Abstract
Listeria monocytogenes is an important food-borne pathogen whose ability to form disinfectant-tolerant biofilms on a variety of surfaces presents a food safety challenge for manufacturers of ready-to-eat products. We developed here a high-throughput biofilm assay for L. monocytogenes and, as a proof of principle, used it to screen an 80-compound protein kinase inhibitor library to identify molecules that perturb biofilm development. The screen yielded molecules toxic to multiple strains of Listeria at micromolar concentrations, as well as molecules that decreased (≤50% of vehicle control) or increased (≥200%) biofilm formation in a dose-dependent manner without affecting planktonic cell density. Toxic molecules—including the protein kinase C antagonist sphingosine—had antibiofilm activity at sub-MIC concentrations. Structure-activity studies of the biofilm inhibitory compound palmitoyl-d,l-carnitine showed that while Listeria biofilm formation was inhibited with a 50% inhibitory concentration of 5.85 ± 0.24 μM, d,l-carnitine had no effect, whereas palmitic acid had stimulatory effects. Saturated fatty acids between C9:0 and C14:0 were Listeria biofilm inhibitors, whereas fatty acids of C16:0 or longer were stimulators, showing chain length specificity. De novo-synthesized short-chain acyl carnitines were less effective biofilm inhibitors than the palmitoyl forms. These molecules, whose activities against bacteria have not been previously established, are both useful probes of L. monocytogenes biology and promising leads for the further development of antibiofilm strategies.
INTRODUCTION
Among the key issues in the food industry is the prevention of the proliferation of food-borne pathogens, including Listeria monocytogenes, on food contact surfaces and ready-to-eat products. Once ingested, L. monocytogenes can surmount three biological obstacles: the blood-brain barrier, the maternal-fetal barrier, and the intestinal barrier (8, 45), leading to complications such as gastroenteritis, meningitis, still-birth, or spontaneous abortions (3, 41, 45). In addition, L. monocytogenes can form mono- or multispecies biofilm communities on inert surfaces (6, 20). L. monocytogenes biofilms can be found in a variety of sites in food-processing facilities. The biofilms are highly resistant to UV light, desiccation, and sanitizing chemicals typically used for sterilization, providing opportunity for spread of L. monocytogenes to food (13, 27, 53). The addition of nisin, potassium/sodium lactate, and sodium acetate/diacetate to packaging material and/or food products to prevent the growth of L. monocytogenes has not eradicated infection, as demonstrated by the frequent recalls of L. monocytogenes-contaminated food products in North America (1, 14, 37a, 37b, 51, 53) and outbreaks of listeriosis in Europe (3). To identify new ways of preventing food product contamination, it is necessary to better understand the mechanisms underlying L. monocytogenes biofilm development.
A limited number of factors required for L. monocytogenes biofilm formation have been identified (recently reviewed by Renier et al. [39]). Biofilm development begins with initial attachment (reversible, then irreversible) to a surface, with the activation of genes involved in attachment, surface protein expression, and extracellular polysaccharide (EPS) production. Further development from small microcolonies to mature biofilms is typically controlled by quorum sensing (17, 39, 40). Lastly, biofilm dispersal can result from shear forces, depletion of nutrients and accumulation of waste products. Degradation of the EPS matrix and/or upregulation of motility (26) allows dispersed cells to attach to a new site or to existing biofilms to restart the cycle.
Many studies aimed at identifying pathways involved in biofilm formation have used genetic approaches, such as screening mutant libraries for those defective in biofilm formation (30, 32, 35, 52). Although genetic approaches are useful, disadvantages include the difficulty of creating mutants in species not amenable to genetic manipulation and the under-representation of mutations in essential genes. The stresses imposed by some lesions can lead to downstream effects, including the accumulation of suppressor mutations (2, 49). In contrast, small molecules provide a way to conditionally inhibit (or stimulate) function—even that of essential targets—over a range of concentrations, potentially providing novel insights into biological pathways. However, many small molecules inhibit more than a single cellular process (36), and identifying their targets can be challenging (7).
Given the number of potential pathways and genes contributing to biofilm development, we reasoned that use of small molecules as reagents to manipulate biofilm formation was warranted. Many studies have shown that it is possible to reduce the formation of food pathogen biofilms using specific small molecule food additives. In the presence of sodium levulinate, sodium lactate, or fatty acids, the growth of L. monocytogenes on ready-to-eat food was inhibited (46, 53). In addition, thyme essential oils, culinary herb extracts, or high-molecular-weight extracellular DNA can prevent adhesion of cells to a surface (21, 38, 44). Low concentrations of EDTA reduced initial cell attachment of L. monocytogenes to polyvinyl chloride without affecting planktonic cell density and inhibited cell-to-cell interactions (12). Quorum sensing in L. innocua was inhibited by natural compounds such as ambuic acid through repression of peptide biosynthesis (34). Thus, natural compounds or small molecules that target mechanisms involved in biofilm formation could be used to prevent their formation on food-contact surfaces.
In this work, we optimized a high-throughput biofilm assay for L. monocytogenes to make it suitable for small molecule screening, and as a proof of principle, used it to test the effects of a collection of 80 eukaryotic protein kinase inhibitors on biofilm development. We reasoned that such molecules, which are largely based on chemical scaffolds that interact with the ATP-binding site of kinases and thus have the potential to interact with many proteins, may have unexpected activity in biofilm biology. We hypothesized that the use of molecules with known modes of action could provide useful clues to aid in identifying the targets of those with effects on L. monocytogenes biofilm formation.
Several molecules that altered biofilm development in a dose-dependent manner were identified, including the inhibitors sphingosine and palmitoyl-d,l-carnitine, both characterized by polar head groups and saturated 16-carbon acyl chains. Structure-activity studies using saturated fatty acids of defined acyl chain length showed that those from C9:0 to C14:0 were effective biofilm inhibitors with activity in the low micromolar range, while those from C16:0 to C18:0 stimulated biofilm formation. The inhibitory effects of select compounds on L. monocytogenes biofilm development on food-grade stainless steel were confirmed using scanning electron microscopy. We demonstrate that sphingosine and palmitoyl-d,l-carnitine also inhibit Staphylococcus aureus biofilm formation, showing that they have activity against other Gram-positive pathogens. These small molecules are useful tools for characterizing the process of Listeria biofilm development and promising lead compounds for new antibiofilm strategies.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
L. monocytogenes food isolates belonging to serotypes 1/2a and1/2b were provided by Burton Blais of the Canadian Food Inspection Agency (CFIA; Ottawa, Ontario, Canada), and L. monocytogenes 568 (serotype 1/2a) was a gift from Lisbeth Truelstrup-Hansen (Dalhousie University). S. aureus 15981 was a gift from Julian Davies (University of British Colombia). The glycerol stocks of L. monocytogenes and S. aureus were stored at −80°C prior to streaking them onto Difco tryptic soy agar (BD Biosciences), and LB-agar (BioShop), respectively, and incubated at 37°C overnight. After incubation, L. monocytogenes strains were used to inoculate 10 ml of tryptic soy broth (TSB; EMD Chemicals) at 37°C with agitation overnight. The overnight cultures were diluted in TSB to standardize the cultures to obtain an optical density at 600 nm (OD600) of ∼0.03 (Thermo Scientific BioMate3). S. aureus 15981 was inoculated in 5 ml of 66% TSB plus 0.2% dextrose overnight, with agitation at 37°C. After incubation, the culture was standardized to an OD600 of ∼0.8 and subsequently diluted 1:200 in 25% TSB plus 0.2% dextrose prior to setting up the biofilm assay.
Preparation of test compounds.
Compounds used for the present study were the Screen-Well kinase inhibitor library (ENZO Life Sciences), palmitoyl-d-carnitine hydrochloride (Crystal Chem, Inc.), palmitoyl-d,l-carnitine hydrochloride, palmitoyl-l-carnitine hydrochloride, d,l-carnitine hydrochloride, myristoyl-d,l-carnitine hydrochloride, saturated fatty acids (C9:0 to C18:0) (all from Sigma-Aldrich), and ZM 449829 (Tocris). Stock solutions (≥10 mM in dimethyl sulfoxide [DMSO]; Caledon) were stored at −20°C and diluted in DMSO for the initial test concentrations (≤100 μM).
Determination of MICs.
L. monocytogenes strains were inoculated overnight at 37°C in 10 ml of TSB with agitation at 200 rpm. The overnight cultures were standardized to an OD600 of ∼0.05 (4.3 × 107 CFU ml−1) in TSB. S. aureus 15981 was inoculated in 5 ml of 66% TSB (2/3 strength of the manufacturer's recommendation) plus 0.2% dextrose and then incubated overnight at 37°C and 200 rpm. After incubation, the culture was standardized to an OD600 of ∼0.05 (5.7 × 106 CFU ml−1) in 25% TSB plus 0.2% dextrose. The initial test concentrations of the compounds were diluted (1:100) in the culture (1 μl of compound in 99 μl of culture) and incubated at 37°C. The cultures were monitored at 24 and 48 h, and the lowest concentration resulting in no growth after 48 h compared to the control samples was defined as the MIC for L. monocytogenes 568, 1/2a, and 1/2b and S. aureus 15981. MIC determination did not follow the standard Clinical and Laboratory Standards Institute MIC guidelines because the cells did not grow in Mueller-Hinton broth (MHB), normally used for MIC determination, to an OD that was different from MHB sterility control.
L. monocytogenes and S. aureus biofilm assays.
L. monocytogenes 568 was inoculated in 10 ml of TSB at 37°C overnight, with shaking at 200 rpm, and subsequently standardized to an OD600 of ∼0.03 in TSB. The initial test concentrations of the compounds were diluted (1:100) in standardized culture (1.5 μl of compound in 148.5 μl of culture). Control wells contained TSB plus 1% DMSO as a sterility control or standardized overnight culture plus 1% DMSO as a growth control. To prevent plate edge effects due to dehydration, the wells at the periphery of the plate were inoculated with 150 μl of sterile distilled H2O (dH2O). Biofilms were grown on polystyrene peg lids (Nunc), a method that produced more reproducible biofilms compared to using the surfaces of the wells. After placement of the peg lid, the plate was sealed with parafilm to prevent evaporation and incubated for 24 h at 37°C, 200 rpm. After 24 h of incubation, the planktonic growth was measured at OD600, and the lid was transferred to a new microtiter plate with the same layout of TSB plus 1% DMSO, TSB with compounds, and water. The plate was resealed with parafilm and incubated at 37°C, with shaking at 200 rpm, for 24 h. This step was repeated again for a total incubation time of 72 h (total of three passages, once every 24 h).
To quantify the amount of biofilms formed on the lid, the 96-peg lid was stained with crystal violet (CV) using a modified protocol (24). After 72 h, the lid was transferred to a new microtiter plate containing 200 μl of 1× phosphate-buffered saline (PBS) per well for 10 min to wash off any loosely adherent bacterial cells. After the wash step, the lid was transferred to a microtiter plate filled with 200 μl of 0.1% (wt/vol) CV for 15 min. To wash off the excess CV, the lid was washed with 70 ml of dH2O, in a single well tray, for 10 min. This step was repeated four times to ensure complete removal of excess CV. To solubilize the CV, the lid was transferred to a 96-well plate containing 200 μl of 95% ethanol or 33% (vol/vol) acetic acid per well for 15 min. The absorbance of the eluted CV was measured at 600 nm (BioTek ELx800).
The S. aureus 15981 biofilm assay was set up with the test compounds in the same manner as for the L. monocytogenes 568 assay, but biofilms were formed directly on the walls of each well of the 96-well plate. The control wells were filled with either (i) standardized culture plus 1% DMSO or (ii) 25% TSB plus 0.2% dextrose plus 1% DMSO, and then 150 μl of water was added to the wells at the periphery to prevent edge effects. The 96-well plate was covered with a MicroWell lid (Nunc), sealed with parafilm, and incubated for 8 h at 37°C without agitation. After incubation and prior to staining of the biofilms, the OD600 of the planktonic culture was measured. The culture was removed from the wells, and the wells were washed with 200 μl of 1× PBS for 5 min. This step was repeated prior to staining the wells with 200 μl of 0.1% CV for 15 min. The wells were washed with excess dH2O to remove unbound CV and air dried in an inverted position for 30 min. Afterward, 200 μl of 95% ethanol was added to the wells and incubated for 15 min at room temperature to elute bound CV, followed by measuring the absorbance of eluted CV at 600 nm.
The planktonic density and CV absorbance data were generated using GraphPad Prism 5 (GraphPad Software, Inc.). The IC50s, defined as the half-maximal inhibitory concentration at which biofilm formation was inhibited by 50% compared to vehicle control, were calculated using GraphPad Prism 5 or GraFit 4 (Erithacus Software, Ltd.). Statistical values (P values) were calculated using a one-way analysis of variance (ANOVA) test and Dunnett's post test on GraphPad Prism.
Scanning electron microscopy of L. monocytogenes 568 on stainless steel.
L. monocytogenes 568 biofilms were grown on food-grade, type 304H stainless-steel coupons (1 by 0.5 cm; Storm Copper Components Co.) in the absence (TSB plus 1% DMSO) or presence of select compounds (sphingosine and palmitoyl-d,l-carnitine) at the indicated concentrations. Stainless steel coupons were placed in the wells of a 96-well plate with 200 μl of medium, and the plate was covered with a MicroWell lid. After 72 h (three passages, once every 24 h, as described above), the coupons were rinsed with PBS as described above and fixed in 2% glutaraldehyde (2% [vol/vol]) in 0.1 M sodium cacodylate buffer (pH 7.4; primary fixative) overnight. The coupons were then rinsed twice in buffer solution and postfixed for 1 h in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer. After the second fixation step, the samples were dehydrated through a graded ethanol series (50, 70, 70, 95, 95, 100, and 100%) and then transferred to the critical point dryer and allowed to dry. The coupons were mounted onto scanning electron microscopy (SEM) stubs, sputter coated with gold, and viewed under the VEGA/TESCAN LSU SEM. The images were acquired at ×5,000 magnification using the VEGA/TESCAN software.
Synthesis of acyl carnitines.
The synthesis of acyl carnitines was carried out using a modified version of the procedure described by Cervenka et al. (11). Briefly, 2.2 mM carbonylimidazole was added to a 2 mM solution of fatty acid in anhydrous toluene (1 ml). Activation was carried out until no more starting material could be detected using liquid chromatography-mass spectrometry (LC/MS). Then, 2 ml of carnitine perchloride (prepared as described in the reference above) was added to the reaction mixture, followed by triethylamine (0.2 ml). The reaction was carried out for 1 to 2 days at 45°C. The progress of the reaction was monitored using LC/MS. At the end of the reaction time, 2 ml of methanol was added, and the solvent was removed under reduced pressure. The remaining oily residue was extracted twice with 5 ml of hexane. The hexane was discarded, and the remaining oil was dissolved in 1 ml of methanol and further extracted twice with 10 ml of hexane. The hexane was discarded, and the methanol was removed under vacuum. The remaining liquid was dissolved in 1 ml of 5% acetic acid and purified using a reverse-phase Sep-Pak cartridge. The final products were eluted with 5-ml aliquots of water-methanol, and the product's purity was confirmed using LC/MS, high-resolution mass spectrometry (HRMS), and one-dimensional (1D) and 2D nuclear magnetic resonance (NMR) experiments.
LC-ESI-MS analysis.
Liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) data were obtained by using an Agilent 1100 Series LC system (Agilent Technologies Canada, Inc.) and a QTRAP LC/MS/MS system (Applied Biosystems). Analytical reversed-phase high-pressure liquid chromatography was performed using a C18 column (Sunfire; 5 μm, 4.6 by 50 mm; Waters) and a Agilent 1100 binary gradient pump system at a flow rate of 1 ml/min, under the following conditions: isocratic 5% solvent B (0.05% formic acid in acetonitrile) and 95% solvent A (0.05% formic acid in water) for 1 min, followed by a linear gradient to 97% solvent B over 7 min.
ESI experiments were performed on a using a Thermo Fisher LTQ-XL-Orbitrap Hybrid mass spectrometer (Thermo Fisher, Bremen, Germany), equipped with an electrospray interface operated in positive ion mode. Sample solution was directly infused into the mass spectrometer at a flow rate of 5 μl/min. The ESI source and MS parameters were automatically optimized and saved in a tune file for the base peak in the mass spectrum. Positive ESI source conditions included a sheath gas flow rate of 15 arbitrary units (AU), auxiliary gas flow rate of 5 AU, an ion spray voltage at 3.9 kV, a capillary temperature of 200°C, a capillary voltage of 23 V, and a tube lens voltage of 70 V. Normalized collision energy was 35%. Helium was used as the collision gas. The LTQ-XL-Orbitrap mass spectrometer experiment was set to perform a FT full scan from 100 to 2,000 m/z with the resolution set at 100,000 (at 500 m/z), followed by linear ion trap tandem MS (MS/MS) scans on the top three ions. Dynamic exclusion was set to 2, and selected ions are placed on an exclusion list for 30 s. The lock-mass option was enabled for the FT full scans using the ambient air polydimethylcyclosiloxane (PCM) ion of m/z = 445.120024 or a common phthalate ion m/z = 391.284286 for real-time internal calibration.
1D and 2D NMR.
1D (1H and 13C) and 2D NMR experiments (correlation spectroscopy, heteronuclear single-quantum correlation spectroscopy, and heteronuclear multiple-bond correlation spectroscopy) were carried out using a Bruker AVIII 700 MHz instrument in methanol-d4. Chemical shifts are reported in ppm relative to tetramethylsilane (TMS) using the residual solvent signals at 3.30 and 49.00 ppm as internal references for the 1H and 13C spectra, respectively. The coupling constants (J) are reported in Hz.
Compound data. (i) (3-Carboxy-2-nonanoyloxy-propyl)-trimethyl-ammonium.
HRMS ES+: C16H32NO4+, calculated 302.2326, found 302.2329. 1H-NMR: 5.59 (m, 1H); 3.72 (m, 2H); 3.16 (s, 9H); 2.62 (dd, 1H, J1 = 4.4, J2 = 4.04); 2.41 (dd, 1H, J1 = J2 = 9.6); 2.35 (m, 2H); 1.61 (p, 2H, J1 = J2 = J3 = J4 = 7.09); 1.31 (m, 10H), 0.96 (t, 3H, J1 = J2 = 7.07). 13C-NMR: 176.43; 174.12; 70.01; 67.82; 54.55; 35.23; 33.05; 30.37; 30.28; 30.22; 25.76; 23.70; 14.41.
(ii) (3-Carboxy-2-decanoyloxy-propyl)-trimethyl-ammonium.
HRMS ES+: C17H34NO4+, calculated 316.2482, found 316.2480. 1H-NMR: 5.59 (m, 1H); 3.71 (m, 2H); 3.16 (s, 9H); 2.61 (dd, 1H, J1 = 4.1, J2 = 4.2); 2.34–2.41 (m, 3H); 1.61 (p, 2H, J1 = J2 = J3 = J4 = 7.1); 1.30 (m, 12H), 0.89 (t, 3H, J1 = J2 = 7.1). 13C-NMR: 175.89; 174.13; 70.02; 67.93; 54.51; 35.22; 33.04; 30.56; 30.42; 30.22; 25.77; 23.74; 14.45.
(iii) (3-Carboxy-2-dodecanoyloxy-propyl)-trimethyl-ammonium.
HRMS ES+: C19H38NO4+, calculated 344.2795, found 344.2790. 1H-NMR: 5.60 (m, 1H); 3.79 (m, 1H); 3.69 (m, 1H); 3.17 (s, 9H); 2.67 (dd, 1H, J1 = J2 = 4.4); 2.54 (dd, 1H, J1 = J2 = 8.5); 2.36 (t, 2H, J1 = J2 = 7.6); 1.61 (p, 2H, J1 = J2 = J3 = J4 = 7.1); 1.29 (m, 16H), 0.89 (t, 3H, J1 = J2 = 7.1). 13C-NMR: 175.96; 174.15; 69.78; 67.17; 54.56; 35.17; 33.10; 30.73; 30.58; 30.46; 30.39; 30.19; 25.75; 23.74; 14.43.
RESULTS
Identification of small-molecule modulators of L. monocytogenes biofilm formation.
Systematic optimization of the medium and growth conditions for the L. monocytogenes biofilm assay resulted in Z′ scores of ≥0.60, making the assay suitable for high-throughput screening (50, 58). A high-quality assay is defined as 0.5 ≤ Z < 1 (50, 58). A pilot screen using the 80-compound Screen-Well kinase inhibitor library at an initial concentration of 50 μM was performed in duplicate. We identified 23 compounds that reproducibly altered L. monocytogenes 568 planktonic cell density and/or biofilm development compared to the vehicle control (Table 1). Five compounds were planktonic growth inhibitors, fifteen compounds inhibited biofilm formation (defined as ≤50% of vehicle-treated control) but not planktonic cell density, and three compounds stimulated biofilm formation (≥200% compared to the vehicle-treated control) without affecting planktonic cell density (Table 1). The MICs of the planktonic growth inhibitors for L. monocytogenes 568 (serotype 1/2a), as well as food isolates belonging to serotypes 1/2a and 1/2b were determined. All three strains of L. monocytogenes had identical MICs for each test compound. Sphingosine had the lowest MIC of 12.5 μM, followed by rottlerin and tyrphostin 9, with MICs of 25 μM. Both GW 5074 and BAY 11-7082 had MICs of 50 μM. To determine whether the planktonic growth inhibitors had activity against other Gram-positive bacteria, they were tested against S. aureus 15981. Sphingosine, rottlerin, and tyrphostin 9 had MICs of 12.5 μM, whereas both GW 5074 and BAY 11-7082 had MICs of 25 μM.
Table 1.
Compounds that modulate L. monocytogenes biofilm development
| Functiona | Compounds |
|---|---|
| Planktonic growth inhibitors | GW 5074, tyrphostin 9, sphingosine, rottlerin, BAY 11-7082 |
| Biofilm inhibitors | U-0126, LFM-A13, SB-202190, BML-257, AG-490, AG-879, ZM 449829, KN-93, staurosporine, hypericin, SP 600125, Ro 31-8220, palmitoyl-d,l-carnitine, indirubin, indirubin-3′-monoxime |
| Biofilm stimulators | Kenpaullone, KN-62, PKC-412 |
Planktonic growth inhibitors were defined as compounds that reduced growth by ≤50% of vehicle control growth at the initial concentration of 50 μM. Biofilm inhibitors were identified as compounds that reduced biofilm formation by ≤50% of vehicle control without affecting planktonic cell density. Biofilm stimulators were defined as those compounds that increased biofilm formation by ≥200% compared to vehicle control without affecting planktonic cell density.
Inhibition of biofilm formation by planktonic growth inhibitors at sub-MIC concentrations.
Compounds that were toxic in the initial screen were tested for their ability to inhibit biofilm formation at sub-MIC concentrations (Table 2). At 3.1 μM, a concentration that does not decrease planktonic cell density, sphingosine reduced biofilm formation to ∼30% of control (Fig. 1A). GW 5074 (IC50 of 3.78 ± 0.16 μM) and BAY 11-7082 (IC50 of 4.12 ± 0.27 μM) were more effective than tyrphostin 9 (IC50 of 4.77 ± 0.86 μM) at inhibiting L. monocytogenes 568 biofilm development. Whereas the planktonic cell density of GW 5074-treated cells increased up to 6.3 μM, then decreased beyond, the planktonic cell density of tyrphostin 9-treated cultures decreased slightly with increasing concentrations (see Fig. S1 in the supplemental material). The planktonic cell density of BAY 11-7082 was unaffected at low concentrations (≤12.5 μM) (see Fig. S1 in the supplemental material). Rottlerin was tested at sub-MIC concentrations; however, the results were not reproducible (data not shown).
Table 2.
Half-maximal inhibitory concentrations of planktonic growth inhibitors and biofilm inhibitors on biofilm formation
| Test compound | Structure | L. monocytogenes IC50 (μM)a |
|---|---|---|
| Sphingosine | 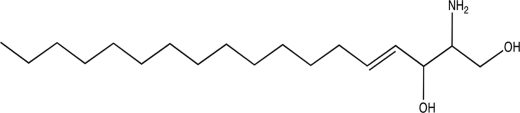 |
2.81 ± 0.21 |
| GW 5074 | 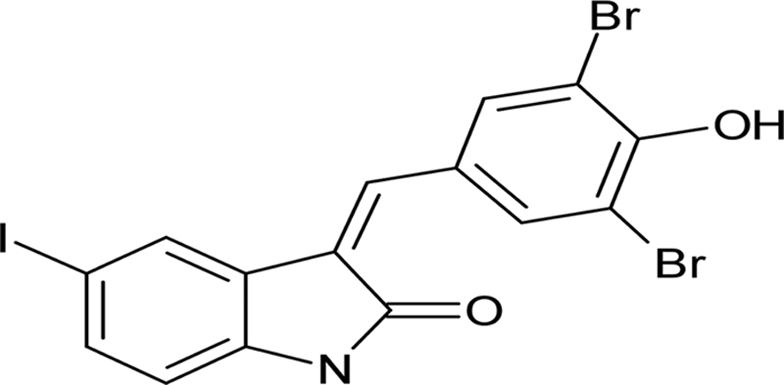 |
3.78 ± 0.16 |
| BAY 11-7082 | 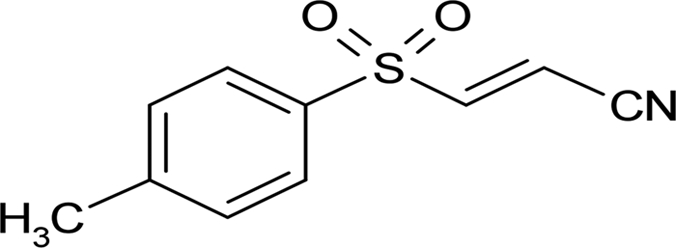 |
4.12 ± 0.27 |
| Tyrphostin 9 | 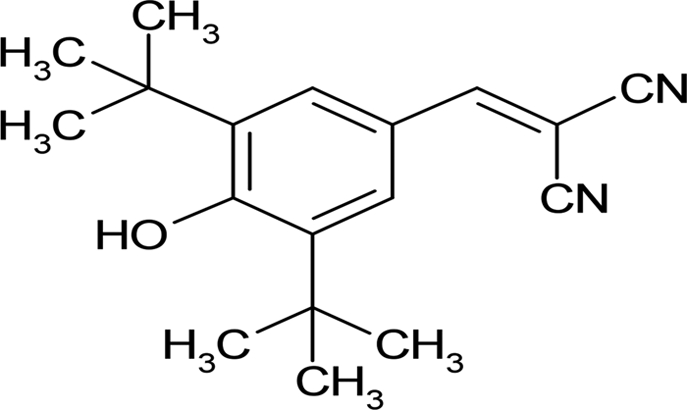 |
4.77 ± 0.86 |
| LFM-A13 | 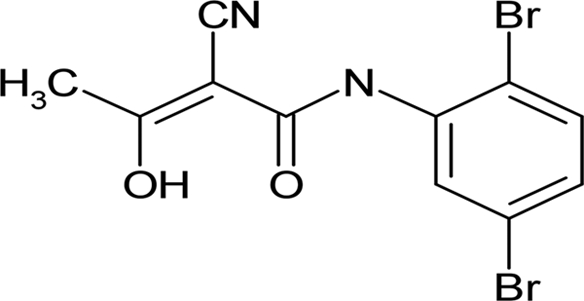 |
3.76 ± 0.16 |
| SP 600125 | 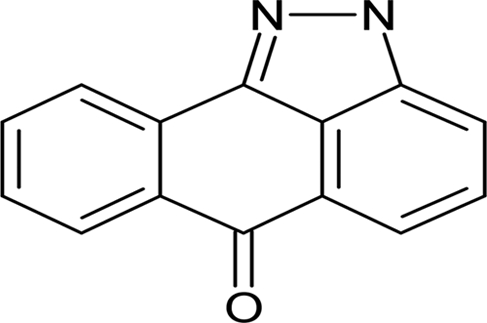 |
5.10 ± 0.36 |
| ZM 449829 | 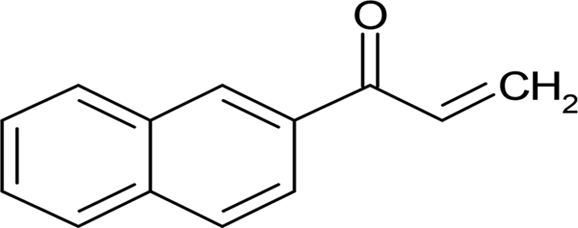 |
5.57 ± 0.24 |
| Staurosporine | 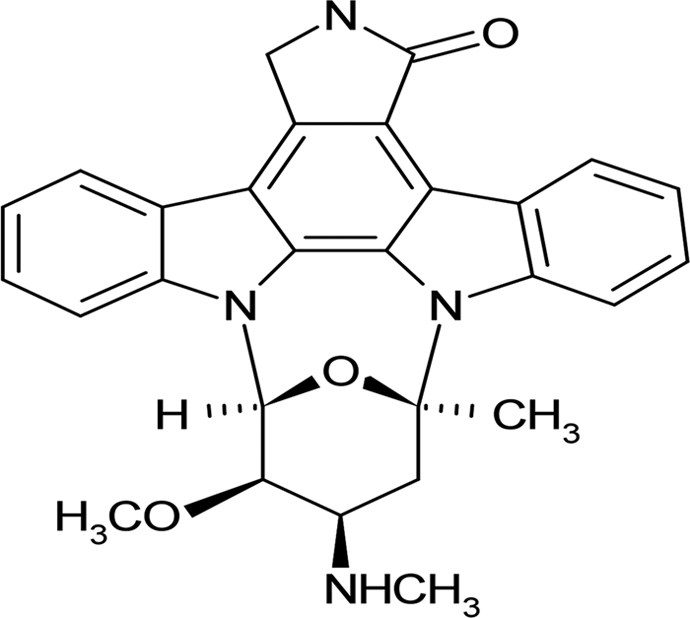 |
9.64 ± 1.95 |
| Ro 31-8220 | 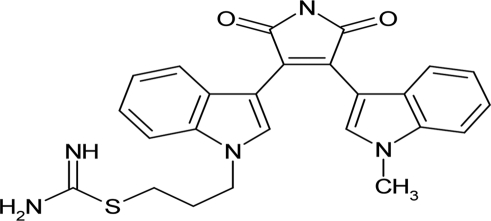 |
22.1 ± 2.36 |
| Indirubin-3′-monoxime | 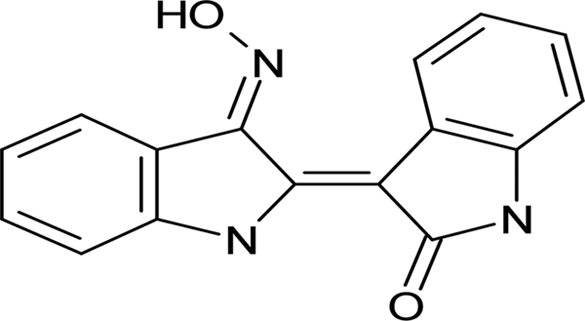 |
22.3 ± 2.43 |
| AG-879 | 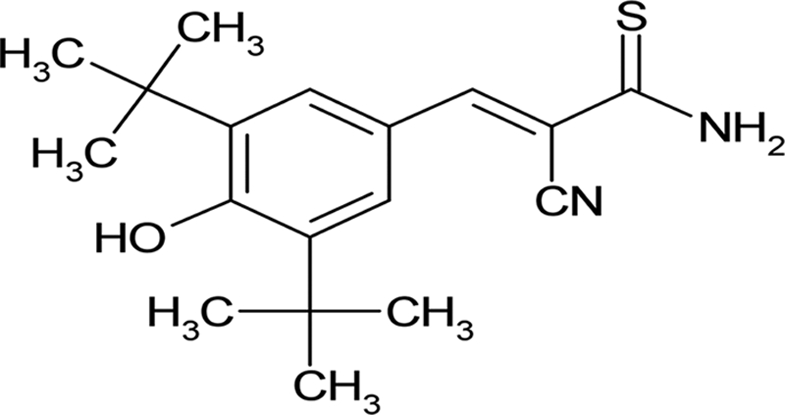 |
29.0 ± 4.04 |
That is, the concentrations at which biofilm formation was inhibited by 50% compared to the vehicle control.
Fig 1.
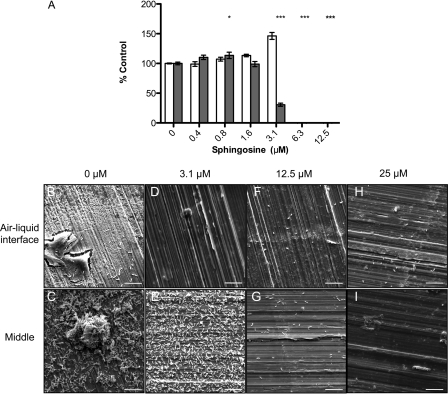
Inhibition of biofilm formation on different surfaces by sphingosine. (A) L. monocytogenes biofilms were grown on polystyrene pegs and quantified using crystal violet staining. Planktonic growth at day 3 (□) and biofilm formation (▩), expressed as a percentage of control (n = 4, with the standard deviations [SD] shown). *, P < 0.01; **, P < 0.001; ***, P < 0.0001. (B to I) Representative SEM images of L. monocytogenes grown on food-grade stainless-steel coupons in the presence or absence of sphingosine at various concentrations. Images were captured near the air-liquid interface and the middle of coupons, where they were submerged in media. Bar, 10 μm. Magnification, ×5,000.
Effects of L. monocytogenes biofilm inhibitors.
Seven of the most effective biofilm inhibitors (Table 2) and palmitoyl-d,l-carnitine (Table 3) identified in the initial screen were further tested in a dose-response assay. The compounds LFM-A13, SP 600125, ZM 449829, and Ro 31-8220 inhibited biofilm formation in a dose-dependent manner (see Fig. S1 in the supplemental material; Table 2). SP 600125 (IC50 of 5.10 ± 0.36 μM) did not affect planktonic cell density, whereas a decrease in planktonic cell density occurred with increasing concentrations of LFM-A13 (IC50 of 3.76 ± 0.16 μM) and Ro 31-8220 (IC50 of 22.1 ± 2.36 μM). Increasing concentrations of ZM 449829 (up to 50 μM) (IC50 of 5.57 ± 0.24 μM) resulted in a dose-dependent increase in planktonic cell density, but a decrease in biofilm formation (see Fig. S1 in the supplemental material), which could indicate repartitioning of cells from the biofilm into the planktonic phase. Both biofilm development and planktonic growth were inhibited at 100 μM.
Table 3.
Half-maximal inhibitory concentrations of carnitine and acylcarnitines on biofilm formation
| Test compound | Structure | L. monocytogenes IC50 (μM)a |
|---|---|---|
| Nonanoyl-d,l-carnitine | 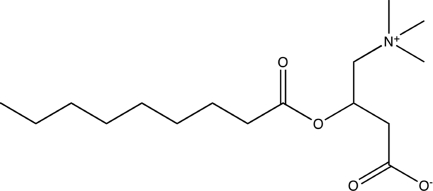 |
57.1 ± 9.61* |
| Decanoyl-d,l-carnitine | 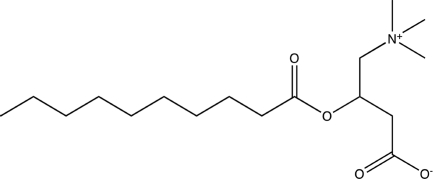 |
78.6 ± 21.2* |
| Lauroyl-d,l-carnitine | 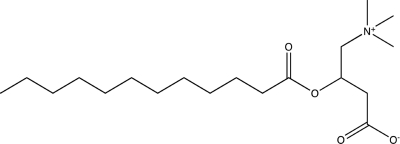 |
10.1 ± 0.75 |
| Myristoyl-d,l-carnitine | 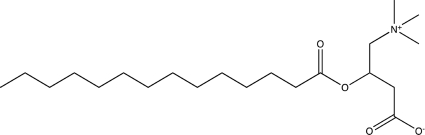 |
17.4 ± 2.13 |
| Palmitoyl-d,l-carnitine | 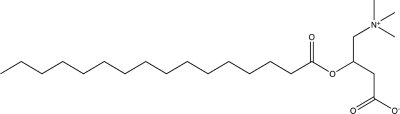 |
5.85 ± 0.24 |
| d,l-Carnitine | 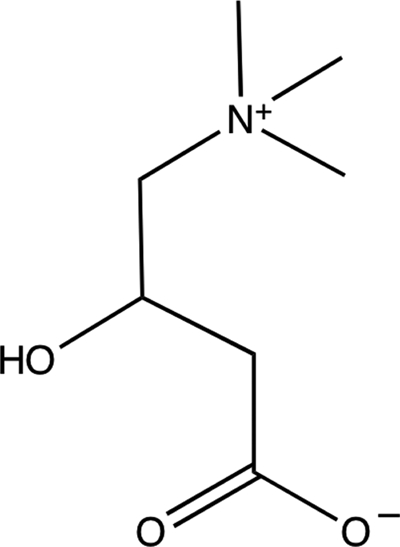 |
Minimal effect |
That is, the concentrations at which biofilm formation was inhibited by 50% compared to vehicle control samples.
, Nonideal behavior, since the data do not go to 0% of the control at highest concentration tested (50 μM).
Indirubin-3′-monoxime inhibited biofilm formation in a dose-dependent manner without affecting planktonic cell density (IC50 of 22.3 ± 2.36 μM). However, at 3.1 μM (the lowest concentration tested) biofilm formation was stimulated (∼140% compared to control) (see Fig. S1 in the supplemental material). Similar results were seen with staurosporine where, at 3.1 μM, biofilm formation was stimulated (∼160% compared to control). Increasing concentrations of staurosporine inhibited biofilm formation without affecting planktonic cell density (IC50 of 9.64 ± 1.95 μM). AG 879, which is structurally related to the planktonic growth inhibitor tyrphostin 9, was less effective at inhibiting biofilm formation (IC50 of 29.0 ± 4.04 μM). With increasing concentrations of AG 879, biofilm formation was reduced, while complete inhibition occurred at 100 μM, likely as a result of a corresponding decrease in planktonic cell density (see Fig. S1 in the supplemental material).
Among the inhibitors identified in our initial screen was the acylated amino acid derivative, palmitoyl-d,l-carnitine, which is comprised of a long acyl-chain and polar head group, similar to sphingosine. Increasing concentrations of palmitoyl-d,l-carnitine (up to 50 μM) resulted in a dose-dependent reduction in biofilm formation with a concomitant increase in planktonic cell density (Table 3; Fig. 2A), similar to the effect of ZM 449829. Even at the lowest concentration tested (3.1 μM), biofilm formation was ∼60% of the control. At 100 μM, both biofilm formation and planktonic growth were inhibited. Because both palmitoyl-d,l-carnitine and sphingosine were effective biofilm inhibitors at sub-MIC concentrations and structurally related, we elected to further investigate their effects on biofilm formation.
Fig 2.
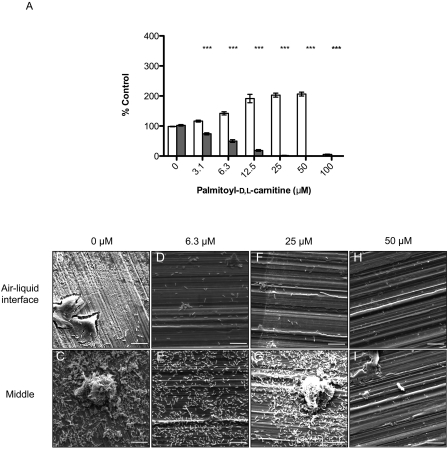
Palmitoyl-d,l-carnitine inhibits L. monocytogenes biofilm formation. (A) L. monocytogenes biofilms were grown on polystyrene pegs using the microtiter assay and quantified with crystal violet staining. Planktonic growth at day 3 (□) and biofilm formation (▩) expressed as a percentage of control (n = 4 with the SD shown). *, P < 0.01; **, P < 0.001; ***, P < 0.0001. (B to I) Representative SEM images of L. monocytogenes grown on food-grade stainless-steel coupons in the absence (1% DMSO) or presence of palmitoyl-d,l-carnitine. Images captured near the air-liquid interface and areas where coupons were submerged in media. Bar, 10 μm. Magnification, ×5,000.
Sphingosine inhibits L. monocytogenes biofilm formation in a concentration-dependent manner.
Sphingosine inhibited L. monocytogenes biofilm formation on polystyrene at sub-MIC concentrations (Fig. 1A). To determine whether the results were independent of the substratum on which biofilms were formed, L. monocytogenes biofilms were also grown on food-grade, type 304H stainless-steel coupons, with or without sphingosine. In the presence of 1% DMSO vehicle control (Fig. 1B and C), many cells adhered to the stainless-steel surface, with some cells in multilayered microcolonies. Interestingly, planktonic cell density of L. monocytogenes was not inhibited at the concentrations that were effective in the polystyrene peg-lid biofilm assay, even when the highest concentration tested was doubled to 25 μM (data not shown). However, in the presence of 3.1 μM sphingosine, the pattern of adherence to stainless steel was altered, with few cells near the air-liquid interface (Fig. 1D), and more in regions where the coupons were submerged in medium (Fig. 1E). In addition, there were no discernible microcolonies present and many of the cells appeared to be shorter or damaged compared to vehicle-treated controls. Although planktonic growth was not inhibited at 12.5 μM in this assay, there was a substantial reduction in the amount of cells adhering throughout the stainless-steel coupons compared to the control samples (Fig. 1F and G). Similar results were obtained at 25 μM (Fig. 1H and I); very few cells attached to the coupons even though planktonic cell density was unaffected. Together, these data show that sub-MIC concentrations of sphingosine reduce L. monocytogenes biofilm formation on both plastic and stainless steel-surfaces and that there are substratum-related differences with respect to its effective concentration.
Palmitoyl-d,l-carnitine inhibits biofilm formation.
As shown in Fig. 2A, biofilm formation on polystyrene was inhibited by palmitoyl-d,l-carnitine. To examine substratum-related effects, biofilms were grown on stainless-steel coupons in the presence of various concentrations of palmitoyl-d,l-carnitine. At 6.3 μM, a reduced fraction of cells adhered to the stainless-steel coupons near air-liquid interface (Fig. 2D) compared to the control samples (Fig. 2B). In areas where the coupons were submerged in media, bacterial cell attachment appeared to be unaffected compared to the control (Fig. 2C and E). In contrast to the polystyrene biofilm assay, where 25 μM palmitoyl-d,l-carnitine blocked biofilm formation, attachment on stainless-steel was comparable to the vehicle control in submerged areas (Fig. 2G). At the air-liquid interface, few cells adhered to the surface compared to control samples (Fig. 2F). At 50 μM, no adherent cells were detected on the stainless-steel coupons, either at the air-liquid interface or below (Fig. 2H and 2I).
Structure-activity studies of palmitoyl-d,l-carnitine.
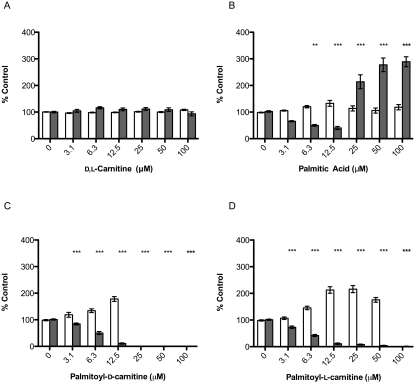
Specific d-amino acids were recently reported to act as small molecule signals to induce dispersal of Gram-positive biofilms (28), with a range of effective concentrations from 3 μM (d-methionine) to 8.5 mM (d-leucine). Based on that report, we hypothesized that the d-carnitine component of palmitoyl-d,l-carnitine might be responsible for its biofilm-inhibitory activity. d,l-Carnitine, palmitic acid, palmitoyl-d-carnitine, and palmitoyl-l-carnitine were tested separately for their effects on L. monocytogenes biofilm development. At concentrations where palmitoyl-d,l-carnitine inhibited biofilm development, neither planktonic cell density nor biofilm formation were affected by d,l-carnitine (Fig. 3A). In contrast to palmitoyl-d,l-carnitine, which inhibited biofilm formation and increased planktonic cell density in a dose-dependent manner (Fig. 2A), increasing concentrations of palmitic acid stimulated biofilm formation without significantly affecting planktonic cell density (≥25 μM) (Fig. 3B). Examination of enantiomer-specific inhibition of biofilm development by palmitoyl-d-carnitine and palmitoyl-l-carnitine showed that both compounds initially caused an increase in planktonic cell density at low micromolar concentrations, but the MIC for palmitoyl-d-carnitine was lower (25 μM) (Fig. 3C) than that of palmitoyl-d,l-carnitine (Fig. 2A) or palmitoyl-l-carnitine (Fig. 3D), both at 100 μM. Interestingly, even though the stereochemistry of palmitoyl-carnitine affects its ability to inhibit planktonic growth, its effect on biofilm development does not appear to be enantiomer-specific, because both palmitoyl-d-carnitine and palmitoyl-l-carnitine inhibited biofilm formation to the same extent as the parent compound (Fig. 3C and D). To examine the effect of acyl-chain length on activity, we synthesized additional acyl carnitines of specific chain length as described in Materials and Methods and tested their effects on biofilm formation. Nonanoyl-d,l-carnitine and decanoyl-d,l-carnitine had minimal effects on L. monocytogenes biofilm formation (Table 3). Lauroyl-d,l-carnitine and myristoyl-d,l-carnitine inhibited L. monocytogenes biofilm formation, but with reduced efficacy (IC50 of 10.1 ± 0.75 μM and 17.4 ± 2.13 μM, respectively) compared to palmitoyl-d,l-carnitine (IC50 of 5.85 ± 0.24 μM) (Table 3).
Fig 3.
Structure-activity studies of palmitoyl-d,l-carnitine. L. monocytogenes biofilms were grown on polystyrene pegs in the presence of d,l-carnitine (A), palmitic acid (B), palmitoyl-d-carnitine (C), or palmitoyl-l-carnitine (D). The amount of biofilm formed was quantified by crystal violet staining. Planktonic growth at day 3 (□) and biofilm formation (▩) are expressed as a percentage of control (n = 4). *, P < 0.01; **, P < 0.001; ***, P < 0.0001.
The effects of fatty acids on Listeria biofilm formation are chain length specific.
Because sphingosine and palmitoyl-d,l-carnitine had similar structures, with a charged head group coupled to a C16:0 acyl chain, we further examined the effects of saturated fatty acids on biofilm formation. Although some fatty acids impair the growth of L. monocytogenes (46, 48), their effects on biofilm formation have not been reported. Saturated fatty acids with chain lengths ranging from C9:0 to C18:0 were tested for their effects on biofilm development (Table 4). The short-chain-length fatty acids C9:0 and C10:0 were less effective at inhibiting biofilm formation compared to the medium-chain-length C12:0, C13:0, and C14:0. Planktonic cell density was unaffected by short- or medium-chain fatty acids at the concentrations tested, whereas C13:0 reduced growth at concentrations above 25 μM (data not shown). Similar to C16:0, both C17:0 and C18:0 stimulated biofilm formation at concentrations ≥25 μM but did not change planktonic cell density relative to the vehicle control (data not shown).
Table 4.
Half-maximal inhibitory concentrations of fatty acids on biofilm formation
| Test compound | Structure | IC50 (μM)a |
|
|---|---|---|---|
| L. monocytogenes | S. aureus | ||
| C9:0 | 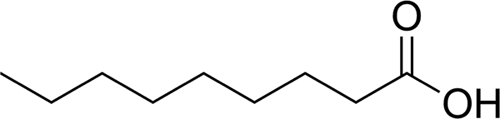 |
33.2 ± 4.67 | 4.75 ± 1.24 |
| C10:0 | 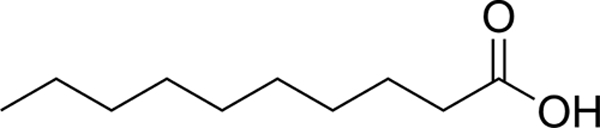 |
20.8 ± 2.11 | 7.81 ± 1.01 |
| C12:0 | 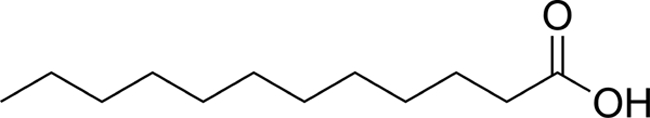 |
4.10 ± 0.27 | 5.81 ± 0.78 |
| C13:0 | 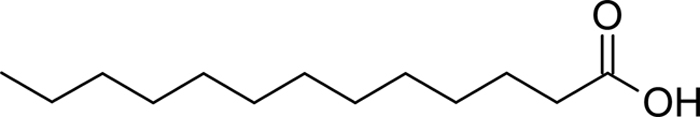 |
4.34 ± 0.23 | 6.53 ± 0.39 |
| C14:0 | 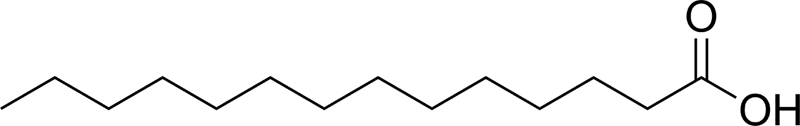 |
2.50 ± 0.26 | 6.38 ± 0.08 |
| C16:0 | 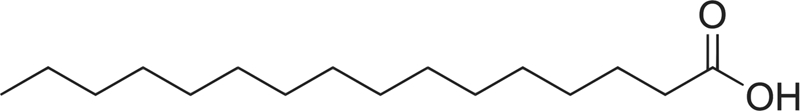 |
3.39 ± 1.95 | Stimulates |
| C17:0 | 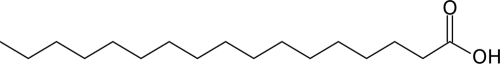 |
Stimulates | 13.8 ± 4.74 |
| C18:0 | 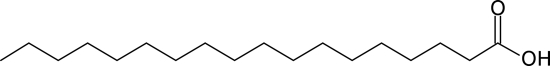 |
Stimulates | 4.02 ± 1.81 |
That is, the concentration at which biofilm formation was inhibited by 50% compared to vehicle control samples. “Stimulates” indicates that the IC50 was not detected at the concentrations tested; biofilm formation was >150% at the highest concentration tested (100 μM) compared to vehicle control samples.
Modulation of S. aureus biofilm formation by fatty acids and their derivatives.
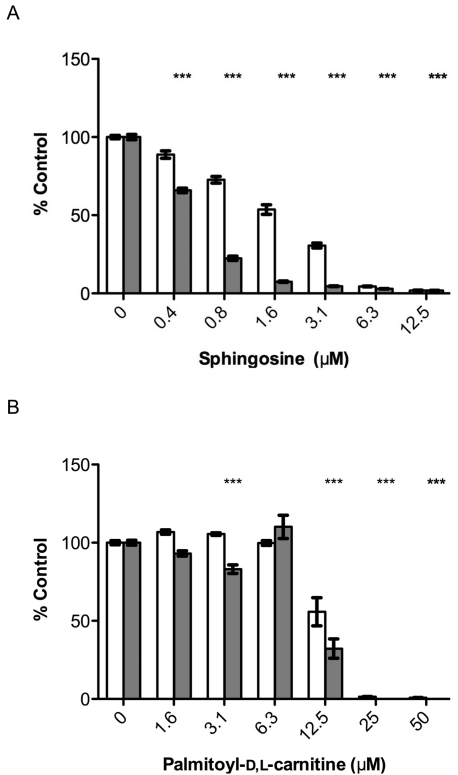
To determine whether the compounds identified as Listeria biofilm inhibitors had activity against other Gram-positive pathogens, specific compounds were tested against S. aureus. Both sphingosine and palmitoyl-d,l-carnitine were effective at reducing S. aureus growth and biofilm formation in a concentration-dependent manner (Fig. 4). Sphingosine had a lower effective concentration against S. aureus (IC50 of 0.49 ± 0.01 μM) than against L. monocytogenes (IC50 of 2.81 ± 0.21 μM).
Fig 4.
S. aureus biofilm development is inhibited by sphingosine and palmitoyl-d,l-carnitine. S. aureus biofilms were grown in microtiter plates in the presence of either sphingosine (A) or palmitoyl-d,l-carnitine (B). Crystal violet staining was used to quantify the amount of biofilm formed. Planktonic growth (□) and biofilm formation (▩) are expressed as a percentage of control samples (n = 3). *, P < 0.01; **, P < 0.001; ***, P < 0.0001.
Palmitoyl-d,l-carnitine was less effective at inhibiting S. aureus biofilm formation compared to that of L. monocytogenes. As shown in Fig. 4B, at low concentrations (1.6 to 6.3 μM), planktonic growth and biofilm formation were comparable to that of the control. At 12.5 μM, planktonic cell density was reduced (∼55% compared to the control), as was biofilm formation (∼30% compared to control). Higher concentrations resulted in complete inhibition of S. aureus planktonic growth. When the constituents of palmitoyl-d,l-carnitine were tested separately, d,l-carnitine had no effect (data not shown), while C16:0 stimulated biofilm formation at higher concentrations, similar to its effects on Listeria (≥25 μM) (Table 4). When the effects of saturated fatty acids on S. aureus were tested, acyl chain length dependency was observed (Table 4). Fatty acids from C9:0 to C18:0 inhibited biofilm formation; however, there was a decrease of at least 50% in planktonic cell density compared to the control at the highest concentration tested (100 μM), which was not observed for L. monocytogenes (data not shown). In contrast, C16:0 to C18:0 increased planktonic cell density with variable effects on S. aureus biofilm development. While C16:0 induced biofilm formation at higher concentrations, C17:0 and C18:0 inhibited biofilm formation. In addition, myristoyl-d,l-carnitine had little effect on biofilm formation, suggesting that specific combinations of acyl chain length and head group affect the potency of the fatty acids and their derivatives against different species.
DISCUSSION
L. monocytogenes biofilms are difficult to remove from industrial surfaces that may come into contact with ready-to-eat food products, leading to cross-contamination. We developed here an L. monocytogenes biofilm assay suitable for high-throughput screening and used it to identify small molecules that alter L. monocytogenes biofilm formation.
Similar to results reported for small molecule screens of Pseudomonas aeruginosa biofilms (24, 56), we identified both inhibitors and stimulators of biofilm formation. From our pilot screen of 80 kinase inhibitors, 19% reduced biofilm formation and 4% increased biofilm development, compared to <1% of stimulators and biofilm inhibitors that were identified from the screen of 66,095 compounds by Junker et al. (24). In a recent screen of the same 80-compound collection using P. aeruginosa, Wenderska et al. (56) found only two compounds (2.5%) that inhibited P. aeruginosa biofilm formation without affecting planktonic cell density. The differences in hit rates between screens may reflect the high density of known bioactives in the targeted kinase inhibitor library versus larger collections, and fewer efflux mechanisms in L. monocytogenes (19, 31, 42), compared to P. aeruginosa (29). Of note, the two compounds identified by Wenderska et al. as biofilm inhibitors were sphingosine and palmitoyl-d,l-carnitine, showing that these molecules have broad range antibiofilm activity against both Gram-negative and Gram-positive species. Although obvious homologues of the eukaryotic kinases are absent in prokaryotes, there are a number of potential targets, including histidine kinases belonging to two-component regulatory systems (18), nucleotide-binding proteins and/or phosphotransferases.
Many of the compounds identified as planktonic growth inhibitors also displayed biofilm inhibitory effects at sublethal doses (see Fig. S1 in the supplemental material). Interestingly, two compounds that have dose-dependent biofilm inhibitory activity, staurosporine and indirubin-3′-monoxime, initially stimulated biofilm formation at low doses (see Fig. S1 in the supplemental material). A similar result was reported previously for Escherichia coli and P. aeruginosa, where subinhibitory concentrations of aminoglycosides stimulated biofilm formation (23).
Among the 20 compounds that were further tested in dose-response assays, sphingosine and palmitoyl-d,l-carnitine were selected for more detailed structure-function studies because they were potent and structurally similar inhibitors of biofilm formation. Sphingosine, derived from palmitoyl-coenzyme A and serine, is an antimicrobial agent naturally produced on human skin, where it has been shown to prevent colonization by S. aureus (4, 5). It was previously reported to be an effective planktonic growth inhibitor of a variety of Gram-positive bacteria—including L. monocytogenes and S. aureus (4, 5, 25, 37)—causing a 4-log reduction in planktonic cultures of L. monocytogenes at 25 μM (46). Our data are consistent with those studies, since concentrations above 6.3 μM inhibited L. monocytogenes planktonic growth in the polystyrene biofilm assay. Further, our data show that biofilm formation was impaired at sub-MIC concentrations. Notably, sphingosine was not an effective growth inhibitor when stainless-steel coupons were used as the substratum, even at concentrations that inhibited biofilm formation. This result suggests that exposure to stainless-steel surfaces or their eluates could interfere with the inhibitory property of the compound or modify bacterial physiology in a manner that allows growth even in the presence of increased inhibitor concentrations. This observation has important implications for the food industry, since growth on stainless-steel surfaces may similarly promote increased resistance to other types of disinfectants. We also noted that there were more cells in submerged areas on the stainless-steel coupons in the presence of the compounds. This finding suggests that the target(s) are more highly expressed in cells exposed to aerobic conditions or that the compounds are more effective against rapidly growing cells at the air-liquid interface.
Sphingosine's protonated active form resembles that of quaternary ammonium compounds (37) that affect membrane integrity. Sphingosine is proposed to bind to the cell through electrostatic and hydrophobic interactions and to form pores that disrupt the cytoplasmic membrane (37). Sphingosine is also an inhibitor of protein kinase C (PKC), a family of enzymes involved in eukaryotic signal transduction pathways. It has been hypothesized that a protein kinase analogous to that of mammalian cells may also be responsible for the antibacterial effect of sphingosine (37, 46), but this has yet to be experimentally demonstrated. As shown in Fig. 1B to I, the integrity of attached L. monocytogenes cells appears to be compromised in the presence of increasing concentrations of sphingosine, even though planktonic cell density was not affected.
Palmitoyl-d,l-carnitine is also a palmitic-acid derived PKC inhibitor (33, 57). Palmitoyl-d,l-carnitine inhibited L. monocytogenes biofilm formation with a corresponding increase in planktonic cell density, possibly due to repartitioning of the cells into the planktonic phase. This phenotype was different from that caused by sphingosine, suggesting that despite their structural similarities, the two molecules act via different mechanisms. S. aureus biofilm formation was also inhibited by palmitoyl-d,l-carnitine, supporting its broad antibiofilm activity. Structure-activity studies showed that neither planktonic growth nor biofilm formation was affected by the d,l-carnitine component, a finding consistent with reports that l-carnitine is used by L. monocytogenes as an osmoprotectant in osmotic stress conditions (15, 54, 55). Because d,l-carnitine had no antibiofilm effect, we hypothesized the active component of palmitoyl-d,l-carnitine would be palmitic acid. Unexpectedly, palmitic acid stimulated biofilm development, for reasons that are not yet clear. Therefore, palmitoyl-d,l-carnitine has unique properties that transcend those of its constituents.
Based on the recent report of d-amino acids inducing biofilm dispersal (28), we tested palmitoyl-d-carnitine and palmitoyl-l-carnitine separately to determine whether the d-enantiomer was more effective. Palmitoyl-d-carnitine inhibited planktonic growth at 25 μM (Fig. 3C) versus palmitoyl-l-carnitine and palmitoyl-d,l-carnitine, which inhibit at 100 μM (Fig. 3D and 2A, respectively), suggesting the d-form is a more potent L. monocytogenes growth inhibitor. However, the effects of palmitoyl-d,l-carnitine on biofilm formation are enantiomer independent, because the level of L. monocytogenes biofilm inhibition by all three compounds was similar. These data suggest that palmitoyl-d,l-carnitine may impair biofilm development through multiple mechanisms, as was reported for its effects on P. aeruginosa (Wenderska et al. [56]).
Based on their amphipathic structures, we also speculated that palmitoyl-d,l-carnitine and sphingosine might reduce bacterial attachment to surfaces via a surfactant or detergent-like effect. However, the length of the lipid tail makes the compounds more likely to form bilayers, rather than micelles, in an aqueous solution (22). To further test the surfactant hypothesis, we tested a variety of common laboratory detergents for antibiofilm activity, revealing that some detergents have inhibitory effects on biofilm formation with various effects on planktonic growth, while others had no effect (see Fig. S2 in the supplemental material). Thus, detergent-like molecules, including palmitoyl-d,l-carnitine and sphingosine, can have biofilm-inhibitory activity that is independent of their surfactant properties.
Fatty acids have chain-length-dependent antimicrobial activity against a variety of bacteria, but their effects on biofilm formation have been less well characterized. A number of recent studies have implicated microbially produced fatty acids or derivatives as diffusible signal factors that control, among other phenotypes, biofilm formation by heterologous species (43). Bovine milk, which contains a variety of fatty acids, has been shown to reduce the amount of viable L. monocytogenes cells in vitro, as well as to prevent intestinal colonization of rats in a chain length-dependent manner (46–48). Sprong et al. showed that shorter-chain-length saturated fatty acids (C4:0, C6:0, and C8:0) lack bactericidal activity toward L. monocytogenes even at 500 μM. In addition, neither C16:0 nor C18:0 had bactericidal activity at 500 μM, which is consistent with our results. In contrast, at 500 μM, long-chain unsaturated fatty acids (C18:1 and C18:2) and medium-chain-length saturated fatty acids (C10:0, C12:0, and C14:0) reduced the number of viable cells (46, 47). Under our experimental conditions, C9:0 to C14:0 fatty acids did not reduce planktonic cell density but were potent inhibitors of biofilm development (Table 4).
The structure-activity relationship of fatty acids and their derivatives is complex. Although palmitoyl-d,l-carnitine was a potent inhibitor of biofilm formation, short-chain acyl carnitines were less effective biofilm inhibitors than their free fatty acid equivalents (Tables 3 and 4). Sugar fatty acid esters were recently shown to inhibit biofilm formation of L. monocytogenes and other food-borne pathogens (16). Increasing sugar fatty ester chain length (i.e., >C12) reduced the amount of L. monocytogenes biofilm formation, but the inhibition was less potent compared to the effects of the same molecules on S. aureus and E. coli. In contrast, a shorter-chain sugar fatty acid ester (C8) did not inhibit biofilm formation (16). Together, these data suggest that the nature of fatty acid modification can significantly modulate effects on biofilm formation.
Biofilms are a major concern in the food industry since they can lead to contamination of food products. Some of the antibiofilm compounds identified through this work—including sphingosine and palmitoyl-d,l-carnitine—or their derivatives may have potential application against food-borne pathogens. Both inhibited both L. monocytogenes and S. aureus biofilm formation in the μM range and reduced the number of cells attaching to food-grade stainless steel and plastic, materials common in the food industry. These or related compounds can potentially be applied to equipment surfaces to prevent bacterial attachment or incorporated into food packaging to prevent bacterial growth. Identifying the mechanisms and targets involved in small-molecule modulation of L. monocytogenes biofilm formation can lead to biofilm inhibitors to be used alone or in conjunction with current sanitation methods used to prevent contamination.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by in part by a Tier I Canada Research Chair (to G.D.W.), by a New Emerging Teams on Antibiotic Adjuvants award from the Canadian Institutes of Health Research (XNE-8705), and through funding provided by the Canadian Food Inspection Agency (CFIA). Notwithstanding, the CFIA does not necessarily endorse the views or practices presented by the authors in the manuscript. I.B.W. was the recipient of a Cystic Fibrosis Canada Studentship, and M.A.C. was the recipient of CFC Summer Studentship awards in 2010 and 2011.
Footnotes
Published ahead of print 22 December 2011
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Abee T, Rombouts FM, Hugenholtz J, Guihard G, Letellier L. 1994. Mode of action of nisin Z against Listeria monocytogenes Scott A grown at high and low temperatures. Appl. Environ. Microbiol. 60:1962–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alaimo PJ, Shogren-Knaak MA, Shokat KM. 2001. Chemical genetic approaches for the elucidation of signaling pathways. Curr. Opin. Chem. Biol. 5:360–367 [DOI] [PubMed] [Google Scholar]
- 3. Allerberger F, Wagner M. 2010. Listeriosis: a resurgent food-borne infection. Clin. Microbiol. Infect. 16:16–23 [DOI] [PubMed] [Google Scholar]
- 4. Arikawa J, et al. 2002. Decreased levels of sphingosine, a natural antimicrobial agent, may be associated with vulnerability of the stratum corneum from patients with atopic dermatitis to colonization by Staphylococcus aureus. J. Investig. Dermatol. 119:433–439 [DOI] [PubMed] [Google Scholar]
- 5. Bibel DJ, Aly R, Shinefield HR. 1992. Antimicrobial activity of sphingosines. J. Investig. Dermatol. 98:269–273 [DOI] [PubMed] [Google Scholar]
- 6. Borucki MK, Peppin JD, White D, Loge F, Call DR. 2003. Variation in biofilm formation among strains of Listeria monocytogenes. Appl. Environ. Microbiol. 69:7336–7342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burdine L, Kodadek T. 2004. Target identification in chemical genetics: the (often) missing link. Chem. Biol. 11:593–597 [DOI] [PubMed] [Google Scholar]
- 8. Camejo A, et al. 2009. In vivo transcriptional profiling of Listeria monocytogenes and mutagenesis identify new virulence factors involved in infection. PLoS Pathog. 5:e1000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reference deleted.
- 10. Reference deleted.
- 11. Cervenka J, Osmundsen H. 1982. Synthesis of unsaturated carnitine esters with N-acyl imidazoles. J. Lipid Res. 23:1243–1246 [PubMed] [Google Scholar]
- 12. Chang Y, Gu W, McLandsborough LA. 2012. Low concentration of ethylenediaminetetraacetic acid (EDTA) affects biofilm formation of Listeria monocytogenes by inhibiting its initial adherence. Food Microbiol. 29:10–17 [DOI] [PubMed] [Google Scholar]
- 13. Chmielewski R, Frank J. 2003. Biofilm formation and control in food processing facilities. Comp. Rev. Food Sci. Food Safety 2:22–32 [DOI] [PubMed] [Google Scholar]
- 14. Farber JM. 2000. Present situation in Canada regarding Listeria monocytogenes and ready-to-eat seafood products. Int. J. Food Microbiol. 62:247–251 [DOI] [PubMed] [Google Scholar]
- 15. Fraser KR, O'Byrne CP. 2002. Osmoprotection by carnitine in a Listeria monocytogenes mutant lacking the OpuC transporter: evidence for a low affinity carnitine uptake system. FEMS Microbiol. Lett. 211:189. [DOI] [PubMed] [Google Scholar]
- 16. Furukawa S, Akiyoshi Y, O'Toole GA, Ogihara H, Morinaga Y. 2010. Sugar fatty acid esters inhibit biofilm formation by food-borne pathogenic bacteria. Int. J. Food Microbiol. 138:176–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garmyn D, Gal L, Lemaitre JP, Hartmann A, Piveteau P. 2009. Communication and autoinduction in the species Listeria monocytogenes: a central role for the agr system. Commun. Integr. Biol. 2:371–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Glaser P, et al. 2001. Comparative genomics of Listeria species. Science 294:849–852 [DOI] [PubMed] [Google Scholar]
- 19. Godreuil S, Galimand M, Gerbaud G, Jacquet C, Courvalin P. 2003. Efflux pump Lde is associated with fluoroquinolone resistance in Listeria monocytogenes. Antimicrob. Agents Chemother. 47:704–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hall-Stoodley L, Costerton JW, Stoodley P. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2:95–108 [DOI] [PubMed] [Google Scholar]
- 21. Harmsen M, Lappann M, Knochel S, Molin S. 2010. Role of extracellular DNA during biofilm formation by Listeria monocytogenes. Appl. Environ. Microbiol. 76:2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hauser H. 2000. Short-chain phospholipids as detergents. Biochim. Biophys. Acta 1508:164–181 [DOI] [PubMed] [Google Scholar]
- 23. Hoffman LR, et al. 2005. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436:1171–1175 [DOI] [PubMed] [Google Scholar]
- 24. Junker LM, Clardy J. 2007. High-throughput screens for small-molecule inhibitors of Pseudomonas aeruginosa biofilm development. Antimicrob. Agents Chemother. 51:3582–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kabara JJ, Swieczkowski DM, Conley AJ, Truant JP. 1972. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 2:23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karatan E, Watnick P. 2009. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol. Mol. Biol. Rev. 73:310–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keskinen LA, Todd EC, Ryser ET. 2008. Impact of bacterial stress and biofilm-forming ability on transfer of surface-dried Listeria monocytogenes during slicing of delicatessen meats. Int. J. Food Microbiol. 127:298–304 [DOI] [PubMed] [Google Scholar]
- 28. Kolodkin-Gal I, et al. 2010. d-Amino acids trigger biofilm disassembly. Science 328:627–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lister PD, Wolter DJ, Hanson ND. 2009. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 22:582–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mah TF, et al. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306–310 [DOI] [PubMed] [Google Scholar]
- 31. Mata MT, Baquero F, Perez-Diaz JC. 2000. A multidrug efflux transporter in Listeria monocytogenes. FEMS Microbiol. Lett. 187:185–188 [DOI] [PubMed] [Google Scholar]
- 32. Musken M, Di Fiore S, Dotsch A, Fischer R, Haussler S. 2010. Genetic determinants of Pseudomonas aeruginosa biofilm establishment. Microbiology 156:431–441 [DOI] [PubMed] [Google Scholar]
- 33. Nakadate T, Blumberg PM. 1987. Modulation by palmitoylcarnitine of protein kinase C activation. Cancer Res. 47:6537–6542 [PubMed] [Google Scholar]
- 34. Nakayama J, et al. 2009. Ambuic acid inhibits the biosynthesis of cyclic peptide quormones in gram-positive bacteria. Antimicrob. Agents Chemother. 53:580–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O'Toole GA, Kolter R. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Mol. Microbiol. 28:449–461 [DOI] [PubMed] [Google Scholar]
- 36. Peterson RT. 2008. Chemical biology and the limits of reductionism. Nat. Chem. Biol. 4:635–638 [DOI] [PubMed] [Google Scholar]
- 37. Possemiers S, Van Camp J, Bolca S, Verstraete W. 2005. Characterization of the bactericidal effect of dietary sphingosine and its activity under intestinal conditions. Int. J. Food Microbiol. 105:59–70 [DOI] [PubMed] [Google Scholar]
- 37a. Public Health Agency of Canada 2009. Listeria monocytogenes outbreak: media room. Public Health Agency of Canada, Ottawa, Ontario, Canada [Google Scholar]
- 37b. Public Health Agency of Canada 2008. Listeriosis epidemiological curve. Public Health Agency of Canada, Ottawa, Ontario, Canada [Google Scholar]
- 38. Rasooli I, Rezaei MB, Allameh A. 2006. Ultrastructural studies on antimicrobial efficacy of thyme essential oils on Listeria monocytogenes. Int. J. Infect. Dis. 10:236–241 [DOI] [PubMed] [Google Scholar]
- 39. Riedel CU, et al. 2009. AgrD-dependent quorum sensing affects biofilm formation, invasion, virulence and global gene expression profiles in Listeria monocytogenes. Mol. Microbiol. 71:1177–1189 [DOI] [PubMed] [Google Scholar]
- 40. Rieu A, Weidmann S, Garmyn D, Piveteau P, Guzzo J. 2007. Agr system of Listeria monocytogenes EGD-e: role in adherence and differential expression pattern. Appl. Environ. Microbiol. 73:6125–6133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Robertson MH. 1977. Listeriosis. Postgrad. Med. J. 53:618–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Romanova NA, Wolffs PF, Brovko LY, Griffiths MW. 2006. Role of efflux pumps in adaptation and resistance of Listeria monocytogenes to benzalkonium chloride. Appl. Environ. Microbiol. 72:3498–3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ryan RP, Dow JM. 2011. Communication with a growing family: diffusible signal factor (DSF) signaling in bacteria. Trends Microbiol. 19:145–152 [DOI] [PubMed] [Google Scholar]
- 44. Sandasi M, Leonard CM, Viljoen AM. 2010. The in vitro antibiofilm activity of selected culinary herbs and medicinal plants against Listeria monocytogenes. Lett. Appl. Microbiol. 50:30–35 [DOI] [PubMed] [Google Scholar]
- 45. Southwick F, Purich D. 1996. Intracellular pathogenesis of listeriosis. N. Engl. J. Med. 334:770–776 [DOI] [PubMed] [Google Scholar]
- 46. Sprong RC, Hulstein MF, Van der Meer R. 2001. Bactericidal activities of milk lipids. Antimicrob. Agents Chemother. 45:1298–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sprong RC, Hulstein MF, Van der Meer R. 2002. Bovine milk fat components inhibit food-borne pathogens. Int. Dairy J. 12:209–215 [Google Scholar]
- 48. Sprong RC, Hulstein MF, Van der Meer R. 1999. High intake of milk fat inhibits intestinal colonization of Listeria but not of Salmonella in rats. J. Nutr. 129:1382–1389 [DOI] [PubMed] [Google Scholar]
- 49. Stockwell BR. 2000. Chemical genetics: ligand-based discovery of gene function. Nat. Rev. Genet. 1:116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sui Y, Wu Z. 2007. Alternative statistical parameter for high-throughput screening assay quality assessment. J. Biomol. Screen. 12:229–234 [DOI] [PubMed] [Google Scholar]
- 51. Swaminathan B, Gerner-Smidt P. 2007. The epidemiology of human listeriosis. Microbes Infect. 9:1236–1243 [DOI] [PubMed] [Google Scholar]
- 52. Tenorio E, et al. 2003. Systematic characterization of Escherichia coli genes/ORFs affecting biofilm formation. FEMS Microbiol. Lett. 225:107–114 [DOI] [PubMed] [Google Scholar]
- 53. Thompson RL, Carpenter CE, Martini S, Broadbent JR. 2008. Control of Listeria monocytogenes in ready-to-eat meats containing sodium levulinate, sodium lactate, or a combination of sodium lactate and sodium diacetate. J. Food Sci. 73:M239–244 [DOI] [PubMed] [Google Scholar]
- 54. Verheul A, Glaasker E, Poolman B, Abee T. 1997. Betaine and l-carnitine transport by Listeria monocytogenes Scott A in response to osmotic signals. J. Bacteriol. 179:6979–6985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Verheul A, Rombouts FM, Beumer RR, Abee T. 1995. An ATP-dependent l-carnitine transporter in Listeria monocytogenes Scott A is involved in osmoprotection. J. Bacteriol. 177:3205–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wenderska I, Chong M, McNulty J, Wright G, Burrows L. 2011. Palmitoyl-DL-carnitine is a multitarget inhibitor of Pseudomonas aeruginosa biofilm development. Chembiochem 12:2759–2766 [DOI] [PubMed] [Google Scholar]
- 57. Wise BC, Kuo JF. 1983. Modes of inhibition by acylcarnitines, adriamycin, and trifluoperazine of cardiac phospholipid-sensitive calcium-dependent protein kinase. Biochem. Pharmacol. 32:1259–1265 [DOI] [PubMed] [Google Scholar]
- 58. Zhang JH, Chung TD, Oldenburg KR. 1999. A simple statistical parameter for use in evaluation and validation of high-throughput screening assays. J. Biomol. Screening 4:67–73 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.