TOC summary: A major outbreak affected dogs, domestic livestock, and humans.
Keywords: Rabies, epidemic, Bhutan, epidemiology, geographic information systems, spatial distribution, viruses, zoonoses, research
Abstract
From January through July 2008, rabies reemerged in the Chhukha district of southwestern Bhutan. To clarify the distribution and direction of spread of this outbreak, we mapped reported cases and conducted directional tests (mean center and standard deviational ellipse). The outbreak resulted in the death of 97 animals (42 cattle, 52 dogs, and 3 horses). Antirabies vaccine was given free of charge to ≈674 persons suspected to have been exposed. The outbreak spread south to north and appeared to follow road networks, towns, and areas of high human density associated with a large, free-roaming, dog population. The outbreak was controlled by culling free-roaming dogs. To prevent spread into the interior of Bhutan, a well-coordinated national rabies control program should be implemented in disease-endemic areas.
Rabies is a fatal zoonosis caused by rabies virus or rabies-related viruses (genus Lyssavirus) and transmitted by the bite of a rabid animal (1). Domestic dogs are the main (>95%) source of human rabies infection. An estimated 55,000 persons die of rabies in Asia and Africa each year (2), >20,000 in India alone (3).
In Bhutan, rabies is endemic to the southern districts that border India (4,5). Domestic dogs are the main reservoir and are responsible for spillover infection of other domestic animals, especially cattle. Sporadic human deaths have also been reported in south-central and southwestern rabies-endemic areas of Bhutan (6,7).
On January 23, 2008, a clinical case of rabies in a cow in Dala, a subdistrict of the Chhukha district, was reported and later confirmed by fluorescent antibody test (8,9). The cow had reportedly been bitten ≈3 weeks earlier by a stray dog with suspected rabies. On the same day, another case was reported (and later confirmed by fluorescent antibody test) in a stray dog in the town of Tshimalakha, Bjachho subdistrict. A retrospective epidemiologic field investigation found that an unreported rabies outbreak in dogs had occurred in the southern villages of Dala subdistrict during November and December 2007.
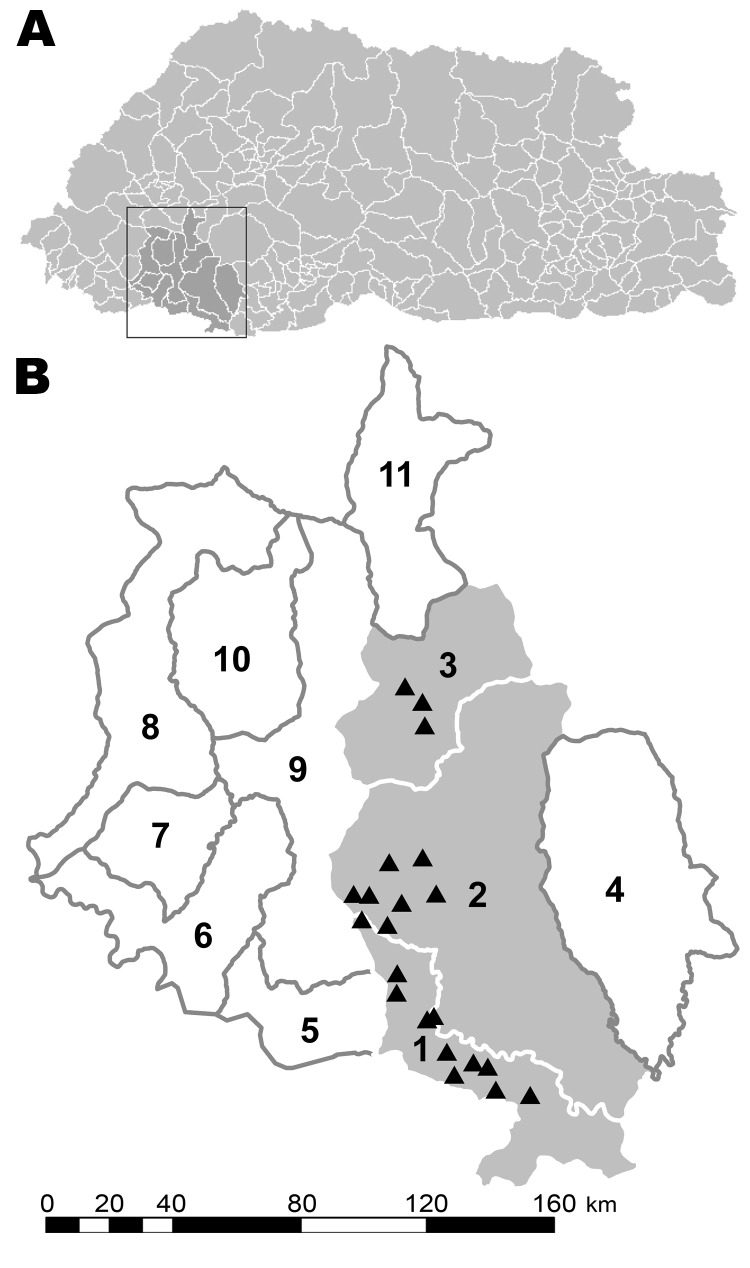
We report a rabies outbreak in the 3 subdistricts of Chhukha district, Bhutan: Dala, Bongo, and Bjachho (Figure 1). To help develop future control programs, our objectives were to 1) describe the spatio–temporal patterns of the outbreak, 2) generate hypotheses about rabies introduction and spread, 3) assess the relationship between animal rabies and public health, and 4) estimate the cost of the outbreak.
Figure 1.
A) Bhutan, with the Chhukha district enclosed. B) The 11 subdistricts of Chukhha district. 1, Dala; 2, Bongo; 3, Bjachho; 4, Genata; 5, Sampheling; 6, Phuentsholing; 7, Logchina; 8, Dungna; 9, Geling; 10, Metap; 11, Chapcha. Gray shading indicates the study areas (1–3); triangles (▲) indicate locations of rabies outbreaks.
Materials and Methods
Data Sources
Outbreak data were obtained from the Veterinary Information System database and included case date, number and species of animals affected, location (village X and Y coordinates), subdistrict, and date and type of intervention activities implemented during the outbreak. Data on number of human exposures, reasons, and type and number of postexposure prophylaxis doses administered were acquired from local hospitals. The study was conducted January 23–July 31, 2008.
Data Analysis
Animal Patterns
The attack rate (no. rabies cases/1,000 animals at risk) was calculated for the outbreak period (10). Animal census data for 2008 or dog population data recorded during a vaccination campaign in 2006 in the main towns of Chhukha district were used to calculate attack rates (Table 1) (11). The χ2 test was used to compare differences in dog and cattle attack rates among subdistricts. We tabulated the number of persons exposed in each subdistrict, number of persons who received postexposure prophylaxis, and reasons for doing so.
Table 1. Attack rates for reported rabies cases, Chhukha district, Bhutan, January 23–July 31, 2008.
| Subdistrict and species | No. cases/total population | Attack rate (95% confidence interval) |
|---|---|---|
| Dala | ||
| Cattle | 18/4,194 | 4 (3–7) |
| Dogs |
12/601 |
20 (11–34) |
| Bongo | ||
| Cattle | 21/2,898 | 7 (5–11) |
| Dogs |
32/1,343 |
24 (17–33) |
| Bjachho | ||
| Cattle | 3/772 | 4 (1–11) |
| Dogs | 8/707 | 11 (6–22) |
Temporal Patterns
The distribution of cases over time was investigated by counting the biweekly number of cases. The relationship between intervention measures (e.g., culling and impounding of free-roaming dogs) and the time series of cases was assessed by counting the number of cases before and after implementation of these control measures.
Spatio-Temporal Patterns
The reported cases were mapped (ArcGIS 9.3; ESRI, Redlands, CA, USA) by using a Bhutan shape file (datum: GRS [Geodectic Reference System] 1980, Spheroid; projection: GCS [Geographic Coordinate System] Bhutan Drukref03, Transverse Mercator). The mean center of cases (average X and Y coordinates, useful for tracking changes in distribution) and a standard deviational ellipse (a measure of directional spread), weighted by date of report of cases (12–16), was calculated (Spatial Statistics Tools; ArcGIS 9.3).
Economics
The cost of the outbreak was analyzed by using 3 simple, direct-cost calculation methods (17). The first calculation was direct cost associated with cattle deaths = number of cattle deaths (n = 42) × average cost of cattle (existing market price of local cattle in Bhutan was Bhutanese ngultrum [Nu.] 10,000). The second calculation was cost of postexposure prophylaxis for humans = total number of human exposures (n = 674) × 5 vaccine doses/person × cost of vaccine (Nu. 450/dose in Bhutan), which was provided free of charge and paid for by the Ministry of Health, Bhutan. The third method was cost of the surveillance and control program, which was calculated on the basis of actual expenditure incurred (removal and impounding of dogs, awareness program), vaccination of ≈200 dogs (at Nu. 25/vaccine dose), and travel and logistics costs for the outbreak response team (paid by the Department of Livestock, Bhutan).
Results
Animal Patterns
During the study period, 97 cases of rabies (42 in cattle, 52 in dogs, 3 in horses) were reported in the subdistricts of Dala (18 cattle, 12 dogs), Bongo (21 cattle, 32 dogs and 3 horses), and Bjachho (3 cattle, 8 dogs) (Table 1). Incidence was 5 (95% confidence interval 4–7) and 20 (95% confidence interval 15–26) cases per 1,000 population at risk for cattle and dogs, respectively. Incidence did not differ significantly between the 3 subdistricts for dogs (χ2 3.65, p = 0.16) or cattle (χ2 3.12, p = 0.21) (Table 1).
Temporal Patterns
The epidemic peak occurred during weeks 11 and 12 (April 3–16), and 65% of cases were reported between weeks 9 and 18 (April and May). The epidemic lasted for 27 weeks and ended in July (Table 2).
Table 2. No. rabies cases in animals reported biweekly by subdistrict, Chhukha district, Bhutan, January 23–July 31, 2008.
| Weeks | DalaV* |
Bongo† |
Bjaccho‡ |
Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cattle | Dogs | Cattle | Dogs | Horses | Cattle | Dogs | ||||
| 1−2 | 2 | 00 | 00 | 00 | 00 | 3 | 3 | 8 | ||
| 3−4 | 1 | 00 | 00 | 00 | 00 | 00 | 00 | 1 | ||
| 5−6 | 00 | 00 | 00 | 00 | 00 | 00 | 2 | 2 | ||
| 7−8 | 5 | 00 | 00 | 00 | 00 | 00 | 00 | 5 | ||
| 9−10 | 1 | 1 | 4 | 3 | 00 | 00 | 1 | 10 | ||
| 11−12 | 4 | 3 | 6 | 4 | 00 | 00 | 1 | 18 | ||
| 13−14 | 1 | 2 | 2 | 5 | 00 | 00 | 1 | 11 | ||
| 15−16 | 00 | 2 | 00 | 9 | 00 | 00 | 00 | 11 | ||
| 17−18 | 3 | 4 | 00 | 6 | 00 | 00 | 00 | 13 | ||
| 19−20 | 1 | 00 | 00 | 3 | 00 | 00 | 00 | 4 | ||
| 21−22 | 00 | 00 | 2 | 00 | 00 | 00 | 00 | 2 | ||
| 23−24 | 00 | 00 | 3 | 1 | 00 | 00 | 00 | 4 | ||
| 25−26 | 00 | 00 | 4 | 1 | 2 | 00 | 00 | 7 | ||
| 27 |
00 |
00 |
|
00 |
00 |
1 |
|
00 |
00 |
1 |
| Total | 18 | 12 | 21 | 32 | 3 | 3 | 8 | 97 | ||
*Free-roaming dogs were culled during weeks 6–9 (Mar 27–Feb 27) and week 24 (Jul 3–9). †Free-roaming dogs in Gedu town were culled during weeks 15–20; dogs were impounded during week 17. ‡Dogs in Tshimalakha and Tshimasham towns were impounded during weeks 16 and 17.
Spatio-Temporal Patterns
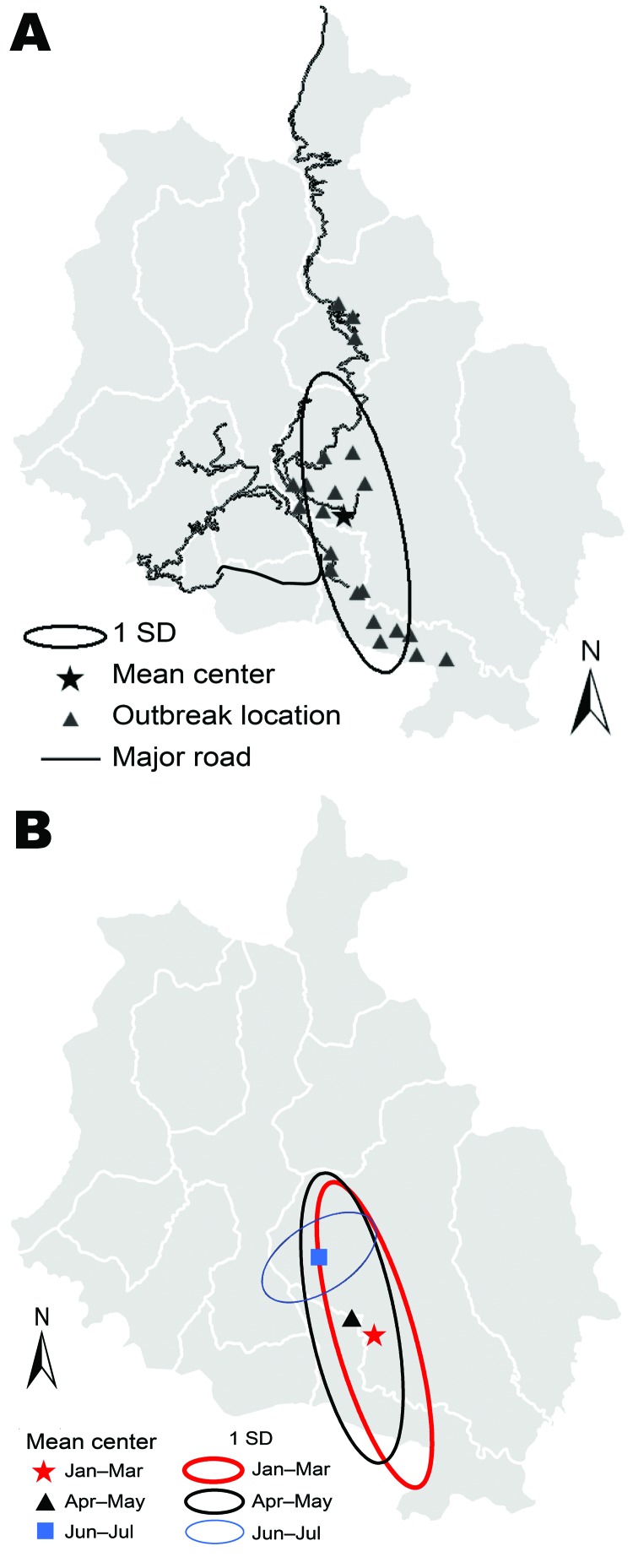
The outbreak (mean center X = 2,700,680 meters; Y = 1,014,350 meters) had an ellipsoid (south-to-north) distribution (Figure 2, panel A). The mean center during consecutive 2-month intervals moved northward (Figure 2, panel B). These distributions overlapped and had a south-to-north direction; however, during the final phase (June–July), the outbreak was distributed west to east and spread in the main town areas of Gedu in Bongo subdistrict and its surrounding villages (Figure 2, panel B). The distribution of cases followed the road network and towns with high human density and high numbers of free-roaming dogs (Figure 2, panel A; some road network data not shown).
Figure 2.
Spatio-temporal patterns of rabies outbreak in the Chhukha district, Bhutan, January 23–July 31, 2008. A) Pattern for the complete outbreak period. B) Patterns during consecutive 2-month intervals during the outbreak period. Jan–Mar period includes January 23–31 (total 69 days; total for other periods 61 days).
Human Exposure Patterns
A total of 674 persons were reported to have been exposed to animals with suspected rabies. Most (77%) exposures were related to contact with rabid animals while either conducting zoosanitary measures or feeding sick animals and by consuming meat and dairy products derived from suspected rabid animals (Table 3). All persons were given antirabies vaccine (5 doses/person) in the hospital. No human deaths were reported during this outbreak.
Table 3. No. human exposures to suspected rabies virus, Chhukha district, Bhutan, January 23–July 31, 2008.
| Subdistrict and exposure |
No. persons exposed |
|---|---|
| Dala | |
| Contact with rabid animals | 30 |
| Bongo | |
| Dog bite | 130 |
| Contact with rabid animals | 132 |
| Consumption of meat or dairy products from rabid animals | 120 |
| Other animal bite |
22 |
| Bjachho | |
| Consumption of meat from rabid animals | 116 |
| Consumption of dairy products from
or contact with rabid animals |
124 |
| Total | 674 |
Outbreak Control
In the outbreak areas, free-roaming dogs were culled during weeks 6–9, 15–20, and 24; a total of 500 dogs were impounded during weeks 16 and 17 and remained in shelters until the outbreak subsided. In the adjacent unaffected areas, ≈200 dogs were vaccinated against rabies. The general public and school students in the outbreak areas were made aware, through public meetings and media announcements, of the dangers of rabies. Culling and impounding of free-roaming dogs is believed to have controlled the outbreak; no cases were detected after July (Table 2).
Outbreak Cost
The direct outbreak cost was estimated to be Nu. 2.75 million (≈US $59,923; 1 US $ = Nu. 46). This cost included cattle deaths (≈Nu. 42,000; 15%); postexposure prophylaxis for humans (≈Nu. 1,516,500; 55%); and implementation of the rabies control program (Nu. 820,000; 30%). The control program cost included ≈Nu. 500,000 for culling, impounding, and awareness programs; ≈Nu. 5,000 for vaccination of domestic dogs; and ≈Nu. 315,000 for the rapid response team (field surveillance and control activities). Because other indirect costs were not taken into account, these costs are likely underestimates.
Discussion
The rabies outbreak in the Chhukha district initially occurred in dogs in the villages in the southern parts of Dala. The index case dog probably bit several other dogs, resulting in sustained animal-to-animal spread among the free-roaming dog population. After this initial focus of infection, infected free-roaming dogs might have spread the disease by biting cattle and other dogs.
The outbreak spread from south to north and seemed to follow the road network and town areas (Figure 2) that had many free-roaming dogs. High numbers of free-roaming dogs would have provided opportunities for infected dogs to transmit the virus to susceptible dogs and then to cattle in the region. Later, the movement of infected free-roaming dogs from some of these towns might have been responsible for the spread of the disease and spillover infection to cattle in surrounding villages (5). However, the culling of free-roaming dogs possibly removed this rabies reservoir from the outbreak areas, resulting in a drastic reduction in the number of cases by June 2008 (18). This corroborates anecdotal field evidence that immediate removal of reservoirs can facilitate the control of a rabies outbreak. In contrast, in a similar large rabies outbreak in eastern Bhutan from May 5, 2005, through the end of 2007, mass culling was not implemented because of the religious sentiments of the local people (5); in this outbreak, widespread dissemination of rabies persisted for much longer.
Postexposure prophylaxis is crucial for preventing rabies in humans after exposure to any rabid animals. Globally, >10 million persons (mostly in Asia) receive postexposure vaccination against rabies (18,19). In the Chhukha district outbreak, ≈674 persons were given full courses (at 0, 3, 7, 14, and 28 days; Essen regimen) of antirabies vaccine, provided free by hospitals. However, most exposures likely carried low risk, e.g., feeding sick (rabid) cattle, touching carcasses while conducting zoosanitary procedures, and consuming cooked meat and dairy products derived from cattle that had died of rabies (because of lack of knowledge about rabies). Except for the few who handled meat or carcasses or were bitten by dogs, others fell under the World Health Organization exposure category I (touching or feeding animals, licks on intact skin); rabies vaccination is usually not recommended for such exposures (19–22). Probably the fear of rabies sensitized the public and ultimately led to mass vaccination of people. Similar mass vaccination after consumption of dairy products from cattle with suspected rabies or handling of rabid animals and contact with confirmed rabies patients has been reported in Bhutan and elsewhere in the world (5,21–23). There are no specific guidelines to assess such nonbite exposure groups. Should a large-scale exposure occur in the future, use of specific criteria and risk assessment for antirabies vaccination may prevent unnecessary use of scarce vaccine resources, whereas public awareness education might prevent future episodes and potential foodborne transmission (18,21,22). Furthermore, in addition to the existing 5-dose intramuscular Essen regimen followed in Bhutan, other postexposure prophylaxis regimens, such as the intradermal method approved by the World Health Organization Expert Committee, should be reviewed because this method is immunogenic, effective, requires fewer doses of vaccine, and costs 70% less than the conventional intramuscular regimen (19,20,24,25).
The estimated cost of this outbreak was large by Bhutan standards and reflects the extent of rabies in a resource-limited country (2,4,17). Globally, it has been estimated that > US$ 1 billion per year is spent on rabies prevention programs, mostly on postexposure prophylaxis (18,19). Similarly, in the Chhukha district outbreak, 55% of the estimated total costs were associated with postexposure prophylaxis for humans. Although vaccinations were free for the recipients, the cost to the Ministry of Health was high. Because the program to eliminate rabies in dogs contributes to the elimination of rabies in humans (or reduces the cost of postexposure prophylaxis), public health and animal health efforts should emphasize the need for control and elimination of rabies in animal reservoirs. In Bhutan, the resources allocated to rabies control in the animal health sector are inadequate and often lead to low vaccination coverage of dogs. Therefore, financial resources should be shared by the public health sector for effective implementation of rabies control and dog management programs.
In conclusion, the Chhukha district rabies outbreak spread consistently from south to north, following the distribution of roads and towns that had large free-roaming dog populations. Rapid culling of in-contact and unvaccinated free-roaming dogs controlled this outbreak. A similar strategy should be considered for any future rabies outbreaks in Bhutan. Because of the risk for spread of rabies from the southern rabies-endemic zone to the rabies-free interior of Bhutan, a well-coordinated national rabies control program should be implemented to prevent and control rabies in Bhutan.
Acknowledgments
We thank the Regional Livestock Development Centre, Tshimasham, for providing outbreak data and the staffs of the livestock sector, Chhukha, for support during the investigation and control program.
Biography
Mr Tenzin is a PhD candidate at the Faculty of Veterinary Science, University of Sydney, Australia, and is supported by University of Sydney International Scholarship. His research interest is the epidemiology of rabies in Bhutan.
Suggested citation for this article: Tenzin, Sharma B, Dhand NK, Timsina N, Ward MP. Reemergence of rabies in Chhukha district, Bhutan, 2008. Emerg Infect Dis [serial on the Internet]. 2010 Dec [date cited]. http://dx.doi.org/10.3201/eid1612.100958
Current affiliation: The University of Sydney, Camden, New South Wales, Australia.
References
- 1.Cleaveland S, Kaare M, Knobel D, Laurenson MK. Canine vaccination—providing broader benefits for disease control. Vet Microbiol. 2006;117:43–50. 10.1016/j.vetmic.2006.04.009 [DOI] [PubMed] [Google Scholar]
- 2.Knobel DL, Cleaveland S, Coleman PG, Fevre EM, Meltzer MI, Miranda MEG, et al. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ. 2005;83:360–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Sudarshan MK, Madhusudana SN, Mahendra BJ, Rao NSN, Narayana ADH, Rahman AS, et al. Assessing the burden of human rabies in India: results of a national multi-center epidemiological survey. Int J Infect Dis. 2007;11:29–35. 10.1016/j.ijid.2005.10.007 [DOI] [PubMed] [Google Scholar]
- 4.Rinzin K. Tenzin, Tebje-Kelly JBU, Tshering P, Stevenson MA. Descriptive spatial and temporal epidemiology of rabies in Bhutan. Proceedings of the 11th International Symposium on Veterinary Epidemiology and Economics. 2006 Aug; Cairns, Queensland, Australia. Fort Collins (CO): International Society for Veterinary Epidemiology and Economics; 2006. [Google Scholar]
- 5.Tenzin, Dhand NK, Dorjee J, Ward MP. Re-emergence of rabies in dogs and other domestic animals in eastern Bhutan, 2005–2007. [Epub ahead of print]. Epidemiol Infect. 2010;:1–6. 10.1017/S0950268810002682 [DOI] [PubMed] [Google Scholar]
- 6.Ministry of Health. Annual health bulletin 2008. [cited 2010 Jun 10]. http://www.health.gov.bt/ahb2008.php
- 7.Second rabies fatality in Gelephu. 2009. Kuensel online [cited 2010 Jun 10]. http://www.kuenselonline.com/modules.php?name=News&file=article&sid=13070.
- 8.Dean DJ, Abelseth MK, Atanasiu P. The fluorescent antobody test. In: Meslin FX, Kaplan MM, Koprowsk H, editors. Laboratory techniques in rabies, 4th ed. Geneva: World Health Organization; 1996. p. 88−95. [Google Scholar]
- 9.McElhinney LM, Fooks AR, Radford AD. Diagnostic tools for the detection of rabies virus. Europran J Comp Animal Prac. 2008;18:224–31. [Google Scholar]
- 10.Smith RD. Veterinary clinical epidemiology—a problem-oriented approach. 2nd ed. Boca Raton (FL): CRC Press, Inc.; 1995. p. 169. [Google Scholar]
- 11.Ministry of Agriculture. Compendium of RNR statistics 2008. [cited 2010 Jun 5]. http://www.rnrstat.bt/csbhutan/documents/Compendium%20of%20RNR%20Stats-2008.pdf
- 12.Ward MP, Carpenter TE. Techniques for analysis of disease clustering in space and time in veterinary epidemiology. Prev Vet Med. 2000;45:257–84. 10.1016/S0167-5877(00)00133-1 [DOI] [PubMed] [Google Scholar]
- 13.Ward MP. Spatio-temporal analysis of infectious disease outbreaks in veterinary medicine: clusters, hotspots and foci. Vet Ital. 2007;43:559–70. [PubMed] [Google Scholar]
- 14.Ward MP, Maftei D, Apostu C, Suru A. Geostatistical visualisation and spatial statistics for evaluation of the dispersion of epidemic highly pathogenic avian influenza subtype H5N1. Vet Res. 2008;39:22. 10.1051/vetres:2007063 [DOI] [PubMed] [Google Scholar]
- 15.Guerra MA, Curns AT, Rupprecht CE, Hanlon CA, Krebs JW, Childs JE. Skunk and raccoon rabies in the eastern United States: temporal and spatial analysis. Emerg Infect Dis. 2003;9:1143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svensson K, Bäck E, Eliasson H, Berglund L, Granberg M, Karlsson L, et al. Landscape epidemiology of tularemia outbreaks in Sweden. Emerg Infect Dis. 2009;15:1937–47. 10.3201/eid1512.090487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meslin FX. The economic burden of rabies in developing countries: cost and benefits from dog rabies elimination. Kenya Vet. 1994;18:523–7. [Google Scholar]
- 18.Expert WHO. Consultation on rabies. First report. World Health Organ Tech Rep Ser. 2005;931:1–121. [PubMed] [Google Scholar]
- 19.World Health Organization. Rabies vaccines WHO position paper. Weekly Epid Record. 2007;82:425–36. [Google Scholar]
- 20.World Health Organization. Recommendations on rabies post-exposure treatment and the correct technique of intradermal immunization against rabies. WHO/EMC/ZOO/96.6. Geneva: The Organization; 1997. [Google Scholar]
- 21.McGuill M, Matyas B, Werner B, DeMaria A Jr. Mass treatment of humans who drank unpasteurized milk from rabid cows—Massachusetts, 1996–1998. JAVMA. 1999;281:1371–2. [Google Scholar]
- 22.Rotz LD, Hensley JA, Rupprecht CE, Childs JE. Large-scale human exposures to rabid or presumed rabid animals in the United States: 22 cases (1990–1996). JAVMA. 1998;212:1198–200. [PubMed] [Google Scholar]
- 23.Rimhanen-Finne R, Järvinen A, Kuusi M, Quiambao BP, Malbas FF Jr, Huovilainen A, et al. Imported human rabies, the Philippines and Finland, 2007. Emerg Infect Dis. 2010;16:1318–9. 10.3201/eid1608.091380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilde H, Khawplod P, Khamoltham T, Hemachudha T, Tepsumethanon V, Lumlerdacha B, et al. Rabies control in south and southeast Asia. Vaccine. 2005;23:2284–9. 10.1016/j.vaccine.2005.01.030 [DOI] [PubMed] [Google Scholar]
- 25.Shantavasinkul P, Tantawichien T, Wilde H, Sawangvaree A, Kumchat A, Ruksaket N, et al. Postexposure rabies prophylaxis completed in 1 week: preliminary study. Clin Infect Dis. 2010;50:56–60. 10.1086/649211 [DOI] [PubMed] [Google Scholar]