains. There is mRNA expression in wild-type Salmonella, non-virulent PhoPc, and E. coli F18. AvrA mRNA is absent in an AvrA mutant Salmonella strain. Mdh is a housekeeping gene. (C) AvrA protein expression was found in the indicated strains. (D) E. coli 18 AvrA association increases the Wnt 2 expression level in ex-germ-free mouse intestinal epithelial cells. Mono-associated mice were originally germ-free IL10−/− mice that were infected with a human commensal enteric bacterium E. coli F18 or E. coli F18AvrA+ by oral swabbing. Total cell lysates were analyzed for protein levels by immunoblotting. (E) Distribution of Wnt 2 (red) in the germ-free and E. coli F18 mono-associated mouse intestine. Please note that enhance Wnt2 staining in the E. coli mono-associated intestine.
We investigated the role of E. coli AvrA on Wnt expression in germ-free mice and mice mono-associated with E. coli F18 or E. coli F18AvrA+ (over-expressing AvrA). We noted that Wnt2 expression was undetectable in the germ-free intestine, upregulated by E. coli F18 colonization, and further enhanced by E. coli F18AvrA+ mono-associated in the ex-germ-free mouse intestine (Fig. 7D). Moreover, we compared germ-free mice and E. coli F18 mono-associated mice to explore the effect of E. coli on modulating Wnt2 distribution. In E. coli F18 mono-associated small intestine, Wnt2 was not only located at the bottom of the crypts but also in the middle of the villus (Fig. 7E). However, Wnt2 staining was very weak in the germ-free mouse intestine. Taken together, our data indicated that enteric bacteria modulate host responses through the Wnt pathway.
Discussion
Our current study attempted to answer two questions: is Wnt2 involved in Salmonella infection, and why is Wnt2 signaling involved in host cells during bacterial-induced apoptosis. We have demonstrated that Salmonella infections induce the elevated expression of Wnt2 mRNA and protein. Over-expressed Wnt2 is able to lower the IL-8 induced by Salmonella infection. In contrast, blocking Wnt2 protein expression in cells led to enhanced IL-8 mRNA levels after Salmonella colonization. Compared to normal cells, we found less Salmonella-induced apoptosis in cells with Wnt2 over-expression. Interestingly, we found that Wnt2 protein degradation is associated with ubiquitination. AvrA is a bacterial protein existing in both E. coli and Salmonella. Our study further demonstrated AvrA stabilized Wnt2 in vitro and in vivo. Pathogenic bacteria regulate Wnt2 expression and location in vivo. In an ex-germ-free system, the expression of Wnt2 was dramatically increased by E. coli F18 expressing AvrA. Hence, we have demonstrated that Wnt2 is directly involved in the regulation of intestinal inflammation induced by enteric bacteria.
Our previous studies have demonstrated that β-catenin is a negative regulator of intestinal NF-κB activity in bacterial-induced epithelial inflammation (5, 24). In the canonical pathway, Wnt binding stabilizes the transcription factor β-catenin, which in turn enters the nucleus to regulate Wnt pathway target genes. Our data indicate that Wnt2 may contribute to host protection in intestine by affecting signaling pathways related to cell proliferation and apoptosis by modulating inflammatory responses. Wnt2 signaling leads to down-regulation of the JNK/AP-1 and NF-κB pathways, up-regulation of β-catenin and promotes viability in intestinal epithelial cells during bacterial infection. Wnt family members have different functions, even if they sometimes compliment each other’s functions. We found Wnt2 had no effects on inhibiting bacterial invasion, whereas Wnt11 significantly decreased Salmonella invasion in epithelial cells (data to be published). Taken together, it is now clear that Wnt, its regulators, and downstream target beta-catenin are all involved in bacterial inflammation in intestine using different strategies.
Our study showed that the Wnt2 protein is regulated by ubiquitination. Proteasome inhibitor treatment is able to stabilize Wnt2. AvrA has been reported as a deubiquitinase (25). Therefore, AvrA may stabilize Wnt2 by removing the ubiquitin from Wnt2. However, in the AvrA− SB1117 infected intestine, Wnt2 protein expression was still higher than the control intestine without Salmonella infection. This result indicates that other bacterial factors also contribute to up-regulation of the Wnt2 protein.
The limitation of our study is that we could only narrow our focus to Wnt2. There are complicated interactions among the 19 Wnt members in this signaling pathway. The other Wnt proteins may synergistically play critical roles in intestinal inflammation. We do not have Wnt2 knockout mice to further investigate Salmonella infections in vivo.
In summary, the current study reports several novel aspects of Wnt2 in host-bacterial interactions: (18) the elevation of Wnt2 by bacterial infection at both the mRNA and protein levels, (2) the protective role of Wnt2 in inhibiting apoptosis and intestinal inflammation during bacterial infection, and (3) the stabilization of Wnt2 by the bacterial protein AvrA from certain Salmonella and E. coli strains. Hence, Wnt2 contributes to host protection in response to pathogenic bacteria in intestine. In addition to providing insights into salmonellosis, our study on the role of Wnt2 in host-bacterial interaction models provides fundamental molecular insights into mucosal inflammation.
Materials and Methods
Ethics statement
All animal protocols were approved by the University of Rochester University Committee on Animal Resources and the Institutional Animal Care (No. 2007-065) and Use Committee of the University of North Carolina at Chapel Hill (04-100.0A).
Bacterial strains and growth condition
Bacterial strains used in this study included wild-type Salmonella typhimurium strain ATCC14028 (WT-SL), commensal Escherichia coli F18 (39), non-pathogenic Salmonella mutant strain PhoPc (40), PhoPc AvrA−, PhoPc AvrA−/AvrA+, PhoPc AvrA+, wild-type Salmonella SL1344 (SB300) and its AvrA mutant strain SB1117. E. coli F18AvrA+ was generated by over-expressing the AvrA gene in a pSKW29 low-copy plasmid (pWSK29 was a gift from Dr. Sidney R. Kushner, University of Georgia). Non-agitated microaerophilic bacterial cultures were prepared by inoculating 10 ml of Luria-Bertani broth with 0.01 ml of a stationary phase culture followed by overnight incubation(~18 h) at 37°C, as previously described (5, 41).
AvrA clone and anti-AvrA antibody
The avrA gene was from wild-type S. typhimurium strain SL3201. Sequence analysis revealed that the avrA allele used in our study was identical to the allele from S. typhimurium LT2 (GenBank accession no. AE008830). Anti-AvrA antibody was custom-made as previously described (42).
Reverse transcription polymerase chain reaction (RT–PCR) for AvrA
Total RNA was extracted from bacteria using a Qiagen RNeasy mini kit (Cat: 74104. Qiagen, Valencia, CA) according to the manufacturer’s protocol. Total RNA was further digested with DNase I (Cat: 18068-015. Invitrogen, Carlsbad, CA, USA). RNA integrity was verified by gel electrophoresis. Extracted RNA yield and purity was then determined by measuring absorbances from the 220 nm to 350 nm range. RNA reverse transcription was performed using a SuperScript III kit (Invitrogen, Cat: 18080-051). cDNA reaction products were then used in a quantitative PCR reaction. The reaction mixture was subjected to PCR amplification using Taq polymerase (Fermentas, Glen Burnie, Maryland. Cat: EP0404) as previously described (43).
Cell culture
Human embryonic kidney 293 cells, epithelial Caco2-BBE, HT29Cl.29A, HeLa, and mouse intestinal epithelial CMT93 cells were maintained in DMEM supplemented with 10% FCS, penicillin-streptomycin and L-glutamine (24, 38, 42, 44). Human colonic epithelial HCT116 cells were cultured in McCoy’s 5A medium supplemented with 10% (v/v) fetal bovine serum (24, 41). The rat small intestinal IEC-18 cell line was grown in DMEM (high glucose, 4.5 g/l) containing 5% (v/v) fetal bovine serum, 0.1 U/ml insulin, 50 μg/ml streptomycin, and 50 U/ml penicillin. MEFs were isolated from E13.5 embryos C57BL/6 mouse breeding (24). Briefly, the embryos were harvested and placed in PBS to remove the internal organs, head, and four limbs. The remaining embryo body was individually minced and digested with 0.5% trypsin and 10 mM EDTA for 0.5 h at 37°C. The digested materials were gently pipetted to a single-cell suspension. The cells were cultured in DMEM (high glucose, 4.5 g/l) containing10% fetal bovine serum, 50 μg/ml streptomycin, and 50U/ml penicillin.
Streptomycin pre-treated mouse model
Animal experiments were performed by using specific pathogen-free female C57BL/6 mice (Taconic, Hudson, NY) that were 6–7 weeks old, as previously described (6). The protocol was approved by the University of Rochester University Committee on Animal Resources (UCAR). Water and food were withdrawn 4 h before oral gavage with 7.5 mg/mouse of streptomycin (75 μl of sterile solution or 75 μl of sterile water in control). Afterward, animals were supplied with water and food ad libitum. Twenty hours after streptomycin treatment, water and food were withdrawn again for 4 hours before the mice were infected with 1×107 CFU of S. typhimurium (100 μl suspension in HBSS) or treated with sterile HBSS (control) by oral gavage as previously described(45–46). At the indicated times post-infection, mice were sacrificed, and tissue samples from the intestinal tracts were removed for analysis.
Germ-free (GF) and mono-associated mouse colon
GF 129/SvEv mice and IL10−/−mice were obtained from the Center for Gastrointestinal Biology and Disease Gnotobiotic Core Facility and the National Gnotobiotic Rodent Resource Center, University of North Carolina, Chapel Hill. Mice were analyzed at the UNC National Gnotobiotic Rodent Resource Center. Sterility of germ-free mice was documented on a monthly basis by fecal Gram stains and aerobic and anaerobic cultures of the feces and bedding. For selected mice, sterility of cecalcontents was documented Gram stain and cultures at the time of necropsy. Mono-associated mice were originally GF mice that were infected with a human commensal enteric bacterium E. coli F18 (39) or E. coli F18AvrA+ by orally swabbing (47).
Mouse colonic epithelial cells
Mouse colonic epithelial cells were collected by scraping from mouse colon including proximal and distal regions (6). Cells were sonicated in lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris (pH 7.4), 1 mM EDTA, 1 mM EGTA (pH 8.0), 0.2 mM sodium ortho-vanadate, protease inhibitor cocktail). The protein concentration was measured using BioRad Reagent (BioRad, Hercules, CA, USA).
Construction of the Plasmid pcDNA3-mWnt2
The pCMV-Myc-hWnt2 (4.8 kb) plasmid was constructed by inserting a SalI-KpnI fragment containing the human Wnt2 complementary DNA (cDNA) (1.08 kb) into the pCMV-Myc plasmid (3.8 kb). PCR primers for generating a SalI-KapI fragment containing the hWnt2 cDNA were as follows: Sal-hWnt2 forward primer: 5-CGGTCGACATGAACGCCCCTCTCGGT-3; hWnt2-KpnI backward primer: 5-GGGGTACCTCATGTAGCGGTTGTCCAGT-3.
Transient transfections
Transient transfections were performed with Lipofectamine 2000 (Invitrogen, San Diego, CA) in accordance with the manufacturer’s instructions. Briefly, 6×105 HCT116 cells were seeded on 60 mm dishes overnight before co-transfection with 4 μg Myc tagged Wnt2. DNA was mixed with the liposome reagent at a ratio of 1:1 before adding to cells. After a 24-h transfection, proteins were extracted with RIPA buffer for immunoblotting (48).
Wnt2 siRNA
Wnt2 siRNA and control siRNA were purchased from Santa Cruz Biotechnology (Wnt2 siRNA: Cat # sc-36841; control siRNA: Cat # sc-37007, Santa Cruz CA, USA). The control siRNA consisted of a scrambled sequence that would not lead to the specific degradation of any cellular mRNA. Caco2-BBE cells were transfected using the DharmaFect 1 transfection reagent (Dharmacon) in Opti-MEM I medium (Invitrogen), according to manufacturer protocols, using final siRNA concentrations of 50 or 100 nM. After siRNA transfection, the plates were incubated for 3–5 days before further analysis.
Immunoblotting
Mouse epithelial cells were lysed in lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris (pH 7.4), 1 mM EDTA, 1 mM EGTA (pH 8.0), 0.2 mM sodium ortho-vanadate, protease inhibitor cocktail). The protein concentration was then measured. MEF cells were rinsed twice in ice-cold HBSS, lysed in protein loading buffer (50 mM Tris (pH 6.8), 100 mM dithiothreitol, 2% SDS, 0.1% bromophenol blue, 10% glycerol), and sonicated. Equal amounts of protein were separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with primary antibodies. The following antibodies were used: anti-c-Myc, anti-PARP (Cell Signal, Beverly, MA), anti-Villin, goat anti-Wnt2 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, U.S.A.), anti-ubiquitin (Biomol, Plymouth Meeting, PA), and anti-β-actin (Sigma-Aldrich, Milwaukee, WI, U.S.A.). They were then visualized by ECL. Membranes probed with more than one antibody were stripped before reprobing.
Immunofluorescence
Colonic tissues from the proximal and distal portion of the colon were freshly isolated and embedded in paraffin wax after fixation with 10% neutral buffered formalin. Immunostaining was performed on paraffin-embedded sections (1 μm) of mouse colons. After preparation of the slides as described previously (25), slides were incubated in 3% hydrogen peroxide for 20 minutes at room temperature to block endogenous peroxidase activity and incubated for 20 min in 5% BSA with 0.1% saponin in PBS to reduce nonspecific background. Cultured cells were rinsed three times in PBS, fixed for 20 min in cold 100% ethanol, and then permeabilized for 10 min with 0.2% Triton X-100. Finally, the cells were rinsed three times with PBS containing 10% bovine serum albumin. The permeabilized cells or tissue samples were incubated with primary antibodies for 90 minutes at room temperature. Samples were then incubated with Dork anti-Goat Alexa Fluor 594 and DAPI (Molecular Probes, Invitrogen) for 1 hour at room temperature. Tissues were mounted with SlowFade (SlowFade® AntiFade Kit, Molecular Probes) followed by coverslipping. The edges were sealed to prevent drying. Specimens were examined with a Leica SP5 Laser Scanning confocal microscope.
Apoptosis assays
After 24 hours of treatment with Salmonella, 1 × 106 adherent cells were trypsinized and incubated with FITC-conjugated annexin V (binds to phosphatidyl serine on the cytoplasmic surface of the cell membrane) and propidium iodide (PI) for 15 minutes in the dark according to the manufacturer’s protocol (Annexin VFITC Apoptosis Detection kit; Oncogene Research Products, San Diego, CA, USA). Cells were analyzed by flow cytometry.
S. typhimurium invasion of human epithelial monolayers
Infection of HCT116 cells was performed by a previously described method (45). Bacterial solutions (~20 bacteria/epithelial cell) were added, and bacterial invasion was assessed after 1 hour. Cell-associated bacteria represent bacteria adhered to and/or internalized into the monolayers. These bacteria were released by incubation with 100 μl of 1% Triton X-100 (Sigma). Bacteria internalized in epithelial cells were released with 1% Triton X-100 20 minutes after the addition of gentamicin (50 μg/ml). Gentamicin, an aminoglycoside antibiotic, does not permeate eukaryotic plasma membranes and is, therefore, cytolytic only to extracellular populations of bacteria; intracellular bacterial populations remain viable (49). For both cell-associated and internalized bacteria, 0.9 ml LB broth was then added, and each sample was vigorously mixed and quantitated by plating for CFUs on MacConkey agar medium (41).
Salmonella-induced human IL-8 secretion
HCT116 cells were cultured in DMEM, followed by Salmonella-containing HBSS (1.6×1010 bacteria/ml) for 30 minutes, washed 3 times in HBSS and incubated at 37°C for 6 hours. Cell supernatants were removed and assayed for IL-8 by ELISA in 96-well plates as described previously (45).
Real-Time quantitative PCR analysis
Total RNA was extracted from epithelial cell monolayers using TRIzol reagent (Invitrogen, Carlsbad, CA). RNA integrity was verified by gel electrophoresis. RNA reverse transcription was performed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA)according to the manufacturer’s directions. The RT cDNA reaction products were subjected to quantitative real-time PCR using the MyiQ single-color real-time PCR detection system (Bio-Rad) and iQ SYBR Green Supermix (Bio-Rad) according to the manufacturer’s directions. IL-8 cDNA was amplified using primers to the human IL-8 gene that were complementary to regions in exon 1 (5′-TGCATAAAGACATACTCCAAACCT) and overlapping the splice site between exons 3 and 4 (5′-AATTCTCAGCCCTCTTCAAAAA). All expression levels were normalized to actin levels or GAPDH of the same sample using forward (5′-AGA GCA AGA GAG GCA TCC TC-3′) and reverse (5′-CTC AAA CAT GAT CTG GGT CA-3′)primers for actin and the forward (5′-GAC GGC CGC ATC TTC TTG T-3′) and reverse (5′-CAC ACC GAC CTT CAC CAT TTT-3′) primers for GAPDH. Percent expression was calculated as the ratio of the normalized value of each sample to that of the corresponding untreated control cells. All real-time PCR reactions were performed in triplicate. All Wnts and Fzs PCR primers are shown in Table 1 (50–51).
Table 1.
Primers for real-time PCR
| Gene | Primer Sequence | Gene | Primer Sequence |
|---|---|---|---|
| RWnt2fw | ccctgatgaatcttcacaacaa | HFz3fw | tggctatgctggatgatcaaag |
| RWnt2bw | tctcccacaacacataacttcg | HFz3bw | tggaggctgccgtggta |
| RWnt2bfw | gatgccaaagagaagaggctta | HFz5fw | ctgaggttctgtgcatggatta |
| RWnt2bbw | cagccttgtccaagacacagta | HFz5bw | gttccatgtcaatgaggaaggt |
| RWnt3afw | tgaatttggaggaatggtctct | HFz6fw | acaagctgaaggtcatttccaaa |
| RWnt3abw | tgggcaccttgaagtatgtgta | HFz6bw | gctactgcagaagtgccatgat |
| RWnt7bfw | atgtaagtgtcacggagtgtcg | HFz9fw | ggttttgactctcacctggttc |
| RWnt7bbw | ggtgtactggtgcgtgttgtag | HFz9bw | atggaaaagactccgattttca |
| RWnt10bfw | cataaccgcaattctggagttt | HFz10fw | gcggtgaagaccatcctg |
| RWnt10bbw | cacttacacacgttgacccact | HFz10bw | gcacggtgtacagcacagag |
Statistical Analysis
Data are expressed as mean ± SD. All statistical tests were 2-sided. P-values of less than 0.05 were considered to be statistically significant. Differences between two samples were analyzed by a Student’s t-test. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
Acknowledgments
We thank Maureen Bower and Bo Liu for technical assistance with gnotobiotic mice and Drs. Nayden Naydenov and Victor Morales for their help with apoptosis assays.
Funding
This work was supported by the National Institutes of Health (DK075386-0251, R03DK089010-01), the American Cancer Society (RSG-09-075-01-MBC), and the IDEAL award from New York State’s Empire State Stem Cell Board (N09G-279) to Jun Sun.
Footnotes
Author Contributions
Conceived and designed the experiments: XL SW RL YX JS.
Performed the experiments: XL SW RL YZ.
Analyzed the data: XL RL SW YX JS.
Contributed reagents/materials/analysis tools: YX RBS JS.
Wrote the paper: XL YX RBS JS.
References
- 1.Ouko L, Ziegler TR, Gu LH, et al. Wnt11 signaling promotes proliferation, transformation, and migration of IEC6 intestinal epithelial cells. J Biol Chem. 2004;279:26707–26715. doi: 10.1074/jbc.M402877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manicassamy S, Reizis B, Ravindran R, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H, Zhang R, Wen S, et al. Nitric oxide increases Wnt-induced secreted protein-1 (WISP-1/CCN4) expression and function in colitis. J Mol Med. 2009;87:435–445. doi: 10.1007/s00109-009-0445-4. [DOI] [PubMed] [Google Scholar]
- 4.Neish AS, Gewirtz AT, Zeng H, et al. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000;289:1560–1563. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- 5.Sun J, Hobert ME, Rao AS, et al. Bacterial activation of beta-catenin signaling in human epithelia. American journal of physiology. 2004;287:G220–227. doi: 10.1152/ajpgi.00498.2003. [DOI] [PubMed] [Google Scholar]
- 6.Duan Y, Liao AP, Kuppireddi S, et al. beta-Catenin activity negatively regulates bacteria-induced inflammation. Laboratory investigation; a journal of technical methods and pathology. 2007;87:613–624. doi: 10.1038/labinvest.3700545. [DOI] [PubMed] [Google Scholar]
- 7.Nava P, Koch S, Laukoetter MG, et al. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity. 32:392–402. doi: 10.1016/j.immuni.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sen M, Ghosh G. Transcriptional outcome of Wnt-Frizzled signal transduction in inflammation: evolving concepts. J Immunol. 2008;181:4441–4445. doi: 10.4049/jimmunol.181.7.4441. [DOI] [PubMed] [Google Scholar]
- 9.You J, Nguyen AV, Albers CG, et al. Wnt pathway-related gene expression in inflammatory bowel disease. Dig Dis Sci. 2008;53:1013–1019. doi: 10.1007/s10620-007-9973-3. [DOI] [PubMed] [Google Scholar]
- 10.Wehkamp J, Wang G, Kubler I, et al. The Paneth cell alpha-defensin deficiency of ileal Crohn’s disease is linked to Wnt/Tcf-4. J Immunol. 2007;179:3109–3118. doi: 10.4049/jimmunol.179.5.3109. [DOI] [PubMed] [Google Scholar]
- 11.Blumenthal A, Ehlers S, Lauber J, et al. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood. 2006;108:965–973. doi: 10.1182/blood-2005-12-5046. [DOI] [PubMed] [Google Scholar]
- 12.Schaale K, Neumann J, Schneider D, et al. Wnt signaling in macrophages: Augmenting and inhibiting mycobacteria-induced inflammatory responses. Eur J Cell Biol. 2010 doi: 10.1016/j.ejcb.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Packey CD, Sartor RB. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr Opin Infect Dis. 2009;22:292–301. doi: 10.1097/QCO.0b013e32832a8a5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sartor RB. Key questions to guide a better understanding of host-commensal microbiota interactions in intestinal inflammation. Mucosal Immunol. 2011;4:127–132. doi: 10.1038/mi.2010.87. [DOI] [PubMed] [Google Scholar]
- 15.Mysorekar IU, Mulvey MA, Hultgren SJ, et al. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J Biol Chem. 2002;277:7412–7419. doi: 10.1074/jbc.M110560200. [DOI] [PubMed] [Google Scholar]
- 16.Voronina VA, Takemaru K, Treuting P, et al. Inactivation of Chibby affects function of motile airway cilia. J Cell Biol. 2009;185:225–233. doi: 10.1083/jcb.200809144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neumann J, Schaale K, Farhat K, et al. Frizzled1 is a marker of inflammatory macrophages, and its ligand Wnt3a is involved in reprogramming Mycobacterium tuberculosis-infected macrophages. FASEB J. 2010;24:4599–4612. doi: 10.1096/fj.10-160994. [DOI] [PubMed] [Google Scholar]
- 18.Lu R, Wu S, Liu X, et al. Chronic effects of a Salmonella type III secretion effector protein AvrA in vivo. PLoS ONE. 2010;5:e10505. doi: 10.1371/journal.pone.0010505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox JG, Feng Y, Theve EJ, et al. Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens. Gut. 2009 doi: 10.1136/gut.2009.183749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gnad T, Feoktistova M, Leverkus M, et al. Helicobacter pylori-induced activation of beta-catenin involves low density lipoprotein receptor-related protein 6 and Dishevelled. Mol Cancer. 9:31. doi: 10.1186/1476-4598-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S, Rhee KJ, Zhang M, et al. Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and gamma-secretase-dependent E-cadherin cleavage. J Cell Sci. 2007;120:1944–1952. doi: 10.1242/jcs.03455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu S, Morin PJ, Maouyo D, et al. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology. 2003;124:392–400. doi: 10.1053/gast.2003.50047. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Lu R, Wu S, et al. Salmonella regulation of intestinal stem cells through the Wnt/beta-catenin pathway. FEBS Lett. 584:911–916. doi: 10.1016/j.febslet.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun J, Hobert ME, Duan Y, et al. Crosstalk between NF-kappaB and beta-catenin pathways in bacterial-colonized intestinal epithelial cells. American journal of physiology. 2005;289:G129–137. doi: 10.1152/ajpgi.00515.2004. [DOI] [PubMed] [Google Scholar]
- 25.Ye Z, Petrof EO, Boone D, et al. Salmonella effector AvrA regulation of colonic epithelial cell inflammation by deubiquitination. The American journal of pathology. 2007;171:882–892. doi: 10.2353/ajpath.2007.070220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gradel KO, Nielsen HL, Schonheyder HC, et al. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology. 2009;137:495–501. doi: 10.1053/j.gastro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Grassl GA, Valdez Y, Bergstrom KS, et al. Chronic enteric salmonella infection in mice leads to severe and persistent intestinal fibrosis. Gastroenterology. 2008;134:768–780. doi: 10.1053/j.gastro.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 28.Claud EC, Zhang X, Petrof EO, et al. Developmentally regulated tumor necrosis factor-alpha induced nuclear factor-kappaB activation in intestinal epithelium. American journal of physiology. 2007;292:G1411–1419. doi: 10.1152/ajpgi.00557.2006. [DOI] [PubMed] [Google Scholar]
- 29.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 30.Planutis K, Planutiene M, Moyer MP, et al. Regulation of norrin receptor frizzled-4 by Wnt2 in colon-derived cells. BMC Cell Biol. 2007;8:12. doi: 10.1186/1471-2121-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karasawa T, Yokokura H, Kitajewski J, et al. Frizzled-9 is activated by Wnt-2 and functions in Wnt/beta -catenin signaling. J Biol Chem. 2002;277:37479–37486. doi: 10.1074/jbc.M205658200. [DOI] [PubMed] [Google Scholar]
- 32.Collier-Hyams LS, Zeng H, Sun J, et al. Cutting edge: Salmonella AvrA effector inhibits the key proinflammatory, anti-apoptotic NF-kappa B pathway. J Immunol. 2002;169:2846–2850. doi: 10.4049/jimmunol.169.6.2846. [DOI] [PubMed] [Google Scholar]
- 33.Grassme H, Jendrossek V, Gulbins E. Molecular mechanisms of bacteria induced apoptosis. Apoptosis. 2001;6:441–445. doi: 10.1023/a:1012485506972. [DOI] [PubMed] [Google Scholar]
- 34.Bafico A, Gazit A, Wu-Morgan SS, et al. Characterization of Wnt-1 and Wnt-2 induced growth alterations and signaling pathways in NIH3T3 fibroblasts. Oncogene. 1998;16:2819–2825. doi: 10.1038/sj.onc.1201797. [DOI] [PubMed] [Google Scholar]
- 35.Smith K, Bui TD, Poulsom R, et al. Up-regulation of macrophage wnt gene expression in adenoma-carcinoma progression of human colorectal cancer. Br J Cancer. 1999;81:496–502. doi: 10.1038/sj.bjc.6690721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu Y, Ye L, Yu S, et al. Rescue of odontogenesis in Dmp1-deficient mice by targeted re-expression of DMP1 reveals roles for DMP1 in early odontogenesis and dentin apposition in vivo. Dev Biol. 2007;303:191–201. doi: 10.1016/j.ydbio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barthel M, Hapfelmeier S, Quintanilla-Martinez L, et al. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003;71:2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao AP, Petrof EO, Kuppireddi S, et al. Salmonella type III effector AvrA stabilizes cell tight junctions to inhibit inflammation in intestinal epithelial cells. PLoS ONE. 2008;3:e2369. doi: 10.1371/journal.pone.0002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen PS, Rossoll R, Cabelli VJ, et al. Relationship between the mouse colonizing ability of a human fecal Escherichia coli strain and its ability to bind a specific mouse colonic mucous gel protein. Infect Immun. 1983;40:62–69. doi: 10.1128/iai.40.1.62-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller SI, Mekalanos JJ. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J Bacteriol. 1990;172:2485–2490. doi: 10.1128/jb.172.5.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma J, Zhang YG, Xia Y, et al. The inflammatory cytokine tumor necrosis factor modulates the expression of Salmonella typhimurium effector proteins. J Inflamm (Lond) 2010;7:42. doi: 10.1186/1476-9255-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu S, Ye Z, Liu X, et al. Salmonella typhimurium infection increases p53 acetylation in intestinal epithelial cells. American journal of physiology. 2010;298:G784–794. doi: 10.1152/ajpgi.00526.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma J, Zhang YG, Xia Y, et al. The inflammatory cytokine tumor necrosis factor modulates the expression of Salmonella typhimurium effector proteins. J Inflamm (Lond) 7:42. doi: 10.1186/1476-9255-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lippert E, Karrasch T, Sun X, et al. Gnotobiotic IL-10; NF-kappaB mice develop rapid and severe colitis following Campylobacter jejuni infection. PLoS ONE. 2009;4:e7413. doi: 10.1371/journal.pone.0007413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCormick BA, Colgan SP, Delp-Archer C, et al. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J Cell Biol. 1993;123:895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu R, Wu S, Liu X, et al. Chronic effects of a Salmonella type III secretion effector protein AvrA in vivo. PLoS ONE. 5:e10505. doi: 10.1371/journal.pone.0010505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu S, Liao AP, Xia Y, et al. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol. 2010;177:686–697. doi: 10.2353/ajpath.2010.090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X, Lu R, Wu S, et al. Salmonella regulation of intestinal stem cells through the Wnt/beta-catenin pathway. FEBS Lett. 2010;584:911–916. doi: 10.1016/j.febslet.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lissner CR, Swanson RN, O’Brien AD. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983;131:3006–3013. [PubMed] [Google Scholar]
- 50.Klein D, Demory A, Peyre F, et al. Wnt2 acts as a cell type-specific, autocrine growth factor in rat hepatic sinusoidal endothelial cells cross-stimulating the VEGF pathway. Hepatology. 2008;47:1018–1031. doi: 10.1002/hep.22084. [DOI] [PubMed] [Google Scholar]
- 51.Okoye UC, Malbon CC, Wang HY. Wnt and Frizzled RNA expression in human mesenchymal and embryonic (H7) stem cells. J Mol Signal. 2008;3:16. doi: 10.1186/1750-2187-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]