Abstract
Serotype replacement in invasive pneumococcal disease has been observed after widespread use of the 7-valent pneumococcal conjugate vaccine (PCV7). Replacement is dominated by penicillin-nonsusceptible serotype 19A in several countries. Antibiotic selection pressure has been proposed to interact with immunization, leading to rapid replacement. In Norway, where prescription of antibiotics is limited, post-PCV7 replacement by serotype 19A is dominated by penicillin-susceptible clones. Hence, serotype 19A replacement occurs, although it is not driven by antibiotic selection pressure.
TEXT
Following licensure and widespread use of the 7-valent pneumococcal conjugate vaccine (PCV7), substantial declines in incidence rates of invasive pneumococcal disease (IPD) have been observed (10, 14, 17). The effect is evident both in the age group targeted by vaccination and as an indirect effect in other age groups (10, 14, 15). However, the overall decline in IPD is tempered to a certain extent by increasing incidence rates of non-vaccine serotype IPD due to serotype replacement (7, 10). In particular, serotype 19A has emerged as a dominating cause of IPD in both children and adults in the post-PCV7 era (10, 14).
The proportion of penicillin-nonsusceptible strains among serotype 19A strains has been reported to be high and increasing and is dominated by a limited number of clonal complexes (CCs), i.e., CC199, CC320, and CC276 (2, 13). However, changes in serotype 19A epidemiology have also been observed in PCV7-naïve populations in South Korea and Israel (3, 4), and temporal trends and selection by antibiotic pressure have been proposed to interact with the PCV7 immune pressure to cause the rapid replacement by serotype 19A observed in the post-PCV7 era.
PCV7 was introduced in Norway in 2006, and by 2008 an early report of post-PCV7 changing epidemiology indicated the beginning of serotype replacement (15). Here, we report the clonal properties and antimicrobial susceptibilities of serotype 19A strains isolated in Norway before and after the introduction of PCV7.
IPD cases reported to the Norwegian Surveillance System for Communicable Diseases (MSIS) in the years 2004 to 2010 were included in the present study. Viable pneumococcal isolates were received at the National Reference Laboratory for Pneumococci from at least 88% of reported cases annually; the proportion of received isolates increased slightly from pre- to post-vaccine introduction. Isolates were serotyped by the Quellung reaction using specific antisera (Statens Serum Institut, Copenhagen, Denmark). Screening for antimicrobial nonsusceptibility was performed with the disc diffusion method. MICs for all isolates with reduced susceptibility in the screening were determined by using the antimicrobial gradient strip diffusion method with materials from AB Biodisk (Solna, Sweden). Since 2009, MICs for all IPD isolates have been determined. Isolates for which benzylpenicillin MICs were >0.064 μg/ml were categorized as penicillin nonsusceptible and those for which MICs were >2.0 μg/ml were categorized as resistant according to breakpoints from EUCAST (http://www.eucast.org/clinical_breakpoints/). All isolates identified as serotype 19A in 2005, 2007, and 2009 were further examined by multilocus sequence typing (MLST), as described by Enright and Spratt (5). Groups of sequence types (ST) sharing 5 of the 7 MLST alleles were assigned to CCs. Incidence rates were calculated with population denominators obtained from Statistics Norway (http://www.ssb.no). To account for missing isolates, the overall incidence rate of IPD was multiplied by the proportion of serotype-specific disease, giving a redistribution of serotypes with the assumption that the serotype distribution for missing isolates was the same as that for available isolates. Incidence rate ratios (IRR) with 95% confidence intervals (95% CI) were calculated using Episheet (http://krothman.byethost2.com/Episheet.xls).
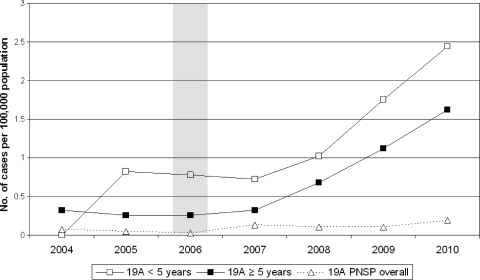
A total of 217 serotype 19A IPD cases were identified in the 7-year period. The overall incidence rate of IPD declined from 24.60 cases per 100,000 population in 2004 to 15.38 cases per 100,000 population in 2010 (IRR, 0.63; 95% CI, 0.57 to 0.69). The incidence rate of serotype 19A IPD increased from 0.30 cases per 100,000 population in 2004 to 1.67 cases per 100,000 population in 2010 (IRR, 5.59; 95% CI, 3.04 to 10.26). The increase among the population aged ≥5 years paralleled the increase among children aged <5 years. The incidence rate of penicillin-nonsusceptible serotype 19A IPD varied from 0.02 cases per 100,000 population (2006) to 0.19 cases per 100,000 population (2010) (Fig. 1).
Fig 1.
Incidence rate of IPD caused by serotype 19A in children aged less than 5 years and in the population aged 5 years or more. The overall incidence rate of penicillin-nonsusceptible (PNSP) serotype 19A IPD is shown by the broken curve. The 7-valent pneumococcal conjugate vaccine was introduced in the national immunization program in 2006, as shown with the shaded column.
Of 83 strains further analyzed by MLST, 66 (79.5%) belonged to CC199, of which 4 strains (6.2%) were nonsusceptible to penicillin (Table 1). One penicillin-resistant strain was identified, belonging to ST320. In 2009, four strains belonging to CC276 were identified, all of them being nonsusceptible to penicillin.
Table 1.
Clonal distribution and nonsusceptibility profiles of serotype 19A isolates recovered from IPD patients in 2005, 2007, and 2009
| Clonal complex and sequence type (no. of isolates) | No. (%) of isolates in: |
Nonsusceptibility profilea (no. of nonsusceptible isolates) in: |
||||
|---|---|---|---|---|---|---|
| 2005 | 2007 | 2009 | 2005 | 2007 | 2009 | |
| CC199 (66) | 10 (83.3) | 10 (62.5) | 46 (83.6) | |||
| ST199 (47) | 7 (58.3) | 8 (50.0) | 32 (58.2) | PNSP-MLS-TC (1) | PNSP (2) | PNSP (1) |
| ST667 (7) | 1 (8.3) | 2 (12.5) | 4 (7.3) | 0 | 0 | 0 |
| ST2220 (10) | 2 (16.7) | 0 | 8 (14.5) | 0 | 0 | 0 |
| ST6958 (1) | 0 | 0 | 1 (1.8) | 0 | 0 | 0 |
| ST416 (1) | 0 | 0 | 1 (1.8) | 0 | 0 | 0 |
| CC276 (4) | 0 | 0 | 4 (7.3) | |||
| ST276 (2) | 0 | 0 | 2 (3.6) | 0 | 0 | PNSP-M-TC (2) |
| ST3772 (2) | 0 | 0 | 2 (3.6) | 0 | 0 | PNSP-MLS-TC (2) |
| Noneb (13) | 2 (16.7) | 6 (37.5) | 5 (9.1) | |||
| ST63 (2) | 1 (8.3) | 1 (6.3) | 0 | 0 | PNSP-MLS-TC (1) | 0 |
| ST172 (1) | 0 | 1 (6.3) | 0 | 0 | PNSP (1) | 0 |
| ST320 (1) | 1 (8.3) | 0 | 0 | PRP-MLS-TC (1) | 0 | 0 |
| ST847 (1) | 0 | 1 (6.3) | 0 | 0 | 0 | 0 |
| ST416 (1) | 0 | 0 | 1 (1.8) | 0 | 0 | MLS-TC (1) |
| ST3017 (2) | 0 | 0 | 2 (3.6) | 0 | 0 | 0 |
| ST3546 (2) | 0 | 1 (6.3) | 1 (1.8) | 0 | MLS-TC (1) | MLS-TC (1) |
| ST3615 (1) | 0 | 1 (6.3) | 0 | 0 | PNSP-M (1) | 0 |
| ST3710 (1) | 0 | 1 (6.3) | 0 | 0 | PNSP-TC (1) | 0 |
| ST5954 (1) | 0 | 0 | 1 (1.8) | 0 | 0 | 0 |
| Total no. of isolates | 12 | 16 | 55 | 2 | 7 | 7 |
PNSP, penicillin nonsusceptibility phenotype (benzylpenicillin MIC > 0.064 μg/ml); MLS, macrolide-lincosamide-streptogramin resistance phenotype (high-level resistance to erythromycin, MIC ≥ 128 μg/ml, and clindamycin, MIC ≥ 128 μg/ml); TC, tetracycline resistance phenotype (MIC > 2.0 μg/ml); PRP, penicillin resistance phenotype (benzylpenicillin MIC > 2.0 μg/ml); M, efflux-mediated macrolide resistance phenotype (low-level resistance to erythromycin [MIC range, 1.0 to 64.0 μg/ml] and susceptibility to clindamycin).
Isolates in this group were not associated with a clonal complex.
The post-PCV7 increase of serotype 19A in Norway was dominated by penicillin-susceptible strains. The increase was driven mainly by expansion of CC199. Strains with intermediate susceptibility to penicillin were only sporadically identified within this CC. CC199 has also been found to dominate the post-PCV7 increase of serotype 19A in the United States (11, 13), although the relative contribution of this CC has appeared to decrease since 2005 (2). In the United States, however, the emergence of CC320 accounted for the major increase in penicillin-resistant strains; ST320 is a double-locus variant of the globally dispersed Taiwan19F-14-ST236 clone, and the majority of CC320 strains in the United States are penicillin resistant. CC320 was not identified in the United States prior to vaccine introduction. CC320 has also been identified in France and Spain (8, 9) and was found to dominate the pre-PCV7 increase of serotype 19A in South Korea (3). In Norway, however, a single isolate of ST320 was identified in 2004, before the introduction of PCV7.
In 2009, CC276 emerged as the dominating contributor to penicillin nonsusceptibility among serotype 19A strains in Norway. ST276 is a single-locus variant of the Denmark14-32-ST230 clone. This CC has been identified in the United States, although its relative contribution to serotype 19A replacement has been modest. In southern Europe, however, expansion of CC276 has been found to dominate the serotype 19A increase, with moderate contribution from CC199 (1, 8, 9).
The clonal characteristics of serotype 19A replacement appear to differ among study sites. In Norway, penicillin-susceptible strains dominated and the major increase was caused by expansion of CC199. However, the emergence of intermediately susceptible strains belonging to the Denmark14-32-ST230 complex, a CC reported to dominate in southern Europe, was observed. Thus, regional differences in circulating clones are evident. Use of antibiotics in Norway is limited by a restrictive prescription policy (6, 12). Thus, selection by antibiotic pressure is likely of minor importance for serotype 19A replacement in our setting. However, multidrug-resistant strains have been identified, although sporadically, and a shift toward a higher proportion of nonsusceptible strains among serotype 19A strains may occur over time. An increase similar to that of serotype 19A has been observed in Norway for serotype 22F, a serotype also predominantly penicillin susceptible. The mechanisms underlying the increase of susceptible clones of non-vaccine serotypes are not fully understood. Both serotypes 19A and 22F were relatively uncommon among asymptomatic carriers both before and after vaccine introduction in Norway (16), and the invasive disease potential is apparently altered in the post-PCV era. The success of certain serotypes and clones thus remains to be explained.
ACKNOWLEDGMENTS
Anne R. Alme, Torill Alvestad, and Gunnhild Rødal are thanked for excellent technical assistance.
Footnotes
Published ahead of print 11 January 2012
REFERENCES
- 1. Aguiar SI, et al. 2010. Denmark14-230 clone as an increasing cause of pneumococcal infection in Portugal within a background of diverse serotype 19A lineages. J. Clin. Microbiol. 48:101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beall BW, et al. 2011. Shifting genetic structure of invasive serotype 19A pneumococci in the United States. J. Infect. Dis. 203:1360–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choi EH, et al. 2008. Streptococcus pneumoniae serotype 19A in children, South Korea. Emerg. Infect. Dis. 14:275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dagan R, Givon-Lavi N, Leibovitz E, Greenberg D, Porat N. 2009. Introduction and proliferation of multidrug-resistant Streptococcus pneumoniae serotype 19A clones that cause acute otitis media in an unvaccinated population. J. Infect. Dis. 199:776–785 [DOI] [PubMed] [Google Scholar]
- 5. Enright MC, Spratt BG. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049–3060 [DOI] [PubMed] [Google Scholar]
- 6. Goossens H, Ferech M, Coenen S, Stephens P, and European Surveillance of Antimicrobial Consumption Project Group. 2007. Comparison of outpatient systemic antibacterial use in 2004 in the United States and 27 European countries. Clin. Infect. Dis. 44:1091–1095 [DOI] [PubMed] [Google Scholar]
- 7. Hicks LA, et al. 2007. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J. Infect. Dis. 196:1346–1354 [DOI] [PubMed] [Google Scholar]
- 8. Mahjoub-Messai F, et al. 2009. Population snapshot of Streptococcus pneumoniae serotype 19A isolates before and after introduction of seven-valent pneumococcal vaccination for French children. J. Clin. Microbiol. 47:837–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marimón JM, et al. 8 September 2011, posting date. Molecular characterization of Streptococcus pneumoniae invasive serotype 19A isolates from adults in two Spanish regions (1994–2009). Eur. J. Clin. Microbiol. Infect. Dis. doi:10.1007/s10096-011-1399-3 [DOI] [PubMed] [Google Scholar]
- 10. Miller E, Andrews NJ, Waight PA, Slack MP, George RC. 2011. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect. Dis. 11:760–768 [DOI] [PubMed] [Google Scholar]
- 11. Moore MR, et al. 2008. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J. Infect. Dis. 197:1016–1027 [DOI] [PubMed] [Google Scholar]
- 12. NORM/NORM-VET 2011. NORM/NORM-VET 2010. Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. NORM/NORM-VET, Tromsø, Norway [Google Scholar]
- 13. Pai R, et al. 2005. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J. Infect. Dis. 192:1988–1995 [DOI] [PubMed] [Google Scholar]
- 14. Pilishvili T, et al. 2010. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J. Infect. Dis. 201:32–41 [DOI] [PubMed] [Google Scholar]
- 15. Vestrheim DF, et al. 2010. Indirect effect of conjugate pneumococcal vaccination in a 2+1 dose schedule. Vaccine 28:2214–2221 [DOI] [PubMed] [Google Scholar]
- 16. Vestrheim DF, Høiby EA, Aaberge IS, Caugant DA. 2010. Impact of a pneumococcal conjugate vaccination program on carriage among children in Norway. Clin. Vaccine Immunol. 17:325–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vestrheim DF, et al. 2008. Effectiveness of a 2+1 dose schedule pneumococcal conjugate vaccination programme on invasive pneumococcal disease among children in Norway. Vaccine 26:3277–3281 [DOI] [PubMed] [Google Scholar]