Abstract
DNA excision repair modulates the mutagenic effect of many genotoxic agents. The recently observed strand specificity for removal of UV-induced cyclobutane dimers from actively transcribed genes in mammalian cells could influence the nature and distribution of mutations in a particular gene. To investigate this, we have analyzed UV-induced DNA repair and mutagenesis in the same gene, i.e. the hypoxanthine phosphoribosyl-transferase (hprt) gene. In 23 hprt mutants from V79 Chinese hamster cells induced by 2 J/m2 UV we found a strong strand bias for mutation induction: assuming that pre-mutagenic lesions occur at dipyrimidine sequences, 85% of the mutations could be attributed to lesions in the nontranscribed strand. Analysis of DNA repair in the hprt gene revealed that more than 90% of the cyclobutane dimers were removed from the transcribed strand within 8 hours after irradiation with 10 J/m2 UV, whereas virtually no dimer removal could be detected from the nontranscribed strand even up to 24 hr after UV. These data present the first proof that strand specific repair of DNA lesions in an expressed mammalian gene is associated with a strand specificity for mutation induction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biernat J., Göbel U. B., Köster H. New bacteriophage vectors for the large scale production of single stranded insert DNA. J Biochem Biophys Methods. 1989 Aug-Sep;19(2-3):155–167. doi: 10.1016/0165-022x(89)90023-7. [DOI] [PubMed] [Google Scholar]
- Biernat J., Göbel U. B., Köster H. Preparation of single stranded insert DNA free of vector sequences. Nucleic Acids Res. 1989 Jan 25;17(2):810–810. doi: 10.1093/nar/17.2.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
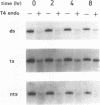
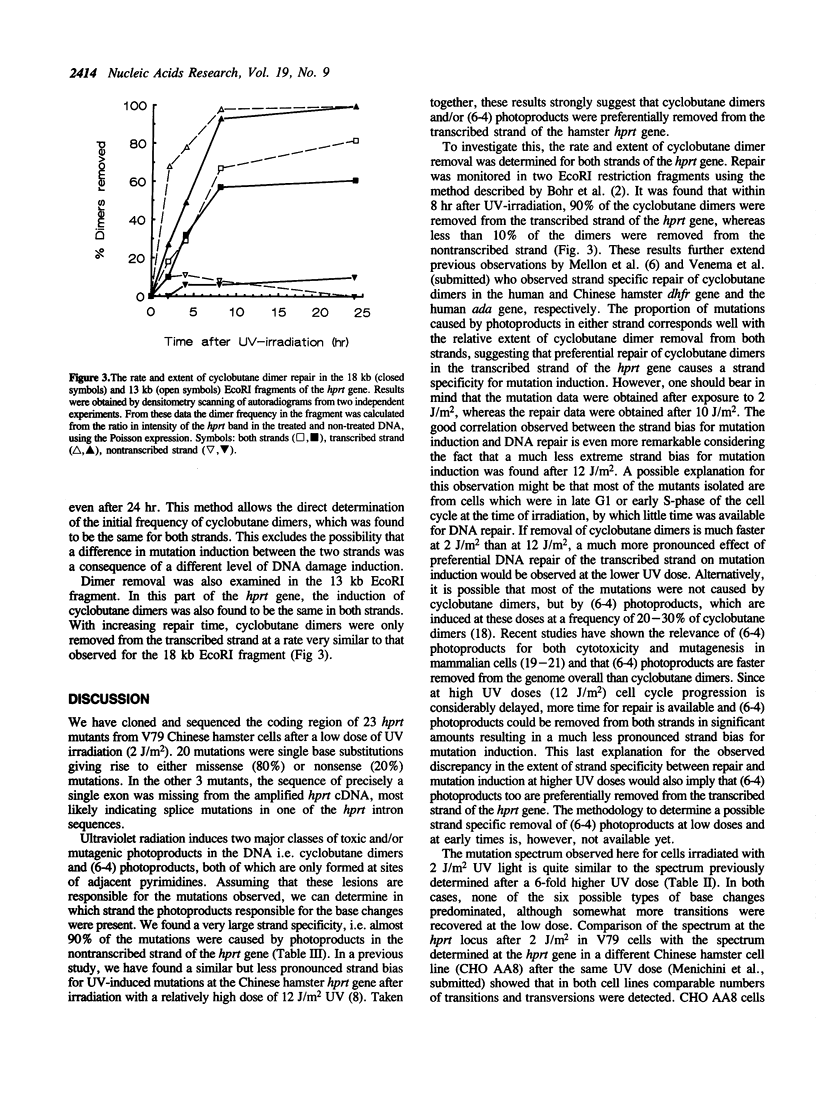
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Cortés F., Lutze L. H., Morgan W. F., Player A. N., Mitchell D. L. Unique DNA repair properties of a xeroderma pigmentosum revertant. Mol Cell Biol. 1987 Sep;7(9):3353–3357. doi: 10.1128/mcb.7.9.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobetsky E. A., Grosovsky A. J., Glickman B. W. The specificity of UV-induced mutations at an endogenous locus in mammalian cells. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9103–9107. doi: 10.1073/pnas.84.24.9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobetsky E. A., Grosovsky A. J., Skandalis A., Glickman B. W. Perspectives on UV light mutagenesis: investigation of the CHO aprt gene carried on a retroviral shuttle vector. Somat Cell Mol Genet. 1989 Sep;15(5):401–409. doi: 10.1007/BF01534891. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Madhani H. D., Bohr V. A., Hanawalt P. C. Differential DNA repair in transcriptionally active and inactive proto-oncogenes: c-abl and c-mos. Cell. 1986 May 9;45(3):417–423. doi: 10.1016/0092-8674(86)90327-2. [DOI] [PubMed] [Google Scholar]
- Mayne L. V., Lehmann A. R. Failure of RNA synthesis to recover after UV irradiation: an early defect in cells from individuals with Cockayne's syndrome and xeroderma pigmentosum. Cancer Res. 1982 Apr;42(4):1473–1478. [PubMed] [Google Scholar]
- Mellon I., Bohr V. A., Smith C. A., Hanawalt P. C. Preferential DNA repair of an active gene in human cells. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8878–8882. doi: 10.1073/pnas.83.23.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Nairn R. S. The biology of the (6-4) photoproduct. Photochem Photobiol. 1989 Jun;49(6):805–819. doi: 10.1111/j.1751-1097.1989.tb05578.x. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L. The relative cytotoxicity of (6-4) photoproducts and cyclobutane dimers in mammalian cells. Photochem Photobiol. 1988 Jul;48(1):51–57. doi: 10.1111/j.1751-1097.1988.tb02785.x. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y., Yamashita K., Sekiguchi M. Purification and characterization of normal and mutant forms of T4 endonuclease V. J Biol Chem. 1982 Mar 10;257(5):2556–2562. [PubMed] [Google Scholar]
- Romac S., Leong P., Sockett H., Hutchinson F. DNA base sequence changes induced by ultraviolet light mutagenesis of a gene on a chromosome in Chinese hamster ovary cells. J Mol Biol. 1989 Sep 20;209(2):195–204. doi: 10.1016/0022-2836(89)90272-6. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Mitchell D. L., Regan J. D., Bouffler S. D., Stewart S. A., Carrier W. L., Nairn R. S., Johnson R. T. CHO mutant UV61 removes (6-4) photoproducts but not cyclobutane dimers. Mutagenesis. 1989 Mar;4(2):140–146. doi: 10.1093/mutage/4.2.140. [DOI] [PubMed] [Google Scholar]
- Venema J., van Hoffen A., Natarajan A. T., van Zeeland A. A., Mullenders L. H. The residual repair capacity of xeroderma pigmentosum complementation group C fibroblasts is highly specific for transcriptionally active DNA. Nucleic Acids Res. 1990 Feb 11;18(3):443–448. doi: 10.1093/nar/18.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieling H., Simons J. W., van Zeeland A. A. Nucleotide sequence determination of point mutations at the mouse HPRT locus using in vitro amplification of HPRT mRNA sequences. Mutat Res. 1988 Mar;198(1):107–113. doi: 10.1016/0027-5107(88)90046-2. [DOI] [PubMed] [Google Scholar]
- Vrieling H., Van Rooijen M. L., Groen N. A., Zdzienicka M. Z., Simons J. W., Lohman P. H., van Zeeland A. A. DNA strand specificity for UV-induced mutations in mammalian cells. Mol Cell Biol. 1989 Mar;9(3):1277–1283. doi: 10.1128/mcb.9.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdzienicka M. Z., van der Schans G. P., Westerveld A., van Zeeland A. A., Simons J. W. Phenotypic heterogeneity within the first complementation group of UV-sensitive mutants of Chinese hamster cell lines. Mutat Res. 1988 Jan;193(1):31–41. doi: 10.1016/0167-8817(88)90005-3. [DOI] [PubMed] [Google Scholar]
- van Zeeland A. A., Smith C. A., Hanawalt P. C. Sensitive determination of pyrimidine dimers in DNA of UV-irradiated mammalian cells. Introduction of T4 endonuclease V into frozen and thawed cells. Mutat Res. 1981 Jun;82(1):173–189. doi: 10.1016/0027-5107(81)90148-2. [DOI] [PubMed] [Google Scholar]