Abstract
Viable methanogens have been detected in dry, aerobic environments such as dry reservoir sediment, dry rice paddies and aerobic desert soils, which suggests that methanogens have mechanisms for long-term survival in a desiccated state. In this study, we quantified the survival rates of the methanogenic archaeon Methanosarcina barkeri after desiccation under conditions equivalent to the driest environments on Earth and subsequent exposure to different stress factors. There was no significant loss of viability after desiccation for 28 days for cells grown with either hydrogen or the methylotrophic substrates, but recovery was affected by growth phase, with cells desiccated during the stationary phase of growth having a higher rate of recovery after desiccation. Synthesis of methanosarcinal extracellular polysaccharide (EPS) significantly increased the viability of desiccated cells under both anaerobic and aerobic conditions compared with that of non-EPS-synthesizing cells. Desiccated M. barkeri exposed to air at room temperature did not lose significant viability after 28 days, and exposure of M. barkeri to air after desiccation appeared to improve the recovery of viable cells compared with that of desiccated cells that were never exposed to air. Desiccated M. barkeri was more resistant to higher temperatures, and although resistance to oxidative conditions such as ozone and ionizing radiation was not as robust as in other desiccation-resistant microorganisms, the protection mechanisms are likely adequate to maintain cell viability during periodic exposure events. The results of this study demonstrate that after desiccation M. barkeri has the innate capability to survive extended periods of exposure to air and lethal temperatures.
INTRODUCTION
As more microorganisms are discovered in extreme environments on Earth that were previously considered to be devoid of life, we have been redefining the physical and chemical parameters for life to exist. One such extreme is the long-term storage of viable cells in a desiccated state, which imposes physiological constraints that many species cannot tolerate. A common characteristic of known desiccation-tolerant microorganisms, which include spore-forming bacteria, heterocyst-forming cyanobacteria, heteropolysaccharide-forming Beijerinckia, and Deinococcus, is the formation of relatively thick outer cell layers (32). The synthesis of an outer cell layer often composed of an extracellular polysaccharide (EPS), in conjunction with other mechanisms such as robust DNA repair and compatible solute formation, enables the cells to retain the minimal intracellular water activity required for survival (32).
The methanogenic Archaea, despite their preference for highly reduced, anoxic conditions in order to grow, are globally distributed in a wide range of anaerobic, aquatic environments, including Antarctic lakes, submarine hydrothermal vents, rice paddies, sewage digestors, and as symbionts in rumen, termites, protozoa, and human large intestines (38). Among the least anticipated environments where viable methanogens have been detected are dry, aerobic environments such as dry reservoir sediment, dry rice paddies, and aerobic desert soils (26, 27, 30). These reports suggested that methanogens could survive desiccation in different environments. In axenic culture studies, Kendrick and Kral (18) and Liu et al. (21) reported that several species of methanogenic Archaea remained viable after up to 30 days of desiccation. In the latter study the authors observed that methanogens remained viable for a longer period of desiccation when the cells were dried with solid particles present, and they hypothesized that microniches within soil particles provided protection from oxygen to cells during periodic desiccation in the environment. However, the cellular mechanisms for desiccation tolerance by methanogens are currently unknown.
Methanosarcina barkeri Fusaro, isolated from sediment from Lago del Fusaro, a freshwater coastal lagoon west of Naples, Italy, can adapt to one of the widest ranges of habitats for an individual methanogenic species (15). In addition to utilizing all known methanogenic substrates, M. barkeri can grow autotrophically in a minimal mineral medium with hydrogen as an energy source, carbon dioxide as a carbon source, and molecular nitrogen as a nitrogen source (5, 22). This species can also adapt to intracellular solute concentrations ranging from freshwater to three times the solute concentration in seawater by synthesis of osmoprotectants, and it exhibits a dichotomous morphology, growing in freshwater as large multicellular aggregates embedded in a heteropolysaccharide matrix or growing in high extracellular solute concentrations as individual cells without an extracellular polymer layer (39). M. barkeri also synthesizes gas vesicles, which have been proposed to be an early organelle of prokaryote motility often regulated by light and oxygen partial pressure (43, 46). The relatively large genome sizes of M. barkeri (4.8 Mb) and of Methanosarcina spp. in general (<5.8 Mb) and the relatively large number of putative coding sequences reflect the versatile nature of these species, with the ability to use a greater range of substrates, adapt to a broader range of environments, and form more complex multicellular structures, compared with the more limited capabilities of most other extremophiles, which generally average around 2 Mb (9, 11, 23). The adaptive success of these species is further evidenced by the occurrence of multiple paralogs in the genomes, including multiple catabolic methyltransferases and carbon monoxide dehydrogenases, all three known types of nitrogenases, and all four known chaperone systems (8, 9, 11, 23).
In laboratory culture the growth of Methanosarcina spp. is inhibited by oxygen and by temperatures above 55°C. However, Methanosarcina barkeri has been reported to tolerate exposure to air for up to 200 h without a detectable loss of viability (10). Recent reports on tolerance of Methanosarcina spp. to stress indicate that these species have enzymes for protection from oxidative stress and repair of DNA damage resulting from ionizing radiation and the ability to endure short periods of desiccation (7, 21, 33, 47). The Methanosarcina morphology of multicellular aggregates embedded in an EPS matrix is unique among the methanogens, and it is remarkably similar to the morphology of other desiccation-resistant prokaryotes. Collectively, the data suggest that the range of environmental conditions in which these species survive has been underestimated. In this report we quantify the viability of M. barkeri under different desiccation conditions and demonstrate that desiccation is an innate survival mechanism that enables cells to remain viable in the presence of potentially lethal stress factors.
MATERIALS AND METHODS
Methanogen strains.
Methanosarcina barkeri strain Fusaro (DSM 804) was obtained from sources described previously and maintained as a frozen stock (41).
Media and cell growth.
Artificial marine mineral medium (M medium) and freshwater mineral medium (F medium) were prepared in Balch-style anaerobe tubes for growth of M. barkeri by methods described previously (40). Growth substrates used were methanol (MeOH) or trimethylamine-HCl (TMA) at a final concentration of 0.1 M or a mix of 80%:20% carbon dioxide-hydrogen in the gas phase. Methanogen medium was prepared anaerobically in an N2-CO2 (4:1) atmosphere by a modified Hungate technique (39). Cultures were incubated in the dark at 35°C.
Desiccation of cells.
Cells were harvested directly in anaerobe culture tubes by centrifugation at 6,700 × g for 15 min in a swinging-bucket centrifuge (model GPR; Beckman Coulter) and transferred to an anaerobic glove box. Inside an anaerobic glove box the supernatant was decanted and the pellets resuspended in approximately 1 ml of supernatant. The resuspended cells were transferred to preweighed microcentrifuge tubes and centrifuged at 6,800 × g for 5 min. The supernatant was decanted, and the tubes with the pellets were weighed. The microcentrifuge tubes with tops open were sealed in screw-cap glass canning jars (0.94 liter) containing 10 g of calcium chloride. For desiccation under aerobic conditions, the jars were removed from the glove box and exposed to the atmosphere for 5 min before resealing.
Substrate experiments.
M. barkeri was grown in F medium with TMA, methanol, or a mix of carbon dioxide-hydrogen as described above. Cells were grown to stationary phase (optical density at 550 nm [OD550] = 0.7 for TMA-grown cells, OD550 = 0.5 for methanol-grown cells, and OD550 = 0.3 for CO2-H2-grown cells). Cultures were harvested at the indicated growth phase by centrifugation of culture tubes as described above. Cells were desiccated in an anaerobic glove box for 1, 7, 14, or 28 days prior to resuspension in medium and enumeration for viable cells as described below. All assays were performed with four replicate cultures.
Growth phase experiments.
M. barkeri was grown in F medium with 0.1 M TMA to three different growth phases: mid-exponential phase, late exponential phase, and stationary phase. The growth phase of the cells was monitored by OD550: mid-exponential-phase cells were harvested at OD550s of 0.45 and 0.6, late-exponential-phase cells were harvested at an OD550 of 0.7 to 0.8, and stationary-phase cells were harvested at an OD550 of greater than 0.83. Four replicates cultures for each growth phase were harvested by centrifugation and desiccated anaerobically for 7 days as described above.
Effect of EPS on cell viability.
M. barkeri was grown in either M medium to generate single cells without extracellular polysaccharide (EPS) or F medium to generate aggregated cells with EPS. Both types of media had 0.1 M TMA as the growth substrate. Cells were grown to stationary phase, harvested by centrifugation as described above, and desiccated anaerobically. Three different desiccation treatments were tested: in treatment 1, cells were desiccated anaerobically for 7 days prior to enumeration of viable cells; in treatment 2, cells were desiccated aerobically in desiccators containing air for 7 days prior to enumeration of viable cells; and in treatment 3, cells were desiccated anaerobically for 7 days and then exposed to air in a desiccator for 7 days prior to enumeration of viable cells. All assays were performed with four replicate cultures.
Treatment with ozone.
M. barkeri was grown in F medium with 0.1 M TMA and harvested in 1-ml aliquots of cells by centrifugation in microcentrifuge tubes. A triplicate set of microcentrifuge tubes containing harvested cells was transferred into four screw-cap glass canning jars containing CaCl2 (desiccated) and four jars without CaCl2 (undesiccated), and the jars were sealed and stored at room temperature. All preparation was performed in an anaerobic glove box at room temperature. After 7 days, 100 ml of 80% ozone from an ozone generator (Guardian, Cocoa, FL) was injected into the jars through a septum in the top, and viable cells were enumerated from triplicate sets of desiccated and undesiccated cells at 0, 1, 24, and 216 h.
Treatment with ionizing radiation.
M. barkeri was grown in 50 ml of F medium with H2-CO2 as the growth substrate. Aliquots (1 ml) of culture were harvested anaerobically in 1.5-ml microcentrifuge tubes at 6,800 × g for 5 min. Six tubes were transferred to a screw-cap glass canning jar containing CaCl2 in an anaerobic glove box and desiccated overnight. An additional six tubes were sealed and stored overnight in a canning jar without desiccant. Duplicate sets of desiccated and undesiccated cells were irradiated to final doses of ionizing radiation of 1.5 and 2.5 kGy using a 60Co gamma source (University of Maryland College Park Gamma Test Facility, College Park, MD) at a dose rate 3 to 13 kGy h−1. One set of control tubes was not irradiated. Viable cells were enumerated, and their survival relative to that of a duplicate set of nonirradiated cells was determined.
Enumeration of viable cells.
Since M. barkeri grows as multicellular aggregates, OD and direct cell count assays are not effective for cell enumeration. To enumerate cells more accurately, most-probable-number (MPN) assays were performed as previously described with modifications described below (4). All procedures were performed in an anaerobic glove box, and all materials were equilibrated in the glove box for 24 h prior to use to remove residual oxygen. For each treatment, replicate samples were assayed as indicated. Each well of a 96-well polycarbonate microtiter plate was filled with 180 μl of the appropriate medium (F medium or M medium). Microcentrifuge tubes containing the desiccated cell pellets were removed from the canning jars and reweighed to determine the final dry mass of the material. The desiccated cell material was resuspended in 1 ml of the appropriate medium (F medium or M medium). After resuspension, replicate 20-μl subsamples of each cell suspension were added to three wells and completely suspended in the medium with a micropipettor. A total of eight 10-fold serial dilutions were created by sequentially transferring a 20-μl subsample to the subsequent row of wells. Plates were covered with clear, sterile aluminum sealing film, and small holes were punctured above each well to allow accumulated methane gas to escape and, in the case of the H2-CO2 assay, to allow the substrate to enter the wells. The plates were placed in stainless steel anaerobe jars (Torbal, Clifton, NJ), and the atmosphere was reduced with hydrogen sulfide generated from Na2S as described previously (2). The anaerobe jars were incubated at 35°C for 60 days. The plates were removed from the jars, and growth was scored based on the appearance of cell pellets. The number of cells per 1 ml was calculated using the 3-dilution MPN table (4).
RESULTS
Effect of growth substrates on the viability of desiccated cells.
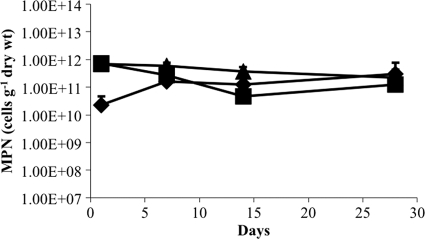
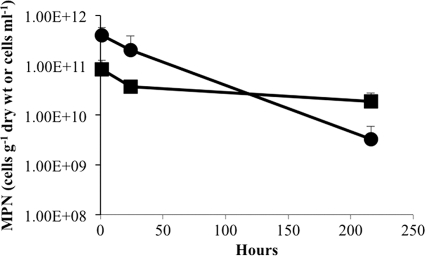
The effect of growth substrates on the viability of M. barkeri after desiccation was determined for trimethylamine (TMA), methanol (MeOH), and H2-CO2. Cultures were grown to stationary phase with each substrate, and the viability of harvested cells was assayed after desiccation for various lengths of time (Fig. 1). Cells grown on TMA and MeOH showed similar patterns of survival over time, with less than 30% loss of viability after 28 days. The apparent increase in viability of cells grown on H2-CO2 within the first 7 days was likely an artifact resulting from the tendency of H2-CO2-grown cells to form large aggregates that were difficult to disperse homogenously even after rigorous resuspension. Regardless of the initial differences, the loss of viability was less than an order of magnitude for each substrate, and the difference in cell viability was not significantly different between substrates after 28 days.
Fig 1.
Effect of growth substrate on recovery of viable cells after desiccation. M. barkeri was grown on H2-CO2 (♦), methanol (■), or trimethylamine (●) prior to desiccation, and the recovery of viable cells was determined over time. Survival is based on an MPN estimation of viable cells per gram (dry weight) of cell material. Values are means and standard deviations for four replicate cultures.
Effect of growth phase on viability of desiccated cells.
A greater percentage of Escherichia coli cells harvested during stationary-phase growth than of cells harvested during exponential growth maintain their viability (36). The recoveries (standard deviations) of viable M. barkeri harvested at mid-exponential, late exponential, and stationary phases of growth prior to desiccation were 3.35 × 108 (4.04 × 108), 5.77 × 109 (3.93 × 109), and 1.33 × 1010 (4.26 × 109) cells g (dry weight)−1, respectively. Cells that were grown to mid-exponential phase retained only 5.8% and 2.5% of the viable cells retained by cells grown to late exponential and stationary phases, respectively. Although there was not a significant difference in recovery of viable cells after desiccation between late-exponential-phase and either mid-exponential- or stationary-phase cultures, there was a significant difference in recovery of viable cells after desiccation between mid-exponential- and stationary-phase cultures (P = 0.0063).
Role of methanochondroitin in maintaining the viability of desiccated cells.
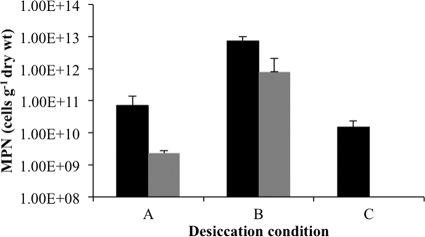
When M. barkeri is grown in medium with high extracellular solute concentrations, such as marine medium, this species no longer synthesizes the methanosarcinal extracellular polysaccharide methanochondroitin and grows as single cells with only a protein S-layer outer wall. To determine whether the methanosarcinal EPS has a role in the survival of M. barkeri during desiccation, cell viabilities of desiccated non-EPS-forming single cells and EPS-forming cell aggregates were compared. As EPS is reported to provide a physical barrier against oxygen diffusion in some nitrogen-fixing bacteria (3, 31, 35), the EPS of M. barkeri was also tested for its potential role in maintaining the viability of cells after exposure to oxygen. Both multicellular aggregates embedded in EPS and single cells without EPS were (i) desiccated and incubated anaerobically, (ii) desiccated anaerobically and incubated aerobically, and (iii) desiccated and incubated aerobically. Figure 2 shows that cell viability was significantly greater for desiccated multicellular aggregates than for single cells under all conditions tested. When desiccated and incubated under anaerobic conditions, cells without EPS retained only 7% of the viability observed for EPS-forming cells, and when desiccated under anaerobic conditions prior to aerobic incubation, non-EPS-forming cells retained 11% of the viability observed for EPS-forming cells. However, no viable cells were detected when non-EPS-forming cells were desiccated and incubated under aerobic conditions. Interestingly, both EPS-synthesizing and non-EPS-synthesizing cells maintained the greatest postdesiccation viability when incubated in air after anaerobic desiccation.
Fig 2.
Effect of EPS on recovery of viable cells after desiccation. M. barkeri was grown as either EPS-forming multicellular aggregates (black bars) or non-EPS-forming single cells (gray bars) prior to desiccation under three different conditions. Anaerobic cells (A) were desiccated anaerobically prior to enumeration of viable cells, anaerobic/aerobic cells (B) were desiccated anaerobically and then exposed to air for 7 days prior to enumeration of viable cells, and aerobic cells (C) were desiccated aerobically for 7 days prior to MPN analysis. Survival is based on an MPN estimation of viable cells per gram (dry weight) of cell material. Values are means and standard deviations for four replicate cultures.
Effects of desiccation on the viability of cells exposed to stress factors.
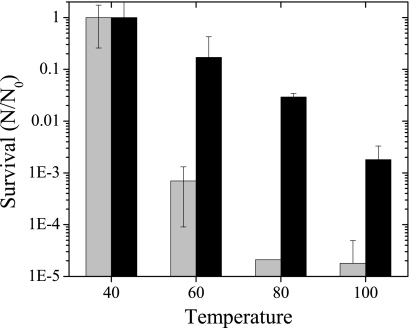
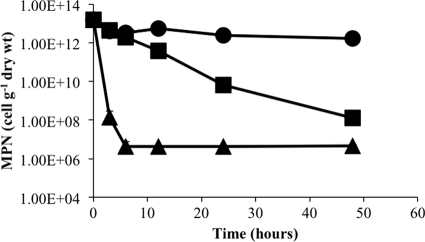
The role of desiccation in protecting M. barkeri from several stress factors commonly encountered in the environment was also tested. Desiccated and actively growing M. barkeri cells were exposed to temperatures ranging from the optimal growth temperature of 40°C to 100°C for 1 h and then assayed for viability. As expected, viability rates decreased as the temperature increased (Fig. 3). However, the rate of recovery of viable desiccated cells was 4 to 5 orders of magnitude higher at temperatures above 40°C than that of undesiccated cells. Since desiccation substantially increased survival of cells after short-term exposure to higher temperatures, desiccated cells were exposed to lethal temperatures for 2 days to determine the longer-term viability (Fig. 4). At 20°C, there was no significant change in the viability of desiccated cells over time. At 55°C, which is 10°C greater than the maximum temperature tolerated for growth of M. barkeri, there was a gradual and constant decrease in cell viability during the exposure period. In contrast, the viability of cells exposed to 90°C was reduced drastically after 3 h of exposure, but viability was constant thereafter for the remaining period of exposure.
Fig 3.
Effect of high temperatures on viability of desiccated and undesiccated cells. Desiccated cells (black bars) were dried anaerobically, and undesiccated cells (gray bars) were grown in liquid medium and pelleted immediately prior to incubation at different temperatures for 1 h. Survival is based on an MPN estimation of viable cells per gram (dry weight) of cell material. Values are means and standard deviations for four replicate culture samples.
Fig 4.
Effect of desiccation on recovery of viable cells after extended exposure to high temperatures. Desiccated M. barkeri cells were incubated at 20°C (●), 55°C (■), and 90°C (▲), and survival based on an MPN estimation of viable cells per gram (dry weight) of cell material was determined over time. Values are means and standard deviations for four replicate culture samples.
The effect of desiccation on survival of M. barkeri under highly oxidative conditions was tested by exposing desiccated and actively growing cells to ozone at an initial concentration similar to that found in the ozone layer around Earth (Fig. 5). The recovery of viable undesiccated cells after exposure to ozone over time was relatively constant, which was likely due to the short half-life (t1/2) in water (t1/2 in air = 20 min) and the presence of residual reductants from the medium. Although the viability of desiccated cells declined over time, the loss of viability was not significant in the first 24 h, when the ozone concentration was greatest (t1/2 in air = 3 days), and cell loss of viability decreased by only 2 orders of magnitude after 10 days.
Fig 5.
Effect of ozone on viability of desiccated and undesiccated cells. Desiccated cells (●) were dried anaerobically, and undesiccated cells (■) were grown in liquid medium and pelleted immediately prior to incubation with ozone. Survival is based on an MPN estimation of viable cells per gram (dry weight) of cell material. Values are means and standard deviations for three replicate culture samples.
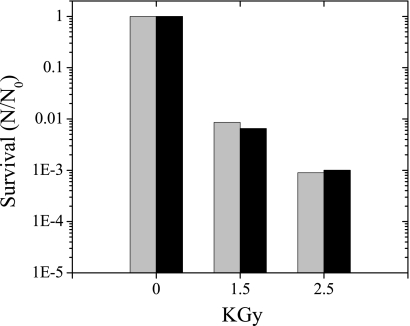
The survival of undesiccated and desiccated cells after exposure to different levels of ionizing radiation was compared (Fig. 6). Recovery of viable cells was similar for desiccated and undesiccated cells, with an overall decline of viable cells of over three orders of magnitude at 2.5 kGy.
Fig 6.
Effect of ionizing radiation on viability of desiccated and undesiccated cells. Desiccated cells (black bars) were dried anaerobically, and undesiccated cells (gray bars) were grown in liquid medium and pelleted prior to exposure to a 60Co gamma source. Survival is based on an MPN estimation of viable cells per gram (dry weight) of cell material. Values are means for two replicate culture samples.
Long-term viability of Methanosarcina spp.
To assess the long-term viability of Methanosarcina spp., a desiccated cell pellet of Methanosarcina thermophila TM-1 harvested in 1984 was located in a frozen culture archive. The culture was grown in a pH auxostat with acetate and harvested anaerobically in late exponential growth as described previously (42). The cell pellet was stored in liquid nitrogen for 12 months and then stored thereafter at −20°C in a plastic container with a loose-fitting top, leading to desiccation and constant exposure to air since 1985. The cell pellet was pink from oxidation of residual resazurin and had a powdery consistency. Samples of 0.01 g of dry pellet material from the surface of the desiccated pellet were resuspended in 1 ml F medium containing 0.1 M TMA. Enumeration by the MPN technique indicated that there were on average 9 × 106 (±2 × 106) viable cells g (dry weight)−1 remaining after exposure to air in a desiccated state for over 25 years.
DISCUSSION
Recovery of M. barkeri desiccated under different growth conditions.
The removal of cell-bound water through air drying and rehydration of air-dried cells can influence the activities and the distribution of bacterial species on both local and global scales. In the current study, M. barkeri was desiccated for as long as 30 days in <10% relative humidity with anhydrous CaCl2 (28), with the goal of simulating dry conditions where viable methanogens have been detected previously (26, 27, 30). The recovery of viable cells after desiccation was equivalent for M. barkeri grown either hydrogenotrophically or methylotrophically with trimethylamine or methanol. The results indicate that the catabolic pathway utilized was not a factor in the mechanisms that mediate postdesiccation viability. Furthermore, there was no significant loss of viability of cells desiccated for 1 day and 28 days, which is consistent with the finding by Mayer and Conrad (26) that the methanogenic population in rice paddies remained constant during dry fallow periods. The latter observation suggests that M. barkeri is innately stable once it is in a state of desiccation and that protection by microaggregation with soil particles is not required to maintain viability over time (12).
Even though recovery from desiccation was not substrate specific, cells in stationary phase at the time of desiccation appeared to recover more efficiently. This likely represents common mechanisms for adaptation to stresses associated with stationary phase and desiccation. Bacterial cells in stationary phase are generally more stress tolerant than metabolically active cells growing exponentially. Exponential phase is a time of rapid growth with the availability of unlimited nutrients, whereas in stationary phase, cell metabolism and growth are minimized in response to various stress factors such as nutrient depletion and toxic product buildup. Studies on other microorganisms have found that when desiccated during stationary phase, cells had a significantly greater survival rate than when desiccated during exponential phase (45). E. coli in stationary phase is adapted to tolerate stress conditions by responses that include modifications of fatty acids, condensation of DNA, synthesis or uptake of osmoprotectants, dimerization of ribosomes, and expression of stress response genes and antioxidants, and these conditions predisposed cells to tolerate the stress conditions imposed by desiccation (36). A study on Saccharomyces cerevisiae (37) also found similarities in the transcriptomes of stationary-phase and desiccated cells, indicating that the process of surviving desiccation is similar to the conditions found during stationary phase. The results of this study suggest that, like for bacterial and eukaryotic cells, the mechanisms enabling adaptation to stress associated with stationary phase and desiccation are similar in the methanogenic Archaea.
Increased resistance of M. barkeri to environmental stressors when desiccated.
Dry sediments or desert soils where methanogens have been detected would potentially expose methanogens to greater temperatures, oxidation, and ionizing radiation than tolerated by M. barkeri in laboratory culture. The M. barkeri genome has genes encoding major known DNA repair mechanisms, including alkyl transfer, photoactivation, base excision repair, nucleotide excision repair, mismatch repair, translesion synthesis, and homologous repair, as well as proteins for preventing oxidative damage, including superoxide dismutase (SOD) and catalase (23). Zhang et al. (47) confirmed that genes encoding putative superoxide dismutase, peroxiredoxin, catalase, and thioredoxin reductase were expressed at various levels in M. barkeri both before and after exposure to air, and Angel et al. (1) showed that the gene encoding catalase was transcribed in desert soil exposed to oxygen. Another report showed that double-strand breaks resulting from exposure of M. barkeri to ionizing radiation were repaired in vivo by homologous recombination, and repair was at least in part the result of eukaryotic poly(ADP-ribose) polymerase activity (34). However, in the current report we show that the sensitivity of desiccated cells to ionizing radiation is similar to that of E. coli, indicating that the mechanisms of adaptation in M. barkeri are not linked to a robust DNA repair associated with desiccation-tolerant microorganisms such as Deinococcus and Halobacterium (20, 25). Desiccated M. barkeri exposed to air at room temperature did not lose significant viability after 28 days, and desiccated M. thermophila remained viable in air at −20°C for 25 years with an estimated loss in viability of 6 orders of magnitude. Interestingly, complete cell death of M. barkeri exposed to 90°C was not observed, and recovery of viable cells remained constant at 106 cells g (dry weight)−1 between 6 and 48 h. Likewise, the viable cell count recovered from archived desiccated M. thermophila was observed at a similar threshold. This apparent threshold of cell recovery might reflect innate recovery/repair processes of desiccation tolerance that minimize sublethal damage to cell macromolecules for a finite portion of the cell population. Overall, the results indicate that both oxidative defense and DNA repair mechanisms are adequate for protecting desiccated M. barkeri from oxidative damage in air, and these mechanisms are most effective when cells are in a desiccated state. The effectiveness of the antioxidant protection mechanisms should not be underestimated, as many critical proteins in M. barkeri contain metal redox centers that are irreversibly inhibited by oxygen. Resistance of M. barkeri to highly oxidative conditions such as ozone and ionizing radiation was not as robust as for other desiccation-resistant microorganisms, such as halophilic Archaea or Deinococcus, but would likely be adequate to maintain cell viability during periodic exposure events.
Role of methanochondroitin in desiccation tolerance.
The secretion of extracellular polysaccharide (EPS) is a common feature of desiccation-tolerant microorganisms. Although it is widely believed that EPS protects cells during desiccation and the protective role has been confirmed for some species, a clear relationship could not be shown in others (19, 29, 44). The Methanosarcina morphology of multicellular aggregates embedded in an EPS matrix is unique among the methanogens, and it is remarkably similar to the morphology of other desiccation-resistant prokaryotes. M. barkeri synthesizes an EPS composed primarily of d-galactosamine and d-glucuronic acid, termed methanochondroitin, that is nearly identical to animal chondroitin except for the order of the monomers and lack of sulfate (16). Because M. barkeri can be grown with or without methanochondroitin synthesis, it was possible to demonstrate that the heteropolysaccharide had a definitive role in desiccation resistance. Regardless of whether cell desiccation/postdesiccation was anaerobic/anaerobic, anaerobic/aerobic, or aerobic/aerobic, cells that synthesized methanochondroitin had a significantly higher survival rate than cells without methanochondroitin. One of the characteristics of chondroitin is the ability to absorb water. This property suggests that methanochondroitin may contribute to cell viability by serving as a humectant, thereby enabling cells to retain a critical level of intracellular water activity while in a desiccated state. In addition to retaining water, extracellular polysaccharides inhibit the fusion of membrane vesicles during desiccation and act as an immobilization matrix for secreted enzymes that remain active during long-term desiccation (13). Another possible role of the EPS is to minimize oxygen diffusion into the cell, which has been shown to prevent oxygen denaturation of nitrogenase in nitrogen-fixing bacteria (3). This conclusion is supported by the observation that methanochondroitin-synthesizing cells desiccated aerobically had nearly the same rate of recovery as cells desiccated anaerobically, but in the absence of methanochondroitin there was no recovery of viable cells from aerobically desiccated cultures. Interestingly, M. barkeri cells grown with and without methanochondroitin that were desiccated anaerobically and then incubated in air had a higher rate of recovery than cells that were maintained anaerobically both pre- and postdesiccation. Although it is possible that postdesiccation exposure to air stimulated an enhanced defense against free radicals by production of catalase, SOD, and other metabolic antioxidants that increased recovery of viable cells after desiccation, the reason for this phenomenon is unknown at this time.
The results of this study demonstrate unequivocally that M. barkeri, in addition to its ability to adapt to the widest ranges of habitats for an individual methanogenic species, also has the innate capability to survive extended periods of desiccation and resistance to oxidative stress from exposure to air. Prior reports showed that Methanosarcina spp. and other methanogens could survive desiccation for limited periods of time (6, 10, 18, 21, 30). This report quantitatively assesses the survival rates of a methanogenic archaeon after desiccation and subsequent exposure to stress factors that simulate the driest environments on Earth, such as the Antarctic dry valleys and hyperarid core of the Atacama Desert in Chile (14, 24). The ability of desiccated cells to tolerate stress factors typically found in the open atmosphere would enable Methanosarcina spp. to be disseminated in high-altitude dust clouds as a mechanism for explaining the ubiquitous nature of these species in both aqueous and terrestrial environments around the globe (17). Future work is necessary to identify the molecular mechanisms utilized by M. barkeri for maintaining viability under desiccation stress and to determine the absolute limits of survival of Methanosarcina spp. in the most extreme environments found on this planet.
ACKNOWLEDGMENTS
This study was funded by the National Aeronautic and Space Administration, Astrobiology: Exobiology and Evolutionary Biology Program, grant number NNX09AM90G.
We thank Jocelyne Diruggiero for irradiating cells at the University of Maryland College Park Gamma Test Facility.
Footnotes
Published ahead of print 22 December 2011
REFERENCES
- 1. Angel R, Metthies D, Conrad R. 2011. Activation of methanogenesis in arid biological soil crusts despite the presence of oxygen. PLoS One 6:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Apolinario EA, Sowers KR. 1996. Plate colonization of Methanococcus maripaludis and Methanosarcina thermophila in a modified canning jar. FEMS Microbiol. Lett. 145:131–137 [Google Scholar]
- 3. Barbosa HR, Alterthum F. 1992. The role of extracellular polysaccharide in cell viability and nitrogenase activity of Beijerinckia derxii. Can. J. Microbiol. 38:986–988 [Google Scholar]
- 4. Blodgett R. 2006. Bacteriological analytical manual online, appendix 2. Most probable number determination from serial dilutions, 8A ed U.S. Food and Drug Adminstration, Washington, DC [Google Scholar]
- 5. Bomar M, Knoll K, Widdel F. 1985. Fixation of molecular nitrogen by Methanosarcina barkeri. FEMS Microbiol. Lett. 31:47–55 [Google Scholar]
- 6. Boon PI, Mitchell A. 1995. Methanogenesis in the sediments of an Australian freshwater wetland: comparison with aerobic decay, and factors controlling methanogenesis. FEMS Microbiol. Ecol. 18:175–190 [Google Scholar]
- 7. Brioukhanov AL, Netrusov AI, Eggen RI. 2006. The catalase and superoxide dismutase genes are transcriptionally up-regulated upon oxidative stress in the strictly anaerobic archaeon Methanosarcina barkeri. Microbiology 152:1671–1677 [DOI] [PubMed] [Google Scholar]
- 8. Conway de Macario E, Maeder DL, Macario AJL. 2003. Breaking the mould: archaea with all four chaperoning systems. Biochem. Biophys. Res. Commun. 301:811–812 [DOI] [PubMed] [Google Scholar]
- 9. Deppenmeier U, et al. 2002. The genome of Methanosarcina mazei: evidence for lateral gene transfer between Bacteria and Archaea. J. Mol. Microbiol. Biotechnol. 4:453–461 [PubMed] [Google Scholar]
- 10. Fetzer S, Bak F, Conrad R. 1993. Sensitivity of methanogenic bacteria from paddy soil to oxygen and desiccation. FEMS Microbiol. Ecol. 12:107–115 [Google Scholar]
- 11. Galagan JE, et al. 2002. The genome of Methanosarcina acetivorans reveals extensive metabolic and physiological diversity. Genet. Res. 12:532–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heijnen CE, van Veen JA. 1991. A determination of protective microhabitats for bacteria introduced into soil. FEMS Microbiol. Lett. 85:73–80 [Google Scholar]
- 13. Helm RF, et al. 2000. Structural characterization of the released polysaccharide of desiccation tolerant Nostoc commune DRH-1. J. Bacteriol. 182:974–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horowitz N, Cameron R, Hubbard J. 1972. Microbiology of the dry valleys of Antarctica. Sci. 176:242–245 [DOI] [PubMed] [Google Scholar]
- 15. Kandler O, Hippe H. 1977. Lack of peptidoglycan in the cell walls of Methanosarcina barkeri. Arch. Microbiol. 113:57–60 [DOI] [PubMed] [Google Scholar]
- 16. Kandler O, Konig H. 1978. Chemical composition of the peptidoglycan-free cell walls of methanogenic bacteria. Arch. Microbiol. 118:141–152 [DOI] [PubMed] [Google Scholar]
- 17. Kellogg C, Griffin D. 2006. Aerobiology and the global transport of desert dust. Trends Ecol. Evol. 21:638–644 [DOI] [PubMed] [Google Scholar]
- 18. Kendrick MG, Kral TA. 2006. Survival of methanogens during desiccation: implications for life on Mars. Astrobiology 6:546–551 [DOI] [PubMed] [Google Scholar]
- 19. Kilbertus G, Proth J, Vervier B. 1979. Effets de la dessiccation sur les bacteries gram-nagatives d'un sol. Soil Biol. Biochem. 11:109–114 [Google Scholar]
- 20. Kottemann M, Kish A, Iloanusi C, Bjork S, Diruggiero J. 2005. Physiological responses of the haolphilic archaeon Halobacterium sp. strain NRC1 to desiccation and gamma irradiation. Extremophiles 9:219–227 [DOI] [PubMed] [Google Scholar]
- 21. Liu C-T, Miyaki T, Aono T, Oyaizu H. 2008. Evaluation of methanogenic strains and their ability to endure aeration and water stress. Curr. Microbiol. 56:214–218 [DOI] [PubMed] [Google Scholar]
- 22. Lobo AL, Zinder SH. 1988. Diazotrophy and nitrogenase activity in the archaebacterium Methanosarcina barkeri 227. Appl. Environ. Microbiol. 54:1656–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maeder DL, et al. 2006. The Methanosarcina barkeri genome: comparative analysis with Methanosarcina acetivorans and Methanosarcina mazei reveals extensive rearrangement within methanosarcinal genomes. J. Bacteriol. 188:7922–7931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maier R, et al. 2004. Microbial life in the Atacama Desert. Science 306:1289–1290 [DOI] [PubMed] [Google Scholar]
- 25. Mattimore V, Battista JR. 1996. Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J. Bacteriol. 178:633–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mayer HP, Conrad R. 1990. Factors infuencing the population of methanogenic bacteria and the initiation of methane production upon flooding of paddy soil. FEMS Microbiol. Ecol. 73:103–112 [Google Scholar]
- 27. Mitchell AM, Baldwil DS. 1999. The effects of sediment desiccation on the potential for nitrification, denitrification and methanogenesis in an Australian reservoir. Hydrobiologia 392:3–11 [Google Scholar]
- 28. Nelson GO. 1971. Controlled test atmospheres: principles and techniques. Ann Arbor Science Publishers, Inc., Ann Arbor, MI [Google Scholar]
- 29. Osa-Afiana LO, Alexander M. 1982. Differences among cowpea rhizobia in tolerance to high temperature and desiccation in soil. Appl. Environ. Microbiol. 43:435–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peters V, Conrad R. 1995. Methanogenic and other strictly anaerobic bacteria in desert soil and other oxic soils. Appl. Environ. Microbiol. 61:1673–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pooja KP, Chandra TS. 2009. Production and partial characterization of a novel capsular polysaccharide KP-EPS produced by Paenibacillus pabuli strain ATSKP. World J. Microbiol. Biotechnol. 25:835–841 [Google Scholar]
- 32. Potts M. 1994. Desiccation tolerance of prokaryotes. Microbiol. Rev. 58:755–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raychaudhuri S, Karmakar P, Choudhary D, Sarma A, Thakur AR. 2003. Effect of heavy ion irradiation on DNA DSB repair in Methanosarcina barkeri. Anaerobe 9:15–21 [DOI] [PubMed] [Google Scholar]
- 34. Raychaudhuri S, Karmakar P, Thakur AR. 2000. Gamma-ray-induced DNA damage and repair in Methanosarcina barkeri. Anaerobe 6:325–331 [DOI] [PubMed] [Google Scholar]
- 35. Sabra W, Zeng AP, Lunsdorf H, Deckwer WD. 2000. Effect of oxygen on formation and structure of Azotobacter vinelandii alginate and its role in protecting nitrogenase. Appl. Environ. Microbiol. 66:4037–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Siegele DA, Kolter R. 1992. Life after log. J. Bacteriol. 174:345–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singh N, Kendall MM, Liu Y, Boone DR. 2005. Isolation and characterization of methylotrophic methanogens from anoxic marine sediments in Skan Bay, Alaska: description of Methanococcoides alaskense sp. nov., and emended description of Methanosarcina baltica. Int. J. Syst. Evol. Microbiol. 55:2531–2538 [DOI] [PubMed] [Google Scholar]
- 38. Sowers K. 2009. Methanogenesis, p 265–286 In Schaechter M. (ed), Encylopedia of microbiology, 3rd ed Elsevier/Academic Press, Philadelphia, PA [Google Scholar]
- 39. Sowers KR, Boone JE, Gunsalus RP. 1993. Disaggregation of Methanosarcina spp. and growth as single cells at elevated osmolarity. Appl. Environ. Microbiol. 59:3832–3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sowers KR, Gunsalus RP. 1988. Adaptation for growth at various saline concentrations by the archaebacterium Methanosarcina thermophila. J. Bacteriol. 170:998–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sowers KR, Johnson JL, Ferry JG. 1984. Phylogenetic relationships among the methylotrophic methane-producing bacteria and emendation of the family Methanosarcinaceae. Int. J. Syst. Bacteriol. 34:444–450 [Google Scholar]
- 42. Sowers KR, Nelson MJ, Ferry JG. 1984. Growth of acetotrophic, methane-producing bacteria in a pH auxostat. Curr. Microbiol. 11:227–230 [Google Scholar]
- 43. Staley JT. 1980. The gas vacuole: an early organelle of prokaryote motility? Orig. Life Evol. Biospheres 10:111–116 [Google Scholar]
- 44. Tamaru Y, Takani Y, Yoshida T, Sakamoto T. 2005. Crucial role of extracellular polysaccharides in desiccation and freezing tolerance in the terrestrial Cyanobacterium nostoc commune. Appl. Environ. Microbiol. 71:7327–7333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vriezen JC, de Bruijn FJ, Nüsslein K. 2006. Desiccation responses and survival of Sinorhizobium meliloti USDA 1021 in relation to growth phase, temperature, chloride and sulfate availability. Lett. Appl. Microbiol. 42:172–178 [DOI] [PubMed] [Google Scholar]
- 46. Walsby AE. 1994. Gas vesicles. Microbiol. Rev. 58:94–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang W, Culley DE, Nie L, Brockman FJ. 2006. DNA microarray analysis of anaerobic Methanosarcina barkeri reveals responses to heat shock and air exposure. J. Ind. Microbiol. Biotechnol. 33:784–790 [DOI] [PubMed] [Google Scholar]