Abstract
In 2008, African swine fever was introduced into Georgia, after which it spread to neighboring Armenia, Azerbaijan, and the Russian Federation. That same year, PCR and sequence analysis identified African swine fever virus in samples from 3 dead female wild boars in northwestern Iran. Wild boars may serve as a reservoir.
Keywords: African swine fever virus, wild boars, pathology, PCR, real-time PCR, viruses, Iran, dispatch
African swine fever (ASF) is a notifiable, highly contagious, lethal, hemorrhagic disease in domestic pigs (1,2). ASF virus (ASFV) (International Committee on Taxonomy of Viruses database no. 00.002.0.01.001), an enveloped double-stranded DNA virus, is the only known DNA arbovirus (3). Maintenance and transmission of ASFV involves cycling of virus between soft ticks of the genus Ornithodoros and wild pigs (warthogs, bush pigs, and giant forest boars) (1,2). The virus can also be acquired through ingestion of contaminated feed.
The syndrome caused by ASFV in pigs was initially described in Kenya and later in most other African countries (1,4). In Africa, it causes a long-term, persistent infection in warthogs and bush pigs (2,3,5). Clinical diagnosis of ASF is difficult because signs of ASF and other hemorrhagic diseases are similar and because virulence varies among ASFV isolates (1,2,5,6).
In June 2007, ASFV was identified in the Caucasus region, including Georgia, Russian Federation, and Armenia (2). Diagnosis near the port of Poti, Georgia, was based on clinical findings, and virus identification was later confirmed by laboratory investigations. ASFV might have been introduced into Georgia by ships carrying contaminated pork or pork products from other countries. After entering Georgia, the virus extended into Armenia in August 2007. The probable route of virus entry into Armenia was movement of infected pigs and wild boars across the border (7). By the end of 2007, an outbreak had occurred in Yerevan and Ararat, after which 1 additional case occurred in February 2008.
In December 2007, the Russian Federation reported its first ASF outbreak since the 1970s. The virus may have entered through neighboring Georgia (7,8). In January 2008, presence of ASF was officially confirmed in northwest Azerbaijan, ≈180 km east of the Georgia border (village of Nic). Because most residents of Nic keep pigs in backyard smallholdings, ASFV may have entered Nic in contaminated pork (or pork products) or in infected wild boars (7,8).
In December 2008 and January 2009, ASFV spread to wild boars in northwestern Iran. As in Georgia, initial diagnosis was based on clinical signs and postmortem examinations. Virus identification was subsequently confirmed by laboratory investigations.
The Study
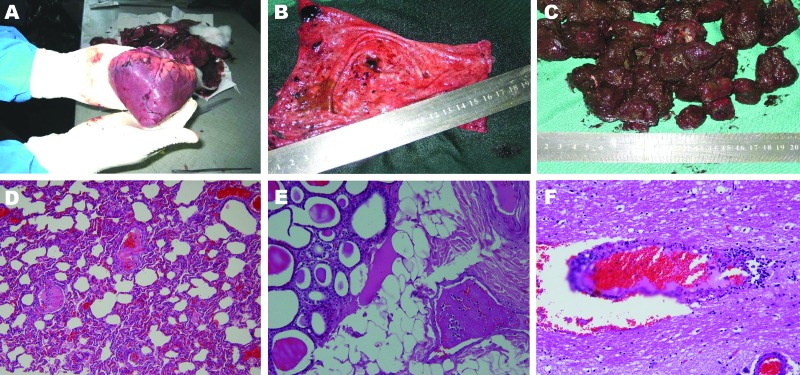
The wild boar population in northwestern Iran is 12,000–13,000. Boars affected by ASFV show weakness, difficulty walking, dragging of the hind legs, dysentery, and sudden death. The first report of dead boars came from 2 villages (Oshdibin and Namngah) in the Jolfa area and then from other cities such as Ahar, Sarab, Maragheh, and Marand. The disease spread to the city of Khoie, located in another province (West Azarbaijan). Postmortem histopathologic investigations of tissue samples of 3 dead, female, wild boars found characteristic signs of ASF, such as disseminated intravascular coagulation with multiple hemorrhages (Figure).
Figure.
Acute form of African swine fever in wild boars. A) Petechial and larger ecchymotic hemorrhages beneath the epicardium. B) Severe hyperemia and petechial and larger ecchymotic hemorrhages in mucosa of urinary bladder. These hemorrhages are common in acute infectious fever and hemorrhagic diathesis. C) Blood-tinged colon contents with fecal balls covered by thick, blood-stained mucus. D) Congestion and fibrinous thromboses in pulmonary vessels and thickening of alveoli (hematoxylin and eosin stain; original magnification ×100). E) Fibrinous thrombus in a venule within interlobular adipose tissue of the thyroid gland (hematoxylin and eosin stain; original magnification ×200). F) Blood vessel congestion, perivascular hemorrhage, lymphocytic perivascular cuffing, and infiltration with degenerating lymphocytes (hematoxylin and eosin stain; original magnification ×200).
Viral DNA from 6 tissue samples (kidney, liver, lung, large intestine, heart, and spleen) from the 3 dead boars was extracted by using the JETQUICK Tissue DNA Spin Kit (GENOMED GmbH, Lohne, Germany) according to the manufacturer’s instructions. ASFV was detected by PCR and SYBR Green real-time PCR on a Rotor-Gene 65H0 (Corbett Life Sciences, Sydney, New South Wales, Australia) in all samples from each boar; primers used were P72 D, U (major capsid protein), and PPA1,2 (in the viral protein 73 coding region of the genome) (6,9). Melting curve analysis showed that an elevated temperature of 86.7°C could generate a specific peak in this curve. The sequences of the PCR products derived by using PPA primer pairs were analyzed by using BLAST (www.ncbi.nlm.nih.gov/blast/Blast.cgi) and showed 100% similarity to submitted sequences of Georgia isolates. A partial sequence of the isolates from Iran has been submitted to GenBank under accession no. FJ897727.
Conclusions
To confirm a specific pathogen and trace the possible sources of infection, genetic characterization of the virus strain associated with disease is crucial (1,2). Real-time PCR and PCR are the most practical ways to differentiate infectious agents that cause similar clinical signs (5,6,9). The 100% similarity between our isolates and those from Georgia suggests that they might have originated from Georgia, probably brought into Iran by infected wild boars crossing the Aras River during the disease incubation period.
The main obstacles to ASF eradication are wildlife reservoirs, limited ability to control movement of infected pigs and wild boars, inadequate laboratory support for rapid and accurate diagnosis, and lack of an effective vaccine (4). Ornithodoros spp. ticks may contribute to virus persistence in the Caucasus region, including northwestern Iran. Although in our study ticks were not detected on the boar carcasses, they should be considered as potential transmission vectors. O. lahorensis ticks are found in areas with a cold climate and are mainly found near sheep and cows, so they are not likely to be isolated from an animal. In the absence of Ornithodoros spp. ticks, transmission to pigs could occur through contact with infected pigs or through feeding of virus-contaminated products (7,8).
The pig industry differs among Caucasus countries. In Azerbaijan, Chechnya, and southern Russian Federation, the pig industry is not as large as it is in Georgia and Armenia (7). In these countries with limited or no pig farms, the virus could be spread by infected wild boars, and the disease could become endemic as it has in Spain and Sardinia (3,7,8,10–12). Because Iran has no commercial pig facilities, the ASFV reservoir would be wild boars, which could transmit disease to neighboring countries that have pig industries, resulting in considerable economic losses.
Acknowledgments
We thank Neda Fakhri Azad and other laboratory technicians of the Veterinary Organization of the Office Centre of Eastern Azerbaijan Province and the Department of Virology of the Pasteur Institute of Iran.
This study was supported by the Pasteur Institute of Iran.
Biography
Dr Rahimi is an assistant professor in the Department of Virology of the Pasteur Institute of Iran. Her research interests are related to enteroviruses and parechoviruses and their pathogenesis. She is also interested in the role of human papillomaviruses in human cancers.
Footnotes
Suggested citation for this article: Rahimi P, Sohrabi A, Ashrafihelan J, Edalat R, Alamdari M, Masoudi M, et al. Emergence of African swine fever virus, northwestern Iran. Emerg Infect Dis [serial on the Internet]. 2010 Dec [date cited]. http://dx.doi.org/10.3201/eid1612.100378
References
- 1.Zsak L, Borca MV, Risatti GR, Zsak A, French RA, Lu Z, et al. Preclinical diagnosis of African swine fever in contact-exposed swine by a real-time PCR assay. J Clin Microbiol. 2005;43:112–9. 10.1128/JCM.43.1.112-119.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowlands RJ, Michaud V, Health L, Hutchings G, Oura C, Vosloo W, et al. African swine fever virus isolate, Georgia, 2007. Emerg Infect Dis. 2008;14:1870–4. 10.3201/eid1412.080591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon LK, Costa JV, Escribano JM, Roch DL, Vinuela E, Wilkinson PJ. Family Asfarviridae. In: Van Regen-Mortel MHV, Fauquet CM, Bishob DHL, Carstens EB, Estes MK, Wickner SM, editors. Virus taxonomy: classification and nomenclature of viruses. Seventh report of the International Committee on Taxonomy of Viruses. San Diego: Academic Press, Inc.; 2000. p. 159–65. [Google Scholar]
- 4.Arias M, Sanchez-Vizcanio JM. African swine fever eradication: the Spanish model. In: Morilla A, Yoon KJ, Zimmerman J, editors. Trends in emerging viral infections of swine. Ames (IA): Iowa State University Press, Inc.; 2002. p. 133–39. [Google Scholar]
- 5.Perez-Filgueira DM, Gonzalez-Camacho F, Gallardo C, Resina-Talavan P, Blanco E, Gomez-Casado E, et al. Optimization and validation of recombinant serological tests for African swine fever diagnosis based on detection of the p30 protein produced in Trichoplusia ni larvae. J Clin Microbiol. 2006;44:3114–21. 10.1128/JCM.00406-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aguero M, Fernandez J, Romero C, Mascaraque S, Arias M, Sanchez-Vizcanio JM. Highly sensitive PCR assay for routine diagnosis of African swine fever virus in clinical samples. J Clin Microbiol. 2003;41:4431–4. 10.1128/JCM.41.9.4431-4434.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Food and Agriculture Organization. African swine fever—outbreak in the Caucasus and why Australia should be aware. 2008. April [cited 2010 Sep 26]. ftp://ftp.fao.org/docrep/fao/011/aj214e/aj214e00.pdf
- 8.Food and Agriculture Organization. African swine fever spread in the Russian Federation and the risk for the region. 2009. Dec [cited 2010 Sep 26]. ftp://ftp.fao.org/docrep/fao/012/ak718e/ak718e00.pdf
- 9.Bastos AD, Penrith ML, Crucière C, Edrich JL, Hutchings G, Roger F, et al. Genotyping field strains of African swine fever virus by partial p72 gene characterization. Arch Virol. 2003;148:693–706. 10.1007/s00705-002-0946-8 [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson PJ. African swine fever virus. In: Pensaert MB, editor. Virus infections of porcines. Amsterdam: Elsevier Science Publishers, Inc.; 1989. p. 17–35. [Google Scholar]
- 11.Office International des Epizooties. African swine fever. In: Manual of diagnostic tests and vaccines for terrestrial animals. Paris: The Office; 2004. p. 1178. [Google Scholar]
- 12.Plowright W, Parker J, Pierce MA. The epizootiology of African swine fever in Africa. Vet Res. 1969;85:668–74. [PubMed] [Google Scholar]