Abstract
Recombinant attenuated Salmonella vaccines have been extensively studied, with a focus on eliciting specific immune responses against foreign antigens. However, very little is known about the innate immune responses, particularly the role of flagellin, in the induction of innate immunity triggered by recombinant attenuated Salmonella in chickens. In the present report, we describe two Salmonella enterica serovar Typhimurium vaccine strains, wild-type (WT) or flagellin-deficient (flhD) Salmonella, both expressing the fusion protein (F) gene of Newcastle disease virus. We examined the bacterial load and spatiotemporal kinetics of expression of inflammatory cytokine, chemokine, and Toll-like receptor 5 (TLR5) genes in the cecum, spleen, liver, and heterophils following oral immunization of chickens with the two Salmonella strains. The flhD mutant exhibited an enhanced ability to establish systemic infection compared to the WT. In contrast, the WT strain induced higher levels of interleukin-1β (IL-1β), CXCLi2, and TLR5 mRNAs in cecum, the spleen, and the heterophils than the flhD mutant at different times postinfection. Collectively, the present data reveal a fundamental role of flagellin in the innate immune responses induced by recombinant attenuated Salmonella vaccines in chickens that should be considered for the rational design of novel vaccines for poultry.
INTRODUCTION
Oral live attenuated Salmonella vaccine vectors expressing recombinant foreign antigens have been shown to induce strong and specific systemic, mucosal, humoral, and cell-mediated immune responses against the foreign antigens in human and animal hosts (5, 19, 27, 55). In addition, Salmonella vectors have the potential to activate innate immune responses and induce inflammatory cytokines and chemokines in mammals and chickens (11, 23, 50, 51). In particular, the cytokine environment of the mucosal or systemic inductive sites dictates the subsequent immune response to the carrier antigens (9, 50, 52). Nonetheless, the innate immune responses in chickens following recombinant attenuated Salmonella immunization are still undetermined.
Flagella are surface appendages of Salmonella enterica serovar Typhimurium that are required for motility and chemotaxis. Flagellin, a constitutive protein of the flagellar apparatus, is a highly evolutionarily conserved molecule that is recognized by host cells through Toll-like receptor 5 (TLR5), contributing to the activation of inflammatory responses and secretion of proinflammatory cytokines and chemokines during Salmonella infections (15, 24). Recently, several studies compared the differences in innate immunity-inducing capacity between wild-type (WT) S. Typhimurium and aflagellar S. Typhimurium in mice and chickens. For instance, Iqbal et al. (21) observed that the aflagellar S. Typhimurium fliM mutant induced less interleukin-1β (IL-1β) mRNA and polymorphonuclear cell infiltration in the chicken gut than did WT S. Typhimurium during the early stages of infection. Further, Simon et al. (38) found no change in the expression of IL-6, IL-12, and gamma interferon (IFN-γ) in the Peyer's patch, spleen, and liver tissues of infected mice following the challenge with WT and aflagellar S. Typhimurium flhC mutant. However, these studies mainly used virulent S. Typhimurium strains. To the best of our knowledge, there is no clear evidence that the expression of flagellin affects the innate responses induced by recombinant attenuated Salmonella vaccine strains.
In the present study, we generated an experimental Newcastle disease (ND) vaccine formulation using two recombinant attenuated S. Typhimurium vaccine strains that differed in the expression of flagellin. The two strains expressed fragments of the fusion protein (F) gene of Newcastle disease virus (NDV). The fusion protein was previously tested in chickens immunized using a DNA vaccine (33). In order to define the contribution of bacterial flagellin to the induction of mediators of the host innate immune response in vivo, we analyzed the relative levels of expression of inflammatory cytokine and chemokine genes in chickens at various times following oral immunization with two recombinant attenuated S. Typhimurium strains.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Bacterial strains Escherichia coli X6212 and S. Typhimurium X4550 and plasmid pYA3334 were kindly provided by R. Curtiss III (The Biodesign Institute, Arizona State University, Tempe, AZ). The bacteria have a chromosomal deletion of the aspartate β-semialdehyde dehydrogenase (asd) gene, which requires complementation by the Asd-positive (Asd+) plasmid pYA3334 expressing heterologous genes (16). E. coli X6212 is an intermediate host used to clone the genes of interest. An aflagellar mutant of X455001 was prepared using the X4550 strain. The flhD gene (flagellum master operon) was mutated by homologous recombination mediated by the suicide plasmid pGMB151 (17). An flhD mutant does not synthesize any flagellar components and therefore has no flagellar secretory apparatus. E. coli and S. Typhimurium cultures were grown at 37°C in Luria-Bertani (LB) broth or on LB agar (Difco, Detroit, MI). When required, the antibiotics nalidixic acid and kanamycin were added to culture media at 50 μg/ml; diaminopimelic acid (DAP) was added (50 μg/ml) for the growth of Asd− strains.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or characteristics | Source or reference |
|---|---|---|
| Strains | ||
| X4550 | SR-11 pStSR101+gyrA1816 Δcrp-1 ΔasdA1 Δ(zhf-4::Tn10) Δcya-1 | R. Curtiss III |
| X6212 | F− λ− ϕ80 Δ(lacZYA-argF) endA1 recA1 hsdR17 deoR thi-1 glnV44 gyrA96 relA1 ΔasdA4 | R. Curtiss III |
| X455001 | ΔflhD derivative of X4550 | 15 |
| KLZ111 | X4550(pYA-F) | This study |
| KLZ112 | X455001 (pYA-F) | This study |
| KLZ113 | X4550(pYA3334) | This study |
| KLZ114 | X455001 (pYA3334) | This study |
| Plasmids | ||
| pYA3334 | Asd+; pUCori | R. Curtiss III |
| pVAX1-F | pVAX1 vector harboring 1.7-kb mature F gene | 31 |
| pYA-F | 0.6-kb DNA encoding the region from amino acid residue 147 to 344 of the mature F protein in pYA3334 | This study |
Expression of recombinant F protein in S. Typhimurium.
DNA manipulations were carried out using methods described by Sambrook et al. (37). Transformation of E. coli and S. Typhimurium was performed by electroporation. Transformants containing Asd+ plasmids were selected on LB agar plates without DAP. Only clones containing the recombinant plasmids were able to grow under these conditions. A fragment of the F gene from amino acid residue 147 to 344 of the mature F protein of NDV (45, 54) was PCR amplified from pVAX1-F (33) template DNA using the primers 5′-AACCATGGGAAATGCTGCCAACATCCTC-3′ (N terminal) and 5′-AAGGATCCATCCAAATCGGTCTCTAC-3′ (C terminal) (restriction sites are underlined and introduced mutations are in bold). The 0.6-kb amplified fragment was cloned into the pYA3334 vector, resulting in pYA-F (Table 1). The plasmids pYA3334 and pYA-F were used to transform competent E. coli X6212. All constructs were verified by restriction enzyme analysis and nucleotide sequencing. Upon confirmation, plasmids pYA3334 and pYA-F were electroporated into the X4550 and X455001 strains and Asd+ transformants were selected on LB plates, resulting in KLZ111 and KLZ112 and vector control strains, KLZ113 and KLZ114. Expression of the F antigen in the cytoplasm and culture supernatants of KLZ111 and KLZ112 was confirmed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, and immunoblot analyses were performed using anti-NDV chicken polyclonal antibody developed in our laboratory. The stability of plasmids was confirmed by the growth of KLZ111 and KLZ112 in LB medium with DAP.
Motility assay.
Bacteria were stab inoculated into motility plates (LB medium containing 0.3% agar). Cultures were incubated upright at 37°C for 12 h and then photographed.
Immunization with S. Typhimurium vaccine strains.
Groups of 100 7-day-old Qingke breeder chickens were orally immunized with 5 × 109 CFU of strain KLZ111 or KLZ112 or mock treated. Four birds from each group were sacrificed at 1, 3, and 5 days postimmunization (p.i.) for postmortem analysis. At each time point, tissue samples of spleen and cecum were aseptically collected into liquid nitrogen for total RNA extraction. Liver, spleen, and cecal contents were obtained for bacterial enumeration. Bacterial culture and enumeration were performed as previously described (39). Briefly, homogenized samples were plated out onto selective Brilliant Green agar (Difco) containing 50 μg of sodium nalidixate/ml with or without 50 μg/ml kanamycin. Plates were incubated at 37°C for 24 h before enumeration of the colonies. Heterophils were isolated from the peripheral blood of 30 chickens per group at each time point. Blood was collected in Vacutainer tubes containing EDTA and mixed thoroughly. Following blood collection, heterophils were isolated using the method described by Redmond et al. (35). All animal experimental protocols were approved by the institute and carried out in accordance with the guidelines for experimental animals established by the Ministry of Science and Technology (Beijing, China).
RNA isolation and qRT-PCR.
Tissues or cells were homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA), and total RNA was prepared as directed by the manufacturer. RNA concentrations were determined by spectrophotometer readings at 260 nm. Quantitative reverse transcriptase PCR (qRT-PCR) was performed to measure mRNA expression levels of IL-1β, IFN-γ, transforming growth factor β4 (TGF-β4), CXCLi2, and TLR5 using SYBR Premix Ex Taq II (Perfect Real Time; TaKaRa Biotechnology, Dalian, China) using an ABI 7500 real-time detection system (Applied Biosystems, Carlsbad, CA). All primer sequences have been previously reported: IL-1β, Y15006 (49); IFN-γ, Y07922 (22); TGF-β4, M31160 (25); CXCLi2, AJ009800 (47); TLR5, AJ626848 (21); and β-actin, L08165 (28). Amplification was performed in a total volume of 20 μl, containing 10 μl of 2× SYBR Premix Ex Taq II, 2 μl of the diluted cDNA, and 0.8 μl of each primer. The real-time PCR program started with denaturing at 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 34 s. Dissociation analysis of amplification products was performed at the end of each PCR to confirm that only one PCR product was amplified and detected. Data were analyzed with ABI 7500 SDS software (ABI), with the baseline being set automatically by the software. The threshold method was used for quantification of the mRNA level (29) and ΔCT values were calculated on the basis of the internal standard β-actin signal. Results were expressed as 2−ΔΔCT (n-fold change compared to the nontreated control group).
Statistical analysis.
Where specified, data were analyzed for statistical significance using an unpaired two-tailed Student t test. A P value of <0.05 was considered significant.
RESULTS
Construction of S. Typhimurium vaccine strains carrying the F gene.
A gene fragment that encodes amino acid residues 147 to 344 (594 bp; 198 amino acids) of the mature F protein (553 amino acids) was selected to use as a test antigen for antigen delivery by a Salmonella carrier. The gene encoding F was introduced into pYA3334. The recombinant plasmid, named pYA-F, was electroporated into the attenuated S. Typhimurium strains X4550 and X455001, which differ in the expression of flagellin, resulting in the selection of the two vaccine strains, named KLZ111 and KLZ112, respectively.
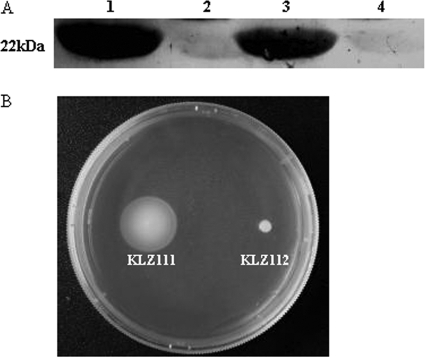
A 22-kDa band reacting with an anti-NDV serum was detected in whole-cell extracts of both strains KLZ111 and KLZ112 (Fig. 1A). Seeding of the vaccine strains in soft agar plates showed that strain KLZ111 was motile while strain KLZ112 was nonmotile (Fig. 1B).
Fig 1.
Expression of NDV F protein and FliC flagellin by the S. Typhimurium vaccine strains. (A) Detection of F protein in whole-cell extracts of the S. Typhimurium vaccine strains. Lanes: 1, KLZ111; 2, KLZ113; 3, KLZ112; 4, KLZ114. (B) Recombinant S. Typhimurium vaccine strains KLZ111 and KLZ112 were stab inoculated into motility agar and photographed.
To examine the stability of pYA-F in X4550 and X455001 in vitro, KLZ111 and KLZ112 were cultured at a daily passage of 1:1,000 dilutions for five consecutive days in LB broth containing DAP. Cells obtained from the last-day culture expressed amounts of the 22-kDa F that were similar to those from the first day (data not shown), suggesting that both Salmonella vectors stably maintained the harbored plasmids.
Recovery of recombinant Salmonella after oral immunization.
Bacterial load is a parameter that may affect the magnitude of the innate immune responses. As an initial step in determining the role of bacterial flagellin in the induction of innate immunity in vivo, chickens were orally inoculated with strain KLZ111 or KLZ112, and bacterial loads in chickens were determined. Chickens were sacrificed at 1, 3, or 5 days p.i. Cecal contents and liver and spleen tissue samples were harvested, and bacterial loads (log CFU/g) were measured.
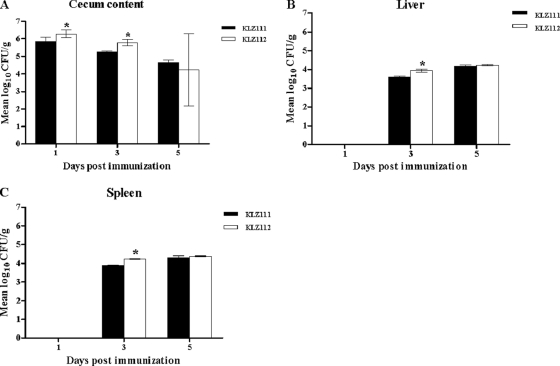
It was determined that the numbers of cells of strains KLZ111 and KLZ112 were high (>104 CFU/g) in cecal contents after immunization, and the level of Salmonella KLZ112 was significantly higher than those found in chickens treated with the KLZ111 strain at 1 and 3 days p.i. (Fig. 2A). However, the numbers of cells of strains KLZ111 and KLZ112 in the cecal contents were equivalent at 5 days p.i. (Fig. 2A). No bacteria were recovered from either the livers or spleens of chickens treated with strains KLZ111 and KLZ112 at 1 day p.i. (Fig. 2B and C). In contrast, the numbers of cells of strain KLZ112 found in the liver and spleen at 3 days p.i. were significantly higher than those for strain KLZ111 (Fig. 2B and C). At 5 days p.i., the numbers of KLZ112 and KLZ111 bacteria in the liver were comparable, indicating that lack of flagella enhances only the initial phases of systemic colonization. The in vitro growth rate of strain KLZ112 in rich medium was similar to that of KLZ111 (data not shown). Taken together, these results suggest that the flagellin-deficient mutant may be able to attain greater numbers upon infection due to an absence of flagellin recognition by the innate immune system of the host.
Fig 2.
Numbers of recombinant S. Typhimurium KLZ111 and KLZ112 bacteria in cecal contents (A), livers (B), and spleens (C) after oral immunization of 7-day-old chickens (4/group). Data shown are CFU/g of Salmonella in each tissue on a logarithmic scale. The error bars indicate standard deviations of the mean. *, statistically significant difference between chickens immunized with Salmonella strains KLZ111 and KLZ112 (P < 0.05).
Quantification of inflammatory cytokine, chemokine, and TLR5 mRNA expression in the cecum, spleen, and heterophils following immunization. (i) Cecum.
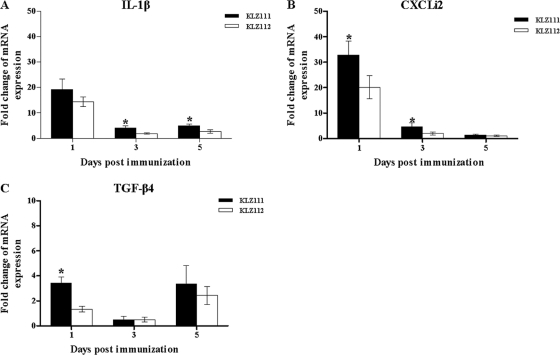
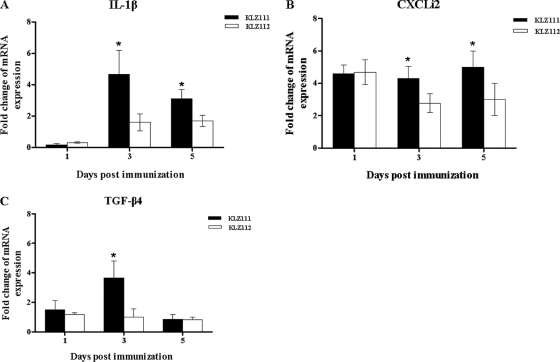
Quantitative analysis of IL-1β expression at 1 day p.i. indicated 19.2- and 14.4-fold increases in KLZ111- and KLZ112-immunized chickens, respectively, compared to mock-treated controls (Fig. 3A). Although reduced expression of IL-1β was found from 1 day p.i. onwards in the ceca of both KLZ111- and KLZ112-immunized chickens, IL-1β levels induced by KLZ111 were significantly higher than those induced by KLZ112 at 3 and 5 days p.i., respectively (Fig. 3A). A statistically significant (P < 0.05) increase in the expression of CXC chemokines (CXCLi2) (32.8- and 20.2-fold increases above control levels, respectively) at 1 day p.i. was found in ceca from KLZ111- and KLZ112-immunized birds compared to mock-treated controls (Fig. 3B). CXCLi2 levels induced by KLZ111 were significantly higher than those induced by KLZ112 at 1 and 3 days p.i. (Fig. 3B). Changes in TGF-β4 mRNA expression were variable over the course of immunization with both recombinant Salmonella strains, and levels of TGF-β4 induced by KLZ111 were significantly higher than those induced by KLZ112 at 1 day p.i. (Fig. 3C). Expression of IFN-γ mRNA was below the detectable threshold for the assay in this experiment.
Fig 3.
Levels of IL-1β (A), CXCLi2 (B), and TGF-β4 (C) mRNAs in ceca of chickens following immunization with recombinant S. Typhimurium KLZ111 or KLZ112. Data shown are the fold changes in mRNA expression compared with that in mock-infected controls, based on triplicate samples from four birds for each time and determined by qRT-PCR. Error bars indicate standard deviations of the means. *, statistically significant difference between chickens immunized with Salmonella strains KLZ111 and KLZ112 (P < 0.05).
(ii) Spleen.
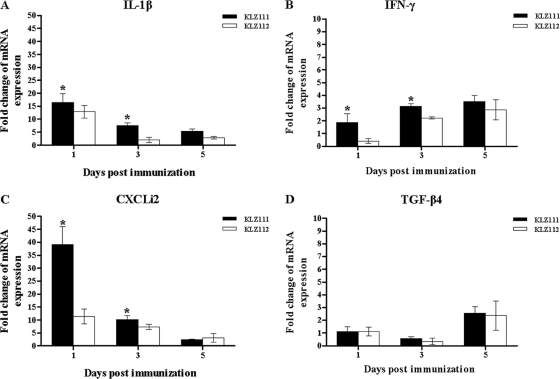
Quantitative analysis of IL-1β and CXCLi2 expression in the spleen showed increases for both KLZ111 (16.5- and 39.1-fold) and KLZ112 (12.9- and 11.4-fold) at 1 day p.i. compared to mock-treated controls (Fig. 4A and C). Reduced expression of IL-1β and CXCLi2 was found from 1 day p.i. onwards in the spleens of both KLZ111- and KLZ112-immunized chickens (Fig. 4A and C). However, IL-1β and CXCLi2 levels induced by KLZ111 were significantly higher than those induced by KLZ112 at 1 and 3 days p.i. (Fig. 4A and C). In contrast, increased expression of IFN-γ was detected from 1 day p.i. onwards in the spleens of both KLZ111- and KLZ112-immunized chickens, and the level induced by KLZ111 was significantly higher than that induced by KLZ112 at 1 and 3 days p.i. (Fig. 4B). The expression level of TGF-β4 in the spleen was not statistically significantly different for the KLZ111- and KLZ112-immunized birds at all time points (Fig. 4D).
Fig 4.
Levels of IL-1β (A), IFN-γ (B), CXCLi2 (C), and TGF-β4 (D) mRNAs in the spleens of chickens following immunization with recombinant S. Typhimurium KLZ111 or KLZ112. Data shown are the fold changes in mRNA expression compared with that in mock-infected controls, based on triplicate samples from four birds for each time determined and by qRT-PCR. Error bars indicate standard deviations of the means. *, statistically significant difference between chickens immunized with Salmonella strains KLZ111 and KLZ112 (P < 0.05).
(iii) Heterophils.
At the three stages investigated, i.e., 1, 3, and 5 days p.i., KLZ111- and KLZ112-immunized animals exhibited a comparably slight increase in the expression of cytokines and chemokines. For IL-1β, enhanced expression was greatest in the KLZ111- and KLZ112-immunized chickens on day 3 (4.7- and 1.7-fold) and diminished on day 5 (Fig. 5A). On days 1, 3, and 5, there was high expression of CXCLi2 in both groups of recombinant Salmonella-treated animals, significantly higher than that found in the controls (Fig. 5B). The levels of IL-1β and CXCLi2 were significantly increased in heterophils of chickens immunized with KLZ111 compared to those treated with KLZ112 at day 3 and 5 days p.i., whereas no difference was observed at day 1 (Fig. 5A and B). The levels of TGF-β4 in the KLZ111-treated chickens were significantly higher than those in the KLZ112-treated chickens at 3 days p.i. (Fig. 5C). However, IFN-γ levels were not significantly different in the heterophils of either KLZ111- or KLZ112-treated birds at all time points (data not shown).
Fig 5.
Levels of IL-1β (A), CXCLi2 (B), and TGF-β4 (C) mRNAs in heterophils of chickens following immunization with recombinant S. Typhimurium KLZ111 or KLZ112. Data shown are the fold changes in mRNA expression compared with that in mock-infected controls, based on triplicate samples from four birds for each time and determined by qRT-PCR. Error bars indicate standard deviations of the means. *, statistically significant difference between chickens immunized with Salmonella strains KLZ111 and KLZ112 (P < 0.05).
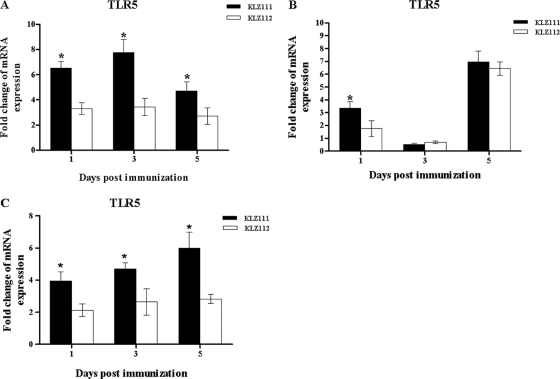
(iv) Quantification of TLR5 mRNA expression following immunization.
TLR5 levels were significantly different in the ceca and heterophils of KLZ111- and KLZ112-treated chickens at all time points (Fig. 6A and C). Expression of TLR5 in the spleens of both groups of recombinant Salmonella-treated chickens was lower on days 1 and 3 than on day 5 (Fig. 6B). However, there was difference in expression in the spleen between KLZ111- and KLZ112-treated chickens at 1 day p.i. (Fig. 6B).
Fig 6.
Levels of TLR5 mRNA in the cecum (A), spleen (B), and heterophils (C) of chickens following immunization with recombinant S. Typhimurium KLZ111 or KLZ112. Data shown are the fold changes in mRNA expression compared with that in mock-infected controls, based on triplicate samples from four birds for each time and determined by qRT-PCR. Error bars indicate standard deviations of the means. *, statistically significant difference between chickens immunized with Salmonella strains KLZ111 and KLZ112 (P < 0.05).
DISCUSSION
Recombinant S. Typhimurium strains have been developed for the delivery of heterologous antigens in chickens, and they induced both mucosal and systemic immune responses (25, 31). In order to develop novel ND vaccines, we generated two recombinant attenuated S. Typhimurium vaccine strains that expressed fragments of the F gene of NDV and determined the inflammatory cytokine and chemokine responses in chickens following recombinant attenuated Salmonella immunizations. In addition, we used WT and flagellin-deficient strains to delineate the role of flagellin in the elicited innate immune responses.
The current study determined that the maximal bacterial numbers were recovered from cecal contents at 1 and 3 days after immunization and that the levels for the WT were significantly lower than those in animals treated with the flhD mutant strain; the loads were comparable on day 5. A wide range of flagellin-deficient strains of S. Typhimurium have been examined for invasion compared with that of wild strains in chickens and rodents after administration by either intravenous, intraperitoneal, or oral routes, with sometimes contradictory conclusions (8, 30, 36, 38, 40). Iqbal et al. (21) showed that the numbers of WT or fliM mutant Salmonella organisms in the cecal contents were equivalent at both 9 and 24 h p.i. in 1-day-old chickens. Allen-Vercoe et al. (4) found that there were no significant differences in the levels of WT or fla mutant strains in the cecal contents at both 1 and 7 days p.i. in 5-day-old chickens. However, consistent with our studies, the number of aflagellate Salmonella organisms recovered from the Peyer's patches was significantly higher than the number of WT bacteria in mice at day 4 p.i. (15).
Despite the growth rates of the two strains being comparable in vitro, higher numbers of flagellin-deficient Salmonella bacteria were detected in the liver and spleen at 3 days p.i. This difference was not observed at 5 days p.i., and the advantage of aflagellar status was short-lived and probably related to an ability to evade early host recognition. Although no Salmonella organisms were detected in the liver or spleen with any of the strains at 1 day p.i. (Fig. 2), the induction of inflammatory genes was observed in the spleen in individual animals (Fig. 4). This induction in the spleen at 1 day after immunization correlated well with the bacterial load at the primary site of infection, the cecum (Fig. 2). The early induction of inflammatory genes seen in the spleen may have been due to the action of cytokines produced in the cecum. Alternatively, induction may also be a result of the release of bacterial pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide, peptidoglycan, or flagellin, from the cecum into the circulatory system, followed by filtering through the hepatic portal system or clearance by the reticuloendothelial system (15, 44).
In chickens, the innate immune response to S. Typhimurium infections is induced by the production of inflammatory cytokines and chemokines, including IL-1, IFN-γ, IL-6, TGF-β4, and CXCLi2, in in vitro and in vivo models (7, 13, 52). However, these studies mainly used WT strains for their analyses, despite the fact that induction of the innate immune response may be needed to protect chickens before the induction of the specific immune response (52). In this study, we found rapid and significant cytokine and chemokine mRNA expression in the cecum and systemic sites upon immunization with both recombinant attenuated Salmonella strains (Fig. 3, 4, and 5). However, there have been contradictory conclusions on the induction of innate immune responses by attenuated Salmonella. A previous study reported an upregulation in both IL-1 and IFN-γ mRNAs in the Peyer's patches and mesenteric lymph nodes in mice following immunization with attenuated Salmonella constructs at day 7 (23). Similarly, Echchannaoui et al. (12) showed that a wide range of genes, including those for chemokines and Th1- and Th2-type cytokines, were upregulated in mice immunized with live attenuated recombinant S. Typhimurium strains compared to control mice at day 1. In contrast, Trebichavsky et al. (46) found that attenuated aroA S. Typhimurium did not induce IL-1β, IL-18, TNF-α, or IFN-γ in the ileum and plasma in gnotobiotic pigs at 24 h after the infection.
In mammals, IL-1β is considered an early-response proinflammatory cytokine produced by many cells in response to microbial challenge. IL-1β is important in the induction of innate response mediators, such as acute-phase proteins and the chemokine CXCLi2 (10). CXCLi2 is important in recruiting neutrophils to sites of inflammation and infection (20). Our study generally demonstrated rapid and significant IL-1β and CXCLi2 mRNA expression upon infection with both recombinant Salmonella strains in the cecum and spleen (Fig. 3 and 4). That is in line with observations from other studies that found prompt expression of IL-1β and CXCLi2 within 2 days of S. Typhimurium infection in the guts and spleens of newly hatched chicks (51). Moreover, the same study found that S. Typhimurium infection results in a pronounced heterophil influx to the cecal lamina propria and liver. It has also been shown that IL-1β mRNA was upregulated in the cecum at day 5 after oral infection with S. Typhimurium (13). In both organs the expression of the chemokine CXCLi2 along with the proinflammatory cytokine IL-1β is indicative of an early inflammatory response (51). Remarkably, Withanage et al. (52) found that infection of chickens with S. Typhimurium induced early expression of chemokines and cytokines in the spleen and gut, accompanied by increased numbers of T cells and the formation of granuloma-like follicular lesions. Therefore, we presume that the chemokines and cytokines induced by attenuated recombinant Salmonella in the cecum and spleen may ultimately enhance the induction of the adaptive immune response.
Heterophils, the avian counterparts of mammalian neutrophils, modulate innate immune response through phagocytosis of invading microbes and foreign particles, production of oxygen intermediates, and release of proteolytic enzymes (42). Therefore, heterophil functional efficiency is of interest for evaluating the potential efficacy of an innate immune response in chickens. Ferro et al. (14) found significant upregulation of proinflammatory cytokine (IL-1β and CXCLi2) mRNA expression in heterophils isolated from line A and D chicks, as supported by the current study, where heterophils isolated from chicken immunized with both recombinant attenuated Salmonella strains showed an increase in IL-1β and CXCLi2 mRNA expression at days 3 and 5 p.i. (Fig. 5). Therefore, an increase in proinflammatory cytokine mRNA expression in heterophils could result in a more efficient and potentially effective immune response.
IFN-γ is a Th1 cytokine that stimulates macrophages to secrete oxidants with antimicrobial activities and is produced by natural killer cells and T lymphocytes (3, 31). Previous studies have reported that the rate of clearance of Salmonella infection correlates with an upregulation of IFN-γ mRNA and a strong T-cell response (6). Surprisingly, transcripts of IFN-γ were not detected in the cecum and heterophils and were observed only in the spleen within 5 days after immunization with both recombinant attenuated Salmonella strains in the current study. In contrast, Withanage et al. (52) showed an upregulation of IFN-γ mRNA of up to 200-fold in the liver, ileum, and cecal tonsils and significant downregulation in the spleen after Salmonella challenge. Therefore, we presume that experimental conditions, such as the age of the animals, bacterial strain, and application dose, may play a critical role in the final experimental outcome.
Expression of the regulatory cytokine TGF-β4 was found after infection, and this may have inhibited inflammatory responses. The conventional paradigm for inflammatory responses is for inverse expression of proinflammatory and anti-inflammatory cytokines (41), as was observed for in vitro experiments using heterophils isolated from commercial lines of birds that differed in resistance to Salmonella infection (43). In the present study, decreased expression of IL-1β and CXCLi2 was found in the spleen and cecum at day 5, and increased expression of TGF-β4 was also found in the same tissues at the same time point, consistent with the role of this cytokine in downregulating inflammatory responses.
The flagella of Salmonella are known to be potent inducers of cytokines. Salmonella flagella can induce rapid de novo synthesis of TNF-α and IL-1β, followed by IL-6 and IL-10, by human peripheral blood mononuclear cells (53). Bacterial flagellin is the major constituent of the bacterial flagellum (34), which can act as an agonist for chicken TLR5 (24) and stimulates chicken TLR5+ cells to upregulate IL-1β, IFN-γ, and CXCLi2 mRNAs (21, 26). Gewirtz et al. (18) have also reported that flagellin of S. Typhimurium is responsible for induction of IL-8 production by intestinal epithelial cells exposed to wild-type organisms.
In this investigation, the levels of proinflammatory cytokines (IL-1β and IFN-γ) and chemokines (CXCLi2) induced by recombinant attenuated Salmonella strain KLZ111 were significantly higher than those induced by the flhD mutant Salmonella strain KLZ112 in various organs and cells. Consistent with these findings, levels of IL-1β mRNA were increased in the cecal tonsils of chicken challenged with fliM mutant or WT S. Typhimurium, with larger amounts induced by flagellum-intact S. Typhimurium. Accordingly, the reduced levels of cecal tonsil IL-1β observed after challenge with aflagellar S. Typhimurium are consistent with the reduced enteric polymorphonuclear cell infiltration (21). Using live Salmonella, chicken kidney cells (CKCs) have been shown to upregulate IL-1β mRNA after exposure to flagellated serovars (S. Typhimurium, S. Enteritidis, and S. Dublin) but not aflagellate S. Gallinarum (22). Gewirtz et al. (18) also showed that an S. Typhimurium flhD mutant failed to induce IL-8 secretion by intestinal epithelial cells but that IL-8 can be induced by the WT strain. These results suggested that flagellin is conducive to the innate immune responses triggered by Salmonella. However, there was no significant difference in IL-6, IL-12, and IFN-γ gene expression in the Peyer's patches and spleen and liver tissues between mice infected with the WT and Salmonella flhC mutant strains (38). It was recently shown that the levels of TNF-α, IL-1β, and IL-12p70 were significantly increased in Peyer's patches and that IFN-γ was highly increased in the medial lymph nodes of mice infected with aflagellate mutants compared to those treated with the WT strain (15). Nevertheless, the disparities between the Salmonella strains, organs, or animals used, together with the types of genes analyzed in these studies, make direct comparisons to our findings difficult.
In the current study, the levels of TLR5 induced by recombinant attenuated Salmonella strains in the spleens of chickens were decreased at day 3 p.i. and increased dramatically at day 5 p.i. In a previous study, TLR5 mRNA expression in the spleens of Leghorn, broiler, and Fayoumi chicken lines was consistently decreased after infection with Salmonella, and there was no significant effect of p.i. time on TLR5 expression level in Salmonella-inoculated and mock-inoculated birds (2). However, Abasht et al. (1) found that male but not female chickens infected with Salmonella showed an increase in the TLR5 RNA expression level in the spleen. Thus, there have been contradictory conclusions about the expression of TLR5 in the spleens of Salmonella-immunized chickens. The downregulation of TLR5 RNA expression in the spleen after Salmonella infection, as observed in this study, might be beneficial for protection of host cells from overstimulation by bacterial flagellin (48). Interestingly, the levels of KLZ111-induced IL-1β, CXCLi2, and IFN-γ mRNAs were not correlated with the level of TLR5 qRT-PCR product detected in our study. In contrast, the levels of TLR5 correlated with the amounts of flagellin-induced IL-1β mRNA in CKC, chicken embryo fibroblast (CEF), and HD11 cell cultures (21), which suggests that there are additional mechanisms responsible for the level of gene expression.
In summary, we have demonstrated that there were differences in the innate responses induced by attenuated recombinant Salmonella and an aflagellate mutant, which pinpoints a critical role of flagellin in the innate immune system in chickens. Recently, the description of TLR5, which recognizes flagellin and has the capacity to activate innate and adaptive immune responses, has intensified the testing of flagellins as vaccine adjuvants for the induction of both antibody and cellular immune responses to foreign antigens (reviewed in reference 32). Therefore, future studies will assess the role of flagellin in the adaptive immune responses to F antigen induced by attenuated recombinant Salmonella.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (no. 30871860 and 31172299), the 863 program (no. 2011AA10A210), the Jangsu Natural Science Foundation (no. BK2010039), the Jiangsu “333” program (no. BRA2011141), the Program for Changjiang Scholars and Innovative Research Team in University (no. IRT0978), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnotes
Published ahead of print 11 January 2012
REFERENCES
- 1. Abasht B, Kaiser MG, Lamont SJ. 2008. Toll-like receptor gene expression in cecum and spleen of advanced intercross line chicks infected with Salmonella enterica serovar Enteritidis. Vet. Immunol. Immunopathol. 123: 314–323 [DOI] [PubMed] [Google Scholar]
- 2. Abasht B, Kaiser MG, van der Poel J, Lamont SJ. 2009. Genetic lines differ in Toll-like receptor gene expression in spleens of chicks inoculated with Salmonella enterica serovar Enteritidis. Poult. Sci. 88: 744–749 [DOI] [PubMed] [Google Scholar]
- 3. Alam MS, et al. 2002. Role of nitric oxide in host defense in murine salmonellosis as a function of its antibacterial and antiapoptotic activities. Infect. Immun. 70: 3130–3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allen-Vercoe E, Woodward MJ. 1999. Colonisation of the chicken caecum by afimbriate and aflagellate derivatives of Salmonella enterica serotype Enteritidis. Vet. Microbiol. 69: 265–275 [DOI] [PubMed] [Google Scholar]
- 5. Ashby D, et al. 2005. Attenuated Salmonella Typhimurium SL3261 as a vaccine vector for recombinant antigen in rabbits. J. Immunol. Methods 299: 153–164 [DOI] [PubMed] [Google Scholar]
- 6. Beal RK, Powers C, Wigley P, Barrow PA, Smith AL. 2004. Temporal dynamics of the cellular, humoral and cytokine responses in chickens during primary and secondary infection with Salmonella enterica serovar Typhimurium. Avian Pathol. 33: 25–33 [DOI] [PubMed] [Google Scholar]
- 7. Berndt A, et al. 2007. Chicken cecum immune response to Salmonella enterica serovars of different levels of invasiveness. Infect. Immun. 75: 5993–6007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carsiotis M, Weinstein DL, Karch H, Holder IA, O'Brien AD. 1984. Flagella of Salmonella typhimurium are a virulence factor in infected C57BL/6J mice. Infect. Immun. 46: 814–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carvajal BG, Methner U, Pieper J, Berndt A. 2008. Effects of Salmonella enterica serovar Enteritidis on cellular recruitment and cytokine gene expression in caecum of vaccinated chickens. Vaccine 26: 5423–5433 [DOI] [PubMed] [Google Scholar]
- 10. Cassatella MA. 1995. The production of cytokines by polymorphonuclear neutrophils. Immunol. Today 16: 21–26 [DOI] [PubMed] [Google Scholar]
- 11. Cho WS, Chae C. 2003. Expression of inflammatory cytokines (TNF-alpha, IL-1, IL-6 and IL-8) in colon of pigs naturally infected with Salmonella typhimurium and S. choleraesuis. J. Vet. Med. A Physiol. Pathol. Clin. Med. 50: 484–487 [DOI] [PubMed] [Google Scholar]
- 12. Echchannaoui H, et al. 2008. Intravaginal immunization of mice with recombinant Salmonella enterica serovar Typhimurium expressing human papillomavirus type 16 antigens as a potential route of vaccination against cervical cancer. Infect. Immun. 76: 1940–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fasina YO, et al. 2008. Intestinal cytokine response of commercial source broiler chicks to Salmonella typhimurium infection. Poult. Sci. 87: 1335–1346 [DOI] [PubMed] [Google Scholar]
- 14. Ferro PJ, Swaggerty CL, Kaiser P, Pevzner IY, Kogut MH. 2004. Heterophils isolated from chickens resistant to extra-intestinal Salmonella enteritidis infection express higher levels of pro-inflammatory cytokine mRNA following infection than heterophils from susceptible chickens. Epidemiol. Infect. 132: 1029–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fournier B, Williams IR, Gewirtz AT, Neish AS. 2009. Toll-like receptor 5-dependent regulation of inflammation in systemic Salmonella enterica serovar Typhimurium infection. Infect. Immun. 77: 4121–4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galan JE, Nakayama K, Curtiss R., III 1990. Cloning and characterization of the asd gene of Salmonella typhimurium: use in stable maintenance of recombinant plasmids in Salmonella vaccine strains. Gene 94: 29–35 [DOI] [PubMed] [Google Scholar]
- 17. Geng SZ, et al. 2010. Construction of flagella mutant by knocking out FlhD gene of Salmonella typhimurium X4550. Chinese Vet. Sci. 40: 1–6 [Google Scholar]
- 18. Gewirtz AT, et al. 2001. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J. Clin. Invest. 107: 99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gunn BM, Wanda SY, Burshell D, Wang C, Curtiss R., III 2010. Construction of recombinant attenuated Salmonella enterica serovar Typhimurium vaccine vector strains for safety in newborn and infant mice. Clin. Vaccine Immunol. 17: 354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hachicha M, Rathanaswami P, Naccache PH, McColl SR. 1998. Regulation of chemokine gene expression in human peripheral blood neutrophils phagocytosing microbial pathogens. J. Immunol. 160: 449–454 [PubMed] [Google Scholar]
- 21. Iqbal M, et al. 2005. Identification and functional characterization of chicken Toll-like receptor 5 reveals a fundamental role in the biology of infection with Salmonella enterica serovar Typhimurium. Infect. Immun. 73: 2344–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaiser P, et al. 2000. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology 146: 3217–3226 [DOI] [PubMed] [Google Scholar]
- 23. Karem KL, Kanangat S, Rouse BT. 1996. Cytokine expression in the gut associated lymphoid tissue after oral administration of attenuated Salmonella vaccine strains. Vaccine 14: 1495–1502 [DOI] [PubMed] [Google Scholar]
- 24. Keestra AM, de Zoete MR, van Aubel RA, van Putten JP. 2008. Functional characterization of chicken TLR5 reveals species-specific recognition of flagellin. Mol. Immunol. 45: 1298–1307 [DOI] [PubMed] [Google Scholar]
- 25. Kogut MH, Rothwell L, Kaiser P. 2003. Differential regulation of cytokine gene expression by avian heterophils during receptor-mediated phagocytosis of opsonized and nonopsonized Salmonella enteritidis. J. Interferon Cytokine Res. 23: 319–327 [DOI] [PubMed] [Google Scholar]
- 26. Kogut MH, Swaggerty C, He H, Pevzner I, Kaiser P. 2006. Toll-like receptor agonists stimulate differential functional activation and cytokine and chemokine gene expression in heterophils isolated from chickens with differential innate responses. Microbes Infect. 8: 1866–1874 [DOI] [PubMed] [Google Scholar]
- 27. Kulkarni RR, Parreira VR, Jiang YF, Prescott JF. 2010. A live oral recombinant Salmonella enterica serovar Typhimurium vaccine expressing Clostridium perfringens antigens confers protection against necrotic enteritis in broiler chickens. Clin. Vaccine Immunol. 17: 205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li S, et al. 2009. Induction of CXC chemokine messenger-RNA expression in chicken oviduct epithelial cells by Salmonella enterica serovar Enteritidis via the type three secretion system-1. Avian Dis. 53: 396–404 [DOI] [PubMed] [Google Scholar]
- 29. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- 30. Lockman HA, Curtiss R., III 1990. Salmonella typhimurium mutants lacking flagella or motility remain virulent in BALB/c mice. Infect. Immun. 58: 137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lowenthal JW, Digby MR, York JJ. 1995. Production of interferon-gamma by chicken T cells. J. Interferon Cytokine Res. 15: 933–938 [DOI] [PubMed] [Google Scholar]
- 32. Mizel SB, Bates JT. 2010. Flagellin as an adjuvant: cellular mechanisms and potential. J. Immunol. 185: 5677–5682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pan ZM, et al. 2005. Safety and efficacy of attenuated Salmonella typhimurium harbouring DNA vaccine against Newcastle disease virus. Acta Microbiol. Sinica 45: 937–941 [PubMed] [Google Scholar]
- 34. Ramos HC, Rumbo M, Sirard JC. 2004. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol. 12: 509–517 [DOI] [PubMed] [Google Scholar]
- 35. Redmond SB, Chuammitri P, Andreasen CB, Palic D, Lamont SJ. 2009. Chicken heterophils from commercially selected and non-selected genetic lines express cytokines differently after in vitro exposure to Salmonella enteritidis. Vet. Immunol. Immunopathol. 132: 129–134 [DOI] [PubMed] [Google Scholar]
- 36. Robertson JM, et al. 2003. Lack of flagella disadvantages Salmonella enterica serovar Enteritidis during the early stages of infection in the rat. J. Med. Microbiol. 52: 91–99 [DOI] [PubMed] [Google Scholar]
- 37. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 38. Simon R, Heithoff DM, Mahan MJ, Samuel CE. 2007. Comparison of tissue-selective proinflammatory gene induction in mice infected with wild-type, DNA adenine methylase-deficient, and flagellin-deficient Salmonella enterica. Infect. Immun. 75: 5627–5639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith HW, Tucker JF. 1975. The effect of antibiotic therapy on the faecal excretion of Salmonella typhimurium by experimentally infected chickens. J. Hyg. (Lond.) 75: 275–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stecher B, et al. 2004. Flagella and chemotaxis are required for efficient induction of Salmonella enterica serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun. 72: 4138–4150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Strober W, et al. 1997. Reciprocal IFN-gamma and TGF-beta responses regulate the occurrence of mucosal inflammation. Immunol. Today 18: 61–64 [DOI] [PubMed] [Google Scholar]
- 42. Swaggerty CL, Kaiser P, Rothwell L, Pevzner IY, Kogut MH. 2006. Heterophil cytokine mRNA profiles from genetically distinct lines of chickens with differential heterophil-mediated innate immune responses. Avian Pathol. 35: 102–108 [DOI] [PubMed] [Google Scholar]
- 43. Swaggerty CL, et al. 2004. Differential cytokine mRNA expression in heterophils isolated from Salmonella-resistant and -susceptible chickens. Immunology 113: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Swank GM, Deitch EA. 1996. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J. Surg. 20: 411–417 [DOI] [PubMed] [Google Scholar]
- 45. Toyoda T, Gotoh B, Sakaguchi T, Kida H, Nagai Y. 1988. Identification of amino acids relevant to three antigenic determinants on the fusion protein of Newcastle disease virus that are involved in fusion inhibition and neutralization. J. Virol. 62: 4427–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Trebichavsky I, et al. 2006. Attenuated aroA Salmonella enterica serovar Typhimurium does not induce inflammatory response and early protection of gnotobiotic pigs against parental virulent LT2 strain. Vaccine 24: 4285–4289 [DOI] [PubMed] [Google Scholar]
- 47. Tsai HJ, Chiu CH, Wang CL, Chou CH. 2010. A time-course study of gene responses of chicken granulosa cells to Salmonella Enteritidis infection. Vet. Microbiol. 144: 325–333 [DOI] [PubMed] [Google Scholar]
- 48. van Aubel RA, Keestra AM, Krooshoop DJ, van Eden W, van Putten JP. 2007. Ligand-induced differential cross-regulation of Toll-like receptors 2, 4 and 5 in intestinal epithelial cells. Mol. Immunol. 44: 3702–3714 [DOI] [PubMed] [Google Scholar]
- 49. Weining KC, Sick C, Kaspers B, Staeheli P. 1998. A chicken homolog of mammalian interleukin-1 beta: cDNA cloning and purification of active recombinant protein. Eur. J. Biochem. 258: 994–1000 [DOI] [PubMed] [Google Scholar]
- 50. Wigley P, et al. 2005. Oral infection with the Salmonella enterica serovar Gallinarum 9R attenuated live vaccine as a model to characterise immunity to fowl typhoid in the chicken. BMC Vet. Res. 1: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Withanage GS, et al. 2004. Rapid expression of chemokines and proinflammatory cytokines in newly hatched chickens infected with Salmonella enterica serovar Typhimurium. Infect. Immun. 72: 2152–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Withanage GS, et al. 2005. Cytokine and chemokine responses associated with clearance of a primary Salmonella enterica serovar Typhimurium infection in the chicken and in protective immunity to rechallenge. Infect. Immun. 73: 5173–5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wyant TL, Tanner MK, Sztein MB. 1999. Salmonella typhi flagella are potent inducers of proinflammatory cytokine secretion by human monocytes. Infect. Immun. 67: 3619–3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yusoff K, et al. 1989. Location of neutralizing epitopes on the fusion protein of Newcastle disease virus strain Beaudette C. J. Gen. Virol. 70: 3105–3109 [DOI] [PubMed] [Google Scholar]
- 55. Zhang XL, Jeza VT, Pan Q. 2008. Salmonella typhi: from a human pathogen to a vaccine vector. Cell Mol. Immunol. 5: 91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]