Abstract
The highly alkaline compound trisodium phosphate (TSP) is used as an intervention to reduce the load of Campylobacter on poultry meat in U.S. poultry slaughter plants. The aim of the present study was to investigate the cellular responses of Campylobacter jejuni NCTC11168 when exposed to sublethal concentrations of TSP. Preexposure of C. jejuni to TSP resulted in a significant increase in heat sensitivity, suggesting that a combined heat and TSP treatment may increase reduction of C. jejuni. A microarray analysis identified a limited number of genes that were differently expressed after sublethal TSP exposure; however, the response was mainly associated with ion transport processes. C. jejuni NCTC11168 nhaA1 (Cj1655c) and nhaA2 (Cj1654c), which encode orthologues to the Escherichia coli NhaA cation/proton antiporter, were able to partially restore TSP, alkaline, and sodium resistance phenotypes to an E. coli cation/proton antiporter mutant. In addition, inhibition of resistance-nodulation-cell division (RND) multidrug efflux pumps by the inhibitor PaβN (Phe-Arg β-naphthylamide dihydrochloride) decreased tolerance to sublethal TSP. Therefore, we propose that NhaA1/NhaA2 cation/proton antiporters and RND multidrug efflux pumps function in tolerance to sublethal TSP exposure in C. jejuni.
INTRODUCTION
Chemical decontamination has been used widely for decades in the U.S. poultry slaughter plants to reduce the presence of human pathogens on the meat (22). Currently, the highly alkaline compound trisodium phosphate (TSP) is applied as a dip or spray of prechilled or chilled poultry carcasses (6, 22) and is effective against Campylobacter, Salmonella, and Listeria (6). In the European Union (EU), an ongoing debate questions the safety of chemical decontamination, and more data, such as the development of resistance, cross protection, and virulence, are needed for the risk assessments required by the EU (8).
The alkalinity of TSP causes bacterial death through disruption of cell membranes (18, 30), and it acts as a detergent that removes fat from the surface of poultry carcasses that further increases the killing action of TSP (13). Sublethal concentrations of TSP may be encountered by the bacteria when the compound is inadequately distributed on the surface of the carcass or inactivated by excessive amounts of organic material or when leftover TSP residues are present on the carcass during storage (1, 2, 31). A major concern is that exposure to sublethal concentrations of TSP may increase bacterial tolerance to food processing interventions, preservation treatments, and antibacterial conditions within the human hosts.
Campylobacter jejuni is one of the most frequently reported causes of bacterial food-borne infections in developed countries (7, 9), leading to self-limiting acute gastroenteritis. The consumption and handling of poultry meat products is the major source of human campylobacteriosis, and the use of TSP during poultry meat processing is known to reduce levels of C. jejuni (4, 28, 29, 37). The aim of the present study was to show whether sublethal TSP exposure affects gene expression and the physiology of C. jejuni, including cross-protection to other environmental stresses.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids are listed in Table 1. C. jejuni strains were routinely grown on blood agar base II (Oxoid) supplemented with 5% calf blood or in brucella broth (BB; Difco, Broendby, Denmark) at 37°C under microaerobic conditions (6% O2, 6% CO2, 4% H2, and 84% N2) or (5% O2, 10% CO2, and 85% N2). De novo protein synthesis was inhibited by chloramphenicol (128 μg/ml). Escherichia coli strains were grown at 37°C in modified Luria-Bertani broth (LBK), where KCl substituted NaCl (10). When appropriate kanamycin (30 μg/ml), erythromycin (200 μg/ml), chloramphenicol (30 μg/ml), or ampicillin (50 or 100 μg/ml) was added. Trisodium phosphate (TSP; Riedel-de Häen 04278) was used at concentrations of weight/volume (%).
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| C. jejuni NCTC11168 | Human clinical isolate | National Collection of Type Cultures (United Kingdom) |
| E. coli | ||
| TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 nupG recA1 araD139 Δ(ara-leu)7697 galE15 galK16 rpsL (Strr) endA1 λ− | Invitrogen |
| KNabc | TG1 nhaA::Kan nhaB::Erm chaA::Cam | 21 |
| ER2566 | F−fhuA2 [lon] ompT lacZ::T7gene1 gal sulA11 Δ(mcrC-mrr)114::IS10 R(mcr-73::miniTn10-Tets)2 R(zgb-210::Tn10)(Tets) endA1 [dcm] λ− | NEB |
| CTN40 | E. coli ER2566 nhaA::Kan nhaB::Erm chaA::Cam | This study |
| CTN15 | CTN40 containing pET-21(+) | This study |
| CTN58 | CTN40 containing pCTN1 | This study |
| CTN3 | CTN40 containing pTLJ03/nhaA1 | This study |
| CTN9 | CTN40 containing pTLJ03 | This study |
| Plasmids | ||
| pCR2.1-TOPO | Cloning vector; Ampr | Invitrogen |
| pCR2.1-TOPO/nhaA1-nhaA2 | pCR2.1-TOPO containing nhaA1 and nhaA2 genes from C. jejuni NCTC11168 | This study |
| pET-21(+) | Transcription vector, T7 promoter, lacI coding sequence; Ampr | Novagen |
| pCTN1 | pET-21(+) containing nhaA1 and nhaA2 genes from C. jejuni NCTC11168 | This study |
| pTLJ03 | Transcription vector, T7 promoter, lacI coding sequence; Ampr | Geneservice (27) |
| pTLJ03/nhaA1 | nhaA1 in pTLJ03, plasmid obtained from C. jejuni NCTC11168 ORF library | Geneservice (27) |
Resistance: Strr (streptomycin), Ampr (ampicillin), Kanr (kanamycin), Ermr (erythromycin), Camr (chloramphenicol). Sensitive: Tets (tetracycline).
E. coli CTN40 carrying cation/proton antiporter mutations from strain KNabc was constructed by P1 transduction and selection for relevant resistance markers and confirmed by lack of growth in LBK 0.2 M NaCl (21). NCTC11168 chromosomal DNA was used as a template for amplification of a 2,346-bp nhaA1 (Cj1655c)-nhaA2 (Cj1654c) fragment using DreamTaq DNA polymerase (Fermentas, St. Leon-Rot, Germany) and the primers (Eurofins MWG GmbH, Ebersberg Germany) CTR1-up (5′-TATGGATCCTTAGGAGCAAGGATGCAAATG-3′; BamHI site underlined) and CTR9-down (5′-TATCTCGAGTATGCCTCATCAATCCCCTTA-3′; XhoI site underlined). The nhaA1-nhaA2 fragment was cloned into pCR2.1 (Invitrogen, Naerum, Denmark) and moved to pET-21(+) (Novagen) using BamHI and XhoI (Fermentas) resulting in pCTN1.
Survival during heat stress after exposure to sublethal TSP.
A 90 ml overnight culture of C. jejuni was adjusted to optical density at 600 nm (OD600) of 0.8, split in two, centrifuged for 5 min at 8,000 rpm, inoculated into BB alone or BB plus 0.6% TSP, and incubated under aerobic conditions at 37°C for 30 min. Cultures were washed twice in phosphate-buffered saline (PBS; pH 7.4 [CM7733; Oxoid, Greve, Denmark]) and resuspended in BB. Cultures were diluted into BB preheated to 48°C, and samples were withdrawn and placed on ice. Samples were 10-fold serial diluted and CFU determined. When no colonies were detected, CFU was set to half the detection limit (1.2 log10 CFU/ml). The mean log10 CFU/ml ± the standard deviation (SD) at each sampling time of three independent experiments is shown.
Collection of samples and RNA extraction.
C. jejuni were inoculated into BB to an OD600 of 0.05 and incubated for 8 h until reaching exponential phase (an OD600 of 0.32 to 0.50). The culture was concentrated and split into two portions, one in BB and another in BB plus 0.6% TSP (pH 9.2). After 5 and 30 min, the samples were collected, and RNAprotect bacterial reagent (Qiagen, Copenhagen, Denmark) was added. Total RNA was extracted using an RNeasy minikit (Qiagen) according to the manufacturer's instructions with an additional on-column DNase I digestion (RNase-Free DNase Set; Qiagen) and the addition of lysozyme. The purity and integrity of the RNA was confirmed with a 2100 Bioanalyzer (Agilent Technologies).
Microarray hybridizations and data analysis.
C. jejuni NCTC11168 whole-genome microarrays (v2.1.0 with PCR reporters for each of 1,654 genes spotted in duplicate) were supplied by the Microarray Group at St. George's Hospital Medical School, London, United Kingdom (BμG@S Group [http://www.bugs.sghms.ac.uk]). Probe labeling and microarray hybridization followed the standard RNA protocols from the BμG@S Group (34). Total RNA (5 μg) derived from C. jejuni both exposed to TSP and unexposed was labeled with Cy3 and Cy5, respectively. Purified Cy3/Cy5-labeled cDNA was hybridized on the microarrays underneath a LifterSlip (Eirie Scientific). Slides were scanned with a GMS418 microarray scanner (Genetic Microsystems). ImaGene software v5.5 (BioDiscovery) was used for quantification of the fluorescence from each spot. The data were further processed using MAVI Pro v2.6.0 software (MWG Biotech) and analyzed using GeneSpring GX v7.3.1 software (Agilent Technologies). The data from triplicate biological experiments at each sampling time were combined. Significant differential gene regulation was identified by using a P value cutoff of 0.05 and application of the Benjamini-Hochberg false discovery rate.
Growth of C. jejuni in the presence of TSP and a multidrug efflux pump inhibitor.
C. jejuni was inoculated to an OD600 of 0.05 into either BB, BB plus 0.4% TSP, BB plus 64 μg of PaβN/ml (Phe-Arg β-naphthylamide dihydrochloride Sigma, Broendby, Denmark), or BB plus 0.4% TSP and 64 μg of PaβN/ml. The mean OD600 ± the SD from three independent experiments is presented.
Functional complementation of the E. coli cation/proton antiporter mutant.
The OD600 of E. coli CTN39, CTN40, CTN15, and CTN58 was adjusted to 0.05 in LKB plus 0.2% TSP, LKB plus 0.2 M NaCl, and LBK (pH 8.5)–(KOH). To CTN15 and CTN58, 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added to induce nhaA1-nhaA2 expression or as a control. The mean OD600 is presented.
RESULTS AND DISCUSSION
Genome-wide gene expression during sublethal TSP exposure.
The gene expression profile of C. jejuni in response to sublethal TSP exposure was investigated by microarray analysis and triplicate biological replicates identified 27 significantly differentially regulated genes (see Table S1 in the supplemental material), 18 of which showed ≥1.5-fold differential expression in at least 1 time point (Table 2). Noteworthy, most gene functions were associated with transport processes, such as iron uptake, DNA uptake, and efflux. The most highly upregulated gene (3.2-fold) was Cj0176c belonging to the operon Cj0173c-Cj0176c that plays a role in uptake of iron from transferrin proteins (19, 20). A gene, Cj1471c, characterized to function in DNA uptake and transformation (38) showed the largest downregulation (2.3-fold) in response to TSP. Thus, in C. jejuni, sublethal TSP exposure elicits a limited transcriptional response that mainly is associated with transport processes.
Table 2.
Microarray analysis of gene expression in C. jejuni NCTC11168 in brucella broth with 0.6% TSP compared to brucella broth (pH 7)
| Systematic | Relative gene expressiona at: |
Product | Additional functional description | |||
|---|---|---|---|---|---|---|
| 5 min |
30 min |
|||||
| Fold change | P | Fold change | P | |||
| Cj0008 | 2.2 | 0.000 | 1.8 | 0.090 | Hypothetical protein Cj0008 | |
| Cj0039c | 1.3 | 0.024 | 1.8 | 0.000 | GTP-binding protein typA homolog | |
| Cj0044c | –1.3 | 0.026 | –2.0 | 0.000 | Hypothetical protein Cj0044c | |
| Cj0045c | 1.0 | 0.859 | –1.7 | 0.000 | Putative iron-binding protein | |
| Cj0176c | 2.5 | 0.001 | 3.2 | 0.000 | Putative lipoprotein | In operon with Cj0173-Cj0175c, which is involved in scavenging iron from lactoferrin and transferrinb |
| Cj0403 | 1.3 | 0.045 | 1.5 | 0.001 | Hypothetical protein Cj0403 | |
| Cj0608 | 2.4 | 0.000 | 1.4 | 0.004 | Putative outer membrane protein | |
| Cj0717 | 1.3 | 0.115 | 1.7 | 0.000 | Hypothetical protein Cj0717 | |
| Cj0770c | 1.1 | 0.298 | 2.0 | 0.000 | Putative periplasmic protein | Contains a conserved domain characteristic of the periplasmic component of an ABC transporterc |
| Cj0771c | 1.3 | 0.089 | 1.8 | 0.000 | Putative periplasmic protein | |
| Cj1000 | 1.8 | 0.018 | 1.7 | 0.000 | Putative transcriptional regulator (LysR family) | |
| Cj1241 | –1.5 | 0.001 | –1.6 | 0.000 | Putative transmembrane transport protein | |
| Cj1436c | –1.4 | 0.025 | –1.6 | 0.000 | Putative aminotransferase | |
| Cj1467 | –1.3 | 0.098 | –2.0 | 0.001 | Hypothetical protein Cj1467 | |
| Cj1471c | –1.7 | 0.006 | –2.3 | 0.001 | Putative type II protein secretion system E protein | Essential for DNA uptake and natural transformationd |
| Cj1483c | –1.4 | 0.326 | –1.8 | 0.000 | Putative lipoprotein | |
| Cj1533c | 1.6 | 0.002 | 1.8 | 0.000 | Putative helix-turn-helix containing protein | |
| Cj1646 | –1.5 | 0.070 | –2.0 | 0.000 | Putative ABC transport system permease protein | |
Relative gene expression is presented as the mean ratio of fluorescence intensity of TSP exposed cells to that of pH 7-exposed cells. Genes with the fold change shown in boldface indicate significant differential expression (Student t test with a P value of <0.05 adjusted with the Benjamini-Hochberg false-discovery-rate multiple testing correction). All P values were calculated using the Student t test.
Information revealed from bioinformatic analysis.
Function described previously (38).
nhaA1 and nhaA2 function as cation/proton antiporters in tolerance to TSP.
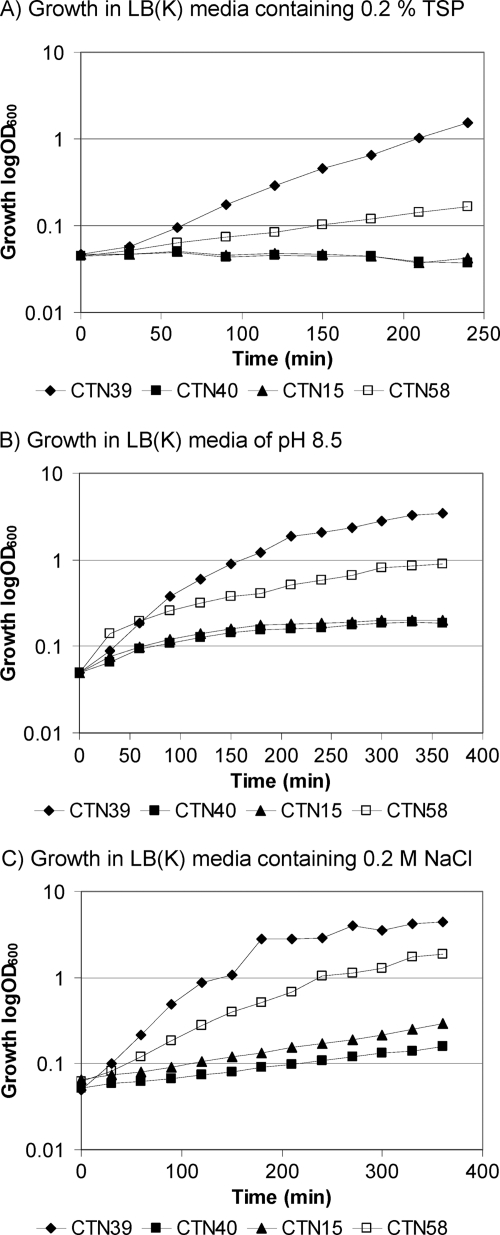
In other bacteria, NhaA cation/proton antiporters play an essential role in alkaline pH homeostasis by exchanging cytoplasmic cations with external protons (23). C. jejuni NCTC11168 encodes two cation/proton antiporter orthologues, NhaA1 and NhaA2 that show 46 and 49% identity to E. coli NhaA, respectively, and 56% similarity to each other. Although an E. coli cation/proton antiporter mutant was unable to grow in 0.2% TSP (pH 8.2), the presence of the C. jejuni genes nhaA1 and nhaA2 partly restored its ability to grow at the wild-type level (Fig. 1A). Furthermore, alkaline conditions (pH 8.5) and NaCl (0.2 M) inhibited growth of the E. coli cation/proton antiporter mutant, but the presence of C. jejuni nhaA1 and nhaA2 partly restored its ability to grow as the wild type (Fig. 1B and C). We attempted to determine the contribution of each of the C. jejuni nhaA genes to TSP tolerance, and even though the plasmids were unstable, we found that nhaA1 partly complemented the growth deficiency of the E. coli cation/proton antiporter mutant in the presence of TSP (0.2%) and NaCl (0.2 M) (data not shown), suggesting that nhaA1 itself is able to function as a cation/proton antiporter. Finally, the ability of nhaA1 and nhaA2 to provide TSP tolerance may be ascribed to their function in tolerance to alkaline as well sodium ions.
Fig 1.
Complementation capacity of C. jejuni NCTC11168 nhaA1 and nhaA2 genes in a TSP, alkali, and Na+-sensitive E. coli cation/proton antiporter mutant. The E. coli strains tested were wt (CTN39, ♦), cation/antiporter ΔnhaA ΔnhaB ΔchaA mutant (CTN40, ■), cation/antiporter ΔnhaA ΔnhaB ΔchaA mutant containing plasmid pCTN1 with the C. jejuni NCTC11168 nhaA1 and nhaA2 genes (CTN58, □), and cation/antiporter ΔnhaA ΔnhaB ΔchaA mutant containing negative control vector pET-21(+) (CTN15, ▲). The E. coli strains were grown in LB(K) medium containing 0.2% TSP (A), pH 8.5 adjusted with KOH (B), or 0.2 M NaCl (C) for up to 360 min at 37°C. One representative of four experiments under each condition is presented, and similar results were obtained in all four experiments.
Our microarray data show that transcription of the C. jejuni nhaA genes are not induced when exposed to TSP. However, noteworthy, changes in the external pH affect the transport activity of E. coli NhaA (3, 24), thus regulating the activity posttranscriptionally, and a similar mechanism may ensure regulation of the activity of the C. jejuni cation/proton antiporters. Interestingly, bioinformatic analysis of sequenced C. jejuni genomes revealed that both NhaA1 and NhaA2 are present and conserved in all genomes, suggesting that both genes have an important role in this species.
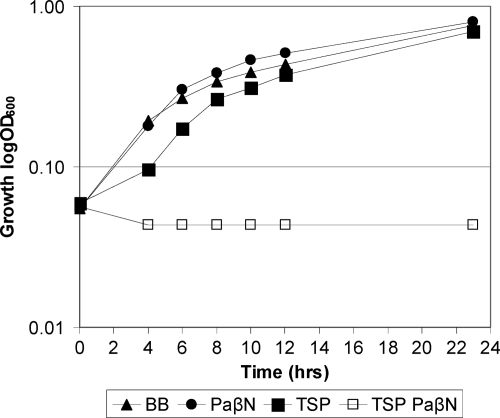
Multidrug efflux pump inhibitor PaβN sensitizes C. jejuni to TSP.
The upregulation of an efflux protein (Cj0608) during sublethal TSP exposure generated the hypothesis that efflux in general may be involved in tolerance to TSP. To further investigate this, we exposed C. jejuni to TSP in the presence of PaβN that inhibits multidrug efflux pumps of the resistance-nodulation-cell division (RND) superfamily (16, 17). We found that C. jejuni NCTC11168 was unable to grow in the presence of 0.4% TSP and 64 μg of PaβN/ml, whereas growth in 64 μg of PaβN/ml was similar to growth in the control media and 0.4% TSP only had a marginally effect on the initial growth rate (Fig. 2). Thus, inhibition of RND multidrug efflux pumps by PaβN decreases C. jejuni tolerance to TSP, suggesting that PaβN directly inhibit TSP transport mediated by RND multidrug efflux pumps. Alternatively, these results may merely reflect that C. jejuni cannot tolerate both the competitive inhibition of transport activity imposed by PaβN and the alkaline TSP that may act as indirect inhibitor of efflux pumps through deprivation of energy (16, 25, 33). In addition, the C. jejuni genome contains functionally uncharacterized putative efflux proteins such as Cj0035c containing the conserved domain of a multidrug efflux pump of the major facilitator superfamily that may have a dual function in a multidrug efflux protein and cation/proton antiporter similar to E. coli Mdfa (15).
Fig 2.
Growth of C. jejuni NCTC11168 in the presence of TSP and the efflux pump inhibitor PaβN. C. jejuni cultures were incubated under microaerobic conditions at 37°C for 24 h in either BB (▲), BB containing 0.4% TSP (■), BB containing 64 μg of PaβN/ml (●), or BB containing 0.4% TSP and 64 μg of PaβN/ml (□). Growth was measured as the OD600, and data are presented as the means from three independent experiments where the SD was below an OD600 of 0.01 in all experiments.
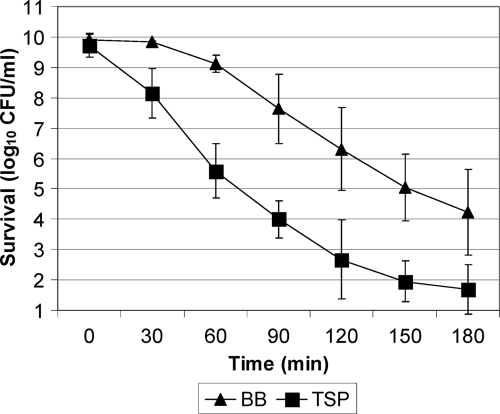
Sublethal TSP exposure increases sensitivity of C. jejuni to heat stress.
In the transmission from farm to fork, C. jejuni most likely encounters high-temperature stress, for instance, hot water or steam treatment in the slaughterhouse (5, 12, 26). During heat stress at 48°C, we observed that the viability of cells pretreated with TSP decreased immediately after onset of the heat stress, whereas cells with no prior TSP treatment were unaffected for the first 30 min of the heat stress (Fig. 3), but survival was not affected by the presence of chloramphenicol (data not shown). A number of additional strains were tested, and they also showed increased sensitivity to heat stress when exposed to TSP (data not shown). In contrast, TSP exposure did not affect survival significantly after 20 min of peroxide stress (data not shown). In conclusion, sublethal exposure to TSP increases the sensitivity of C. jejuni to heat stress under aerobic conditions independently of protein synthesis. In contrast, de novo protein synthesis during alkaline exposure participated in induction of thermotolerance in Salmonella enterica serovar Enteritidis (11, 31), and previous studies of food-borne pathogens (S. enterica serovar Enteritidis, E. coli O157:H7, V. parahaemolyticus, and L. monocytogenes) have demonstrated that cells surviving TSP or a comparable alkaline treatment acquired increased thermotolerance (14, 31, 32, 35). Collectively, the observed differences among bacterial species emphasizes the importance of carefully assessing the impact of sublethal TSP exposure on survival during subsequent stressful interventions for all relevant pathogens on poultry meat.
Fig 3.
Effect of sublethal TSP treatment on the survival of C. jejuni NCTC11168 during heat stress. C. jejuni cells were incubated in BB (▲) or BB containing 0.6% TSP (■) for 30 min in aerobic conditions at 37°C. Subsequently, the cells were resuspended in fresh BB and incubated at 48°C for 3 h, where survival was determined as CFU/ml, and data are presented as the mean log10 CFU/ml ± the SD of three experiments.
Concluding remarks.
In light of our observations, new decontamination procedures may be designed to obtain increased reduction of C. jejuni, such as a combination of TSP and heat treatment. Finally, the importance of efflux pumps as well as cation/proton antiporters such as NhaA1 and Nha2 may be interesting to investigate further to identify inhibitors or combined treatments that will provide a high reduction of C. jejuni even when low concentrations of TSP are used.
Supplementary Material
ACKNOWLEDGMENTS
We kindly thank Tsuchiya and T. Kuroda from Okayama University (Okayama, Japan) for providing the E. coli KNabc strain. We sincerely appreciate the technical assistance from Brenda Birungi, Christel Galschiøt Buerholt, and Jan Pedersen.
The microarray hybridizations and data analysis were financially supported by the European Union-funded Integrated Project (BIOTRACER contract FOOD-2006-CT-036272) under the 6th RTD framework. The Ph.D. scholarship for C.T.R. was provided by the Danish Directorate for Food, Fisheries, and Agri-Business (FFS05-1 [3304-FFS-06-0455]).
Footnotes
Published ahead of print 22 December 2011
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Alonso-Hernando A, Alonso-Calleja C, Capita R. 2009. Comparative analysis of acid resistance in Listeria monocytogenes and Salmonella enterica strains before and after exposure to poultry decontaminants. Role of the glutamate decarboxylase (GAD) system. Food Microbiol. 26:905–909 [DOI] [PubMed] [Google Scholar]
- 2. Alonso-Hernando A, Alonso-Calleja C, Capita R. 2010. Effects of exposure to poultry chemical decontaminants on the membrane fluidity of Listeria monocytogenes and Salmonella enterica strains. Int. J. Food Microbiol. 137:130–136 [DOI] [PubMed] [Google Scholar]
- 3. Arkin IT, et al. 2007. Mechanism of Na+/H+ antiporting. Science 317:799–803 [DOI] [PubMed] [Google Scholar]
- 4. Bashor MP, et al. 2004. Effects of carcass washers on Campylobacter contamination in large broiler processing plants. Poult. Sci. 83:1232–1239 [DOI] [PubMed] [Google Scholar]
- 5. Boysen L, Rosenquist H. 2009. Reduction of thermotolerant Campylobacter species on broiler carcasses following physical decontamination at slaughter. J. Food Prot. 72:497–502 [DOI] [PubMed] [Google Scholar]
- 6. Capita R, Alonso-Calleja C, Garcia-Fernandez MC, Moreno B. 2002. Trisodium phosphate (TSP) treatment for decontamination of poultry. Food Sci. Technol. Int. 8:11–24 [Google Scholar]
- 7. Centers for Disease Control and Prevention 2009. Preliminary FoodNet Data on the incidence of infection with pathogens transmitted commonly through food in 10 states, 2008. MMWR Morb. Mortal. Wkly. Rep. 58:333–337 [PubMed] [Google Scholar]
- 8. European Commission. 2009. Council Decision (EC) no, 121/2009 of the European Council of 18 December 2008 rejecting the proposal from the Commission for a Council Regulation Implementing Regulation (EC) No 853/2004 of the European Parliament and of the Council as regards the use of antimicrobial substances to remove surface contamination from poultry carcasses. OJEC L42/13:1–3 http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:042:0013:0015:EN:PDF [Google Scholar]
- 9. European Food Safety Authority 2009. The community summary report on trends and sources of zoonoses and zoonotic agents in the European Union in 2007. EFSA J. 223:1–320 [Google Scholar]
- 10. Goldberg EB, et al. 1987. Characterization of a Na+/H+ antiporter gene of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 84:2615–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Humphrey TJ, Wallis M, Hoad M, Richardson NP, Rowbury RJ. 1993. Factors influencing alkali-induced heat-resistance in Salmonella enteritidis phage type 4. Lett. Appl. Microbiol. 16:147–149 [Google Scholar]
- 12. James C, et al. 2007. Decontamination of poultry carcasses using steam or hot water in combination with rapid cooling, chilling or freezing of carcass surfaces. Int. J. Food Microbiol. 114:195–203 [DOI] [PubMed] [Google Scholar]
- 13. Kim JW, Slavik MF, Bender FG. 1994. Removal of Salmonella Typhimurium attached to chicken skin by rinsing with trisodium phosphate solution: scanning electron-microscopic examination. J. Food Safety 14:77–84 [Google Scholar]
- 14. Koga T, Katagiri T, Hori H, Takumi K. 2002. Alkaline adaptation induces cross-protection against some environmental stresses and morphological change in Vibrio parahaemolyticus. Microbiol. Res. 157:249–255 [DOI] [PubMed] [Google Scholar]
- 15. Lewinson O, Padant E, Bibi E. 2004. Alkalitolerance: a biological function for a multidrug transporter in pH homeostasis. Proc. Natl. Acad. Sci. U. S. A. 101:14073–14078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lomovskaya O, et al. 2001. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother. 45:105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martinez A, Lin J. 2006. Effect of an efflux pump inhibitor on the function of the multidrug efflux pump CmeABC and antimicrobial resistance in Campylobacter. Foodborne Pathog. Dis. 3:393–402 [DOI] [PubMed] [Google Scholar]
- 18. Mendonca AF, Amoroso TL, Knabel SJ. 1994. Destruction of gram-negative food-borne pathogens by high pH involves disruption of the cytoplasmic membrane. Appl. Environ. Microbiol. 60:4009–4014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller CE, Rock JD, Ridley KA, Williams PH, Ketley JM. 2008. Utilization of lactoferrin-bound and transferrin-bound iron by Campylobacter jejuni. J. Bacteriol. 190:1900–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller CE, Williams PH, Ketley JM. 2009. Pumping iron: mechanisms for iron uptake by Campylobacter. Microbiology 155:3157–3165 [DOI] [PubMed] [Google Scholar]
- 21. Nozaki K, Inaba K, Kuroda T, Tsuda M, Tsuchiya T. 1996. Cloning and sequencing of the gene for Na+/H+ antiporter of Vibrio parahaemolyticus. Biochem. Biophys. Res. Commun. 222:774–779 [DOI] [PubMed] [Google Scholar]
- 22. Oyarzabal OA. 2005. Reduction of Campylobacter spp. by commercial antimicrobials applied during the processing of broiler chickens: a review from the United States perspective. J. Food Prot. 68:1752–1760 [DOI] [PubMed] [Google Scholar]
- 23. Padan E, Bibi E, Ito M, Krulwich TA. 2005. Alkaline pH homeostasis in bacteria: new insights. Biochim. Biophys. Acta 1717:67–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Padan E, Kozachkov L, Herz K, Rimon A. 2009. NhaA crystal structure: functional-structural insights. J. Exp. Biol. 212:1593–1603 [DOI] [PubMed] [Google Scholar]
- 25. Pages JM, Amaral L. 2009. Mechanisms of drug efflux and strategies to combat them: challenging the efflux pump of Gram-negative bacteria. Biochim. Biophys. Acta 1794:826–833 [DOI] [PubMed] [Google Scholar]
- 26. Park SF. 2002. The physiology of Campylobacter species and its relevance to their role as food-borne pathogens. Int. J. Food Microbiol. 74:177–188 [DOI] [PubMed] [Google Scholar]
- 27. Parrish JR, et al. 2004. High-throughput cloning of Campylobacter jejuni ORFs by in vivo recombination in Escherichia coli. J. Proteome Res. 3:582–586 [DOI] [PubMed] [Google Scholar]
- 28. Riedel CT, Brøndsted L, Rosenquist H, Haxgart SN, Christensen BB. 2009. Chemical decontamination of Campylobacter jejuni on chicken skin and meat. J. Food Prot. 72:1173–1180 [DOI] [PubMed] [Google Scholar]
- 29. Rosenquist H, Sommer HM, Nielsen NL, Christensen BB. 2006. The effect of slaughter operations on the contamination of chicken carcasses with thermotolerant Campylobacter. Int. J. Food Microbiol. 108:226–232 [DOI] [PubMed] [Google Scholar]
- 30. Sampathkumar B, Khachatourians GG, Korber DR. 2003. High pH during trisodium phosphate treatment causes membrane damage and destruction of Salmonella enterica serovar Enteritidis. Appl. Environ. Microbiol. 69:122–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sampathkumar B, Khachatourians GG, Korber DR. 2004. Treatment of Salmonella enterica serovar Enteritidis with a sublethal concentration of trisodium phosphate or alkaline pH induces thermotolerance. Appl. Environ. Microbiol. 70:4613–4620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sharma M, Beuchat LR. 2004. Sensitivity of Escherichia coli O157: H7 to commercially available alkaline cleaners and subsequent resistance to heat and sanitizers. Appl. Environ. Microbiol. 70:1795–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sigal N, Cohen-Karni D, Siemion S, Bibi E. 2006. MdfA from Escherichia coli, a model protein for studying secondary multidrug transport. J. Mol. Microbiol. Biotechnol. 11:308–317 [DOI] [PubMed] [Google Scholar]
- 34. Stabler RA, et al. 2006. Comparative phylogenomics of Clostridium difficile reveals clade specificity and microevolution of hypervirulent strains. J. Bacteriol. 188:7297–7305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taormina PJ, Beuchat LR. 2001. Survival and heat resistance of Listeria monocytogenes after exposure to alkali and chlorine. Appl. Environ. Microbiol. 67:2555–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Vliet AH, Ketley JM, Park SF, Penn CW. 2002. The role of iron in Campylobacter gene regulation, metabolism, and oxidative stress defense. FEMS Microbiol. Rev. 26:173–186 [DOI] [PubMed] [Google Scholar]
- 37. Whyte P, Collins JD, McGill K, Monahan C, O'Mahony H. 2001. Quantitative investigation of the effects of chemical decontamination procedures on the microbiological status of broiler carcasses during processing. J. Food Prot. 64:179–183 [DOI] [PubMed] [Google Scholar]
- 38. Wiesner RS, Hendrixson DR, DiRita VJ. 2003. Natural transformation of Campylobacter jejuni requires components of a type II secretion system. J. Bacteriol. 185:5408–5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.