Abstract
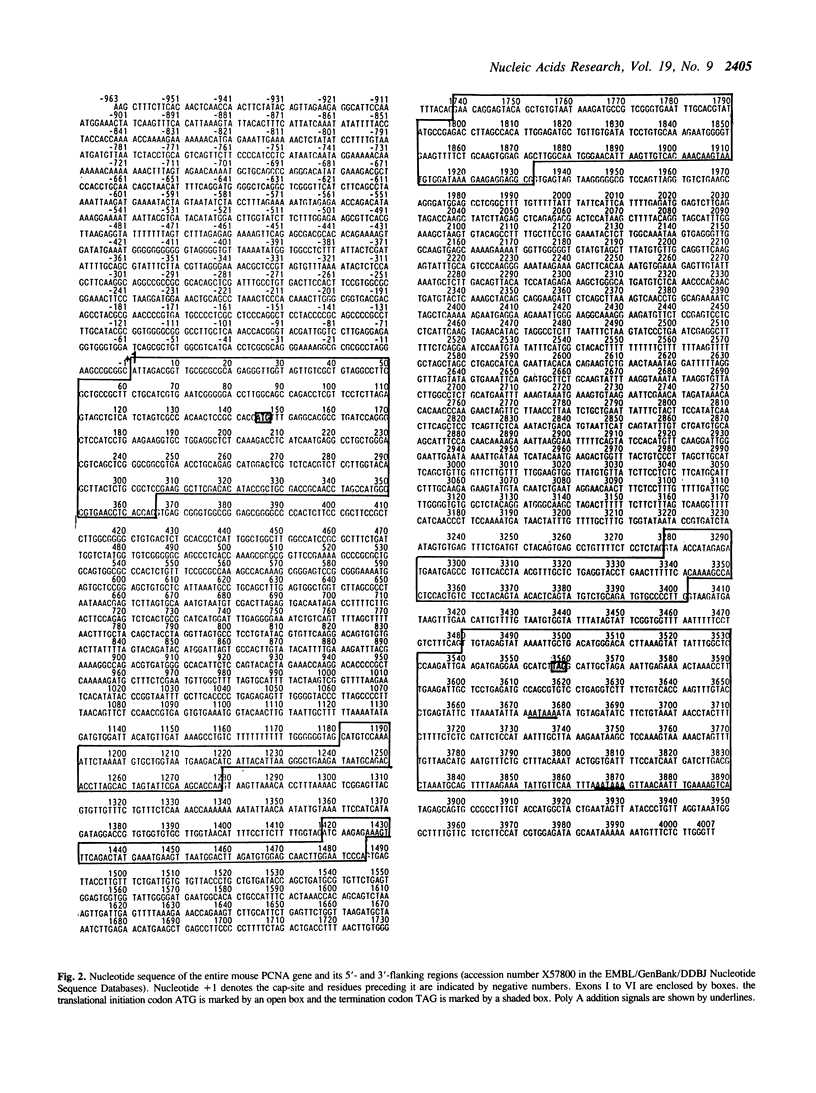
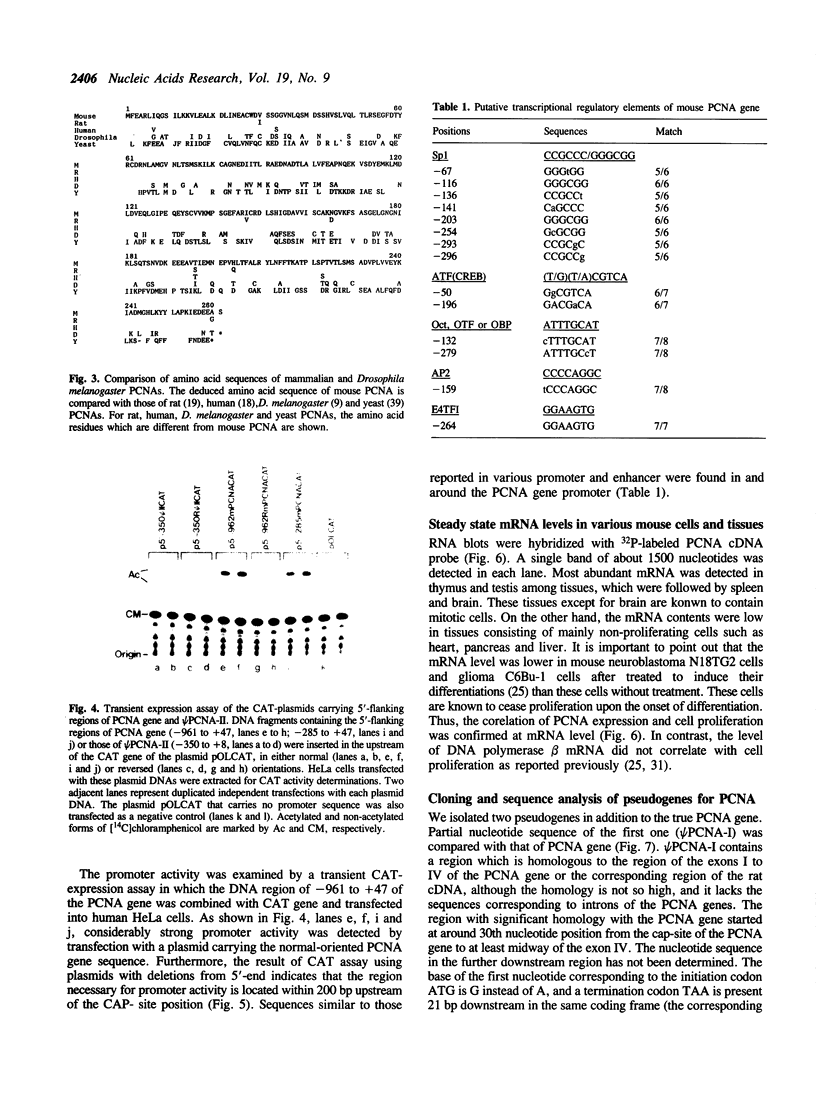
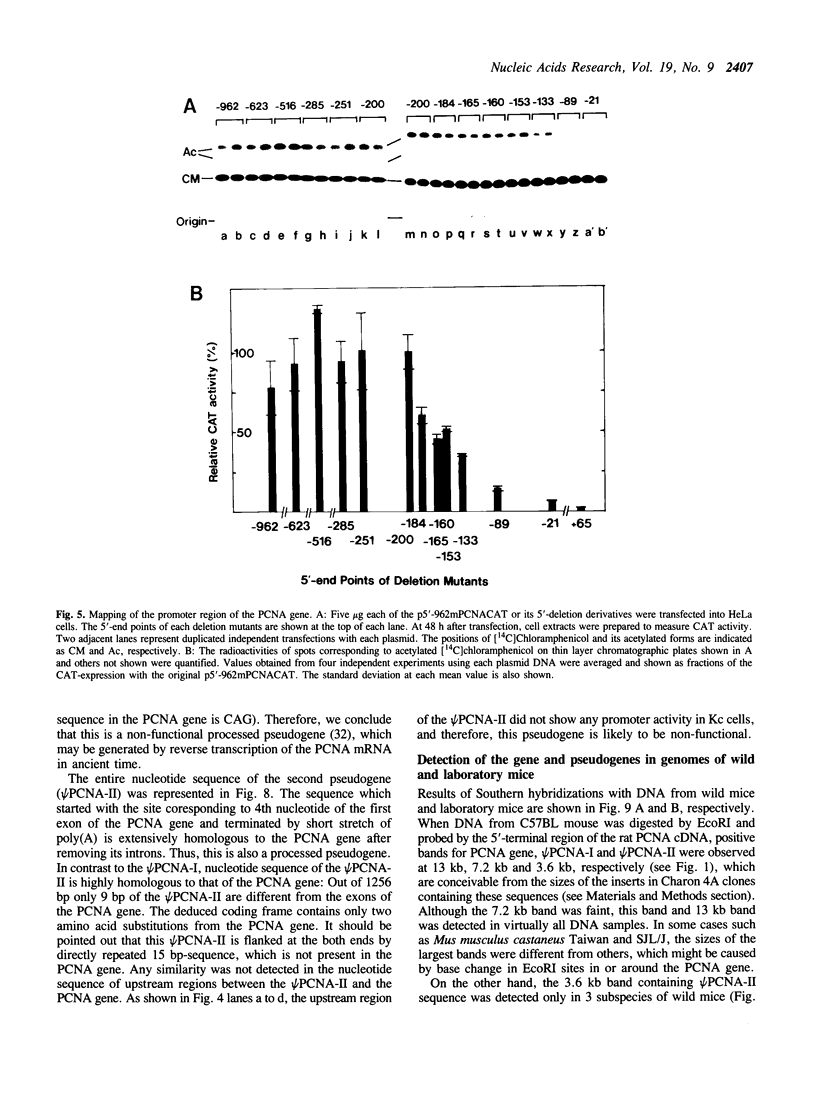
We have isolated clones containing the entire mouse proliferating cell nuclear antigen (PCNA) gene of 3890 bp and flanking sequences using a rat PCNA cDNA as a probe. The mouse gene has 6 exons whose sequences and junction points of exons with introns are extensively homologous to the human gene while sizes and nucleotide sequences of introns are much less conserved than exons. By a transient expression assay of chloramphenicol acetyltransferase, the promoter of this gene is localized within 200 bp upstream of the transcription initiation site. We have also isolated two processed pseudogenes. Homology between the first one (psi PCNA-I) and the exons of the PCNA gene was 76.8% in the region so far sequenced. The second one (psi PCNA-II) consists of a region highly homologous to the entire exons of the PCNA gene, and only 9 out of total 1256 bp are different from the corresponding exon sequence of the gene. The 5'-flanking region of the psi PCNA-II did not function as an active promoter. Surveys in various wild and laboratory mice genomes suggest that the psi PCNA-II was generated through the reverse transcription process of the PCNA mRNA about 5 x 10(5) years ago in the domesticus subspecies of Mus musculus, the house mouse. The psi PCNA-II is tentatively mapped in the chromosome 17 of the C57BL mouse.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Huebsch D., Blundell P. A., Macdonald-Bravo H., Bravo R. Cloning and sequence of the human nuclear protein cyclin: homology with DNA-binding proteins. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1575–1579. doi: 10.1073/pnas.84.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Bauer G. A., Burgers P. M. Molecular cloning, structure and expression of the yeast proliferating cell nuclear antigen gene. Nucleic Acids Res. 1990 Jan 25;18(2):261–265. doi: 10.1093/nar/18.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop C. E., Boursot P., Baron B., Bonhomme F., Hatat D. Most classical Mus musculus domesticus laboratory mouse strains carry a Mus musculus musculus Y chromosome. Nature. 1985 May 2;315(6014):70–72. doi: 10.1038/315070a0. [DOI] [PubMed] [Google Scholar]
- Bravo R., Fey S. J., Bellatin J., Larsen P. M., Arevalo J., Celis J. E. Identification of a nuclear and of a cytoplasmic polypeptide whose relative proportions are sensitive to changes in the rate of cell proliferation. Exp Cell Res. 1981 Dec;136(2):311–319. doi: 10.1016/0014-4827(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Bravo R., Macdonald-Bravo H. Changes in the nuclear distribution of cyclin (PCNA) but not its synthesis depend on DNA replication. EMBO J. 1985 Mar;4(3):655–661. doi: 10.1002/j.1460-2075.1985.tb03679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis J. E., Madsen P. Increased nuclear cyclin/PCNA antigen staining of non S-phase transformed human amnion cells engaged in nucleotide excision DNA repair. FEBS Lett. 1986 Dec 15;209(2):277–283. doi: 10.1016/0014-5793(86)81127-9. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Herr W., Sturm R. A., Clerc R. G., Corcoran L. M., Baltimore D., Sharp P. A., Ingraham H. A., Rosenfeld M. G., Finney M., Ruvkun G. The POU domain: a large conserved region in the mammalian pit-1, oct-1, oct-2, and Caenorhabditis elegans unc-86 gene products. Genes Dev. 1988 Dec;2(12A):1513–1516. doi: 10.1101/gad.2.12a.1513. [DOI] [PubMed] [Google Scholar]
- Jaskulski D., Gatti C., Travali S., Calabretta B., Baserga R. Regulation of the proliferating cell nuclear antigen cyclin and thymidine kinase mRNA levels by growth factors. J Biol Chem. 1988 Jul 25;263(21):10175–10179. [PubMed] [Google Scholar]
- Ku D. H., Travali S., Calabretta B., Huebner K., Baserga R. Human gene for proliferating cell nuclear antigen has pseudogenes and localizes to chromosome 20. Somat Cell Mol Genet. 1989 Jul;15(4):297–307. doi: 10.1007/BF01534969. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M., Franza B. R., Jr, Garrels J. I. Identity of the proliferating cell nuclear antigen and cyclin. Nature. 1984 May 24;309(5966):374–376. doi: 10.1038/309374a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Moriuchi T., Koji T., Nakane P. K. Molecular cloning of cDNA coding for rat proliferating cell nuclear antigen (PCNA)/cyclin. EMBO J. 1987 Mar;6(3):637–642. doi: 10.1002/j.1460-2075.1987.tb04802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyachi K., Fritzler M. J., Tan E. M. Autoantibody to a nuclear antigen in proliferating cells. J Immunol. 1978 Dec;121(6):2228–2234. [PubMed] [Google Scholar]
- Miyata T., Yasunaga T. Rapidly evolving mouse alpha-globin-related pseudo gene and its evolutionary history. Proc Natl Acad Sci U S A. 1981 Jan;78(1):450–453. doi: 10.1073/pnas.78.1.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. S., Sullivan K., Tan E. M., Prystowsky M. B. Proliferating cell nuclear antigen/cyclin is an interleukin 2-responsive gene. J Biol Chem. 1987 Jun 25;262(18):8447–8450. [PubMed] [Google Scholar]
- Odaka T., Ikeda H., Yoshikura H., Moriwaki K., Suzuki S. Fv-4: gene controlling resistance to NB-tropic Friend murine leukemia virus. Distribution in wild mice, introduction into genetic background of BALB/c mice, and mapping of chromosomes. J Natl Cancer Inst. 1981 Nov;67(5):1123–1127. [PubMed] [Google Scholar]
- Ottavio L., Chang C. D., Rizzo M. G., Travali S., Casadevall C., Baserga R. Importance of introns in the growth regulation of mRNA levels of the proliferating cell nuclear antigen gene. Mol Cell Biol. 1990 Jan;10(1):303–309. doi: 10.1128/mcb.10.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelich G., Kostura M., Marshak D. R., Mathews M. B., Stillman B. The cell-cycle regulated proliferating cell nuclear antigen is required for SV40 DNA replication in vitro. Nature. 1987 Apr 2;326(6112):471–475. doi: 10.1038/326471a0. [DOI] [PubMed] [Google Scholar]
- Prelich G., Stillman B. Coordinated leading and lagging strand synthesis during SV40 DNA replication in vitro requires PCNA. Cell. 1988 Apr 8;53(1):117–126. doi: 10.1016/0092-8674(88)90493-x. [DOI] [PubMed] [Google Scholar]
- Prelich G., Tan C. K., Kostura M., Mathews M. B., So A. G., Downey K. M., Stillman B. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987 Apr 2;326(6112):517–520. doi: 10.1038/326517a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuka I., Daidoji H., Matsuoka M., Kadowaki K., Takasaki Y., Nakane P. K., Moriuchi T. Gene for proliferating-cell nuclear antigen (DNA polymerase delta auxiliary protein) is present in both mammalian and higher plant genomes. Proc Natl Acad Sci U S A. 1989 May;86(9):3189–3193. doi: 10.1073/pnas.86.9.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Miyashita N., Moriwaki K., Kominami R., Muramatsu M., Kanehisa T., Bonhomme F., Petras M. L., Yu Z. C., Lu D. Y. Evolutionary implication of heterogeneity of the nontranscribed spacer region of ribosomal DNA repeating units in various subspecies of Mus musculus. Mol Biol Evol. 1986 Mar;3(2):126–137. doi: 10.1093/oxfordjournals.molbev.a040384. [DOI] [PubMed] [Google Scholar]
- Travali S., Ku D. H., Rizzo M. G., Ottavio L., Baserga R., Calabretta B. Structure of the human gene for the proliferating cell nuclear antigen. J Biol Chem. 1989 May 5;264(13):7466–7472. [PubMed] [Google Scholar]
- Vanin E. F. Processed pseudogenes: characteristics and evolution. Annu Rev Genet. 1985;19:253–272. doi: 10.1146/annurev.ge.19.120185.001345. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Hayashi Y., Ajiro K., Matsukage A. Cell-type-specific expression of mouse DNA polymerase beta-gene is regulated by silencer elements. J Cell Physiol. 1989 Nov;141(2):431–436. doi: 10.1002/jcp.1041410225. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Hayashi Y., Matsukage A. Dual cis-acting negative regulatory elements located upstream of the mouse DNA polymerase beta gene. J Biochem. 1989 Jan;105(1):79–83. doi: 10.1093/oxfordjournals.jbchem.a122623. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Hirose F., Hayashi Y., Nishimoto Y., Matsukage A. Murine DNA polymerase beta gene: mapping of transcription initiation sites and the nucleotide sequence of the putative promoter region. Mol Cell Biol. 1987 May;7(5):2012–2018. doi: 10.1128/mcb.7.5.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Nishida Y., Moriuchi T., Hirose F., Hui C. C., Suzuki Y., Matsukage A. Drosophila proliferating cell nuclear antigen (cyclin) gene: structure, expression during development, and specific binding of homeodomain proteins to its 5'-flanking region. Mol Cell Biol. 1990 Mar;10(3):872–879. doi: 10.1128/mcb.10.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekawa H., Moriwaki K., Gotoh O., Miyashita N., Migita S., Bonhomme F., Hjorth J. P., Petras M. L., Tagashira Y. Origins of laboratory mice deduced from restriction patterns of mitochondrial DNA. Differentiation. 1982;22(3):222–226. doi: 10.1111/j.1432-0436.1982.tb01255.x. [DOI] [PubMed] [Google Scholar]
- Zerler B., Roberts R. J., Mathews M. B., Moran E. Different functional domains of the adenovirus E1A gene are involved in regulation of host cell cycle products. Mol Cell Biol. 1987 Feb;7(2):821–829. doi: 10.1128/mcb.7.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmudzka B. Z., Fornace A., Collins J., Wilson S. H. Characterization of DNA polymerase beta mRNA: cell-cycle and growth response in cultured human cells. Nucleic Acids Res. 1988 Oct 25;16(20):9587–9596. doi: 10.1093/nar/16.20.9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber M., Tan E. M., Ryoji M. Involvement of proliferating cell nuclear antigen (cyclin) in DNA replication in living cells. Mol Cell Biol. 1989 Jan;9(1):57–66. doi: 10.1128/mcb.9.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]







