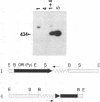
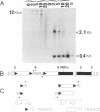
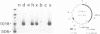Abstract
We attempted to use the polymerase chain reaction (PCR) to monitor in vitro recombination in a plasmid containing directly repeated sequences. Some of the plasmid preparations which had not been exposed to recombination conditions were however found to behave in the PCR test as if they had undergone homologous recombination. We show here that such false positives are attributable to a small degree of nicking and/or breaking of the DNA template. Presumably, such damage allows the formation of hybrid parental duplexes containing at least one truncated strand, the 3' end of which maps within the homology; extension of this 3' end by the polymerase then results in a linkage of sequences identical to that arising from homologous recombination.
Full text
PDF

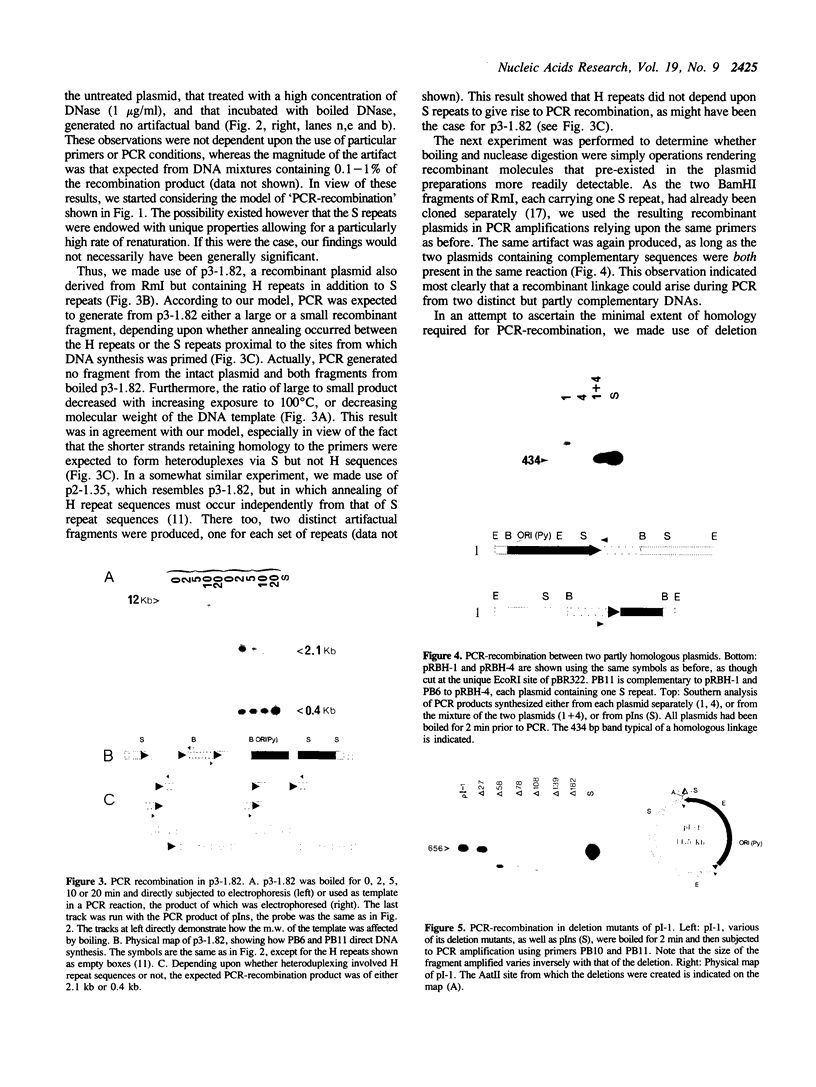

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgaux P., Gendron D., Bourgaux-Ramoisy D. Preferred crossover sites on polyomavirus DNA. J Virol. 1990 May;64(5):2327–2336. doi: 10.1128/jvi.64.5.2327-2336.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgaux P., Sylla B. S., Chartrand P. Excision of polyoma virus DNA from that of a transformed mouse cell: identification of a hybrid molecule with direct and inverted repeat sequences at the viral-cellular joints. Virology. 1982 Oct 15;122(1):84–97. doi: 10.1016/0042-6822(82)90379-8. [DOI] [PubMed] [Google Scholar]
- Finn G. K., Kurz B. W., Cheng R. Z., Shmookler Reis R. J. Homologous plasmid recombination is elevated in immortally transformed cells. Mol Cell Biol. 1989 Sep;9(9):4009–4017. doi: 10.1128/mcb.9.9.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frappier D., Gendron D., Bourgaux-Ramoisy D., Bourgaux P. Alternative homologous and nonhomologous products arising from intramolecular recombination. J Virol. 1990 Oct;64(10):5058–5065. doi: 10.1128/jvi.64.10.5058-5065.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. S., Smithies O. Recombinant fragment assay for gene targetting based on the polymerase chain reaction. Nucleic Acids Res. 1988 Sep 26;16(18):8887–8903. doi: 10.1093/nar/16.18.8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley M. J., Bennett S. E., Mosbaugh D. W. Characterization of the 5' to 3' exonuclease associated with Thermus aquaticus DNA polymerase. Nucleic Acids Res. 1990 Dec 25;18(24):7317–7322. doi: 10.1093/nar/18.24.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhans A., Vartanian J. P., Wain-Hobson S. DNA recombination during PCR. Nucleic Acids Res. 1990 Apr 11;18(7):1687–1691. doi: 10.1093/nar/18.7.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Olsen D. B., Eckstein F. Incomplete primer extension during in vitro DNA amplification catalyzed by Taq polymerase; exploitation for DNA sequencing. Nucleic Acids Res. 1989 Dec 11;17(23):9613–9620. doi: 10.1093/nar/17.23.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piché A., Bourgaux P. Resolution of a polyomavirus-mouse hybrid replicon: release of genomic viral DNA. J Virol. 1987 Mar;61(3):840–844. doi: 10.1128/jvi.61.3.840-844.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piché A., Bourgaux P. Resolution of a polyomavirus-mouse hybrid replicon: viral function required for recombination. J Virol. 1987 Mar;61(3):845–850. doi: 10.1128/jvi.61.3.845-850.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Shuldiner A. R., Nirula A., Roth J. Hybrid DNA artifact from PCR of closely related target sequences. Nucleic Acids Res. 1989 Jun 12;17(11):4409–4409. doi: 10.1093/nar/17.11.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sylla B. S., Allard D., Roy G., Bourgaux-Ramoisy D., Bourgaux P. A mouse DNA sequence that mediates integration and excision of polyoma virus DNA. Gene. 1984 Sep;29(3):343–350. doi: 10.1016/0378-1119(84)90063-5. [DOI] [PubMed] [Google Scholar]
- Tindall K. R., Kunkel T. A. Fidelity of DNA synthesis by the Thermus aquaticus DNA polymerase. Biochemistry. 1988 Aug 9;27(16):6008–6013. doi: 10.1021/bi00416a027. [DOI] [PubMed] [Google Scholar]