Abstract
Tuberculosis (TB) continues to be a major health problem, and there are few biomarkers for predicting prognosis. Indoleamine 2,3-dioxygenase (IDO), a potent immunoregulatory molecule, catalyzes the rate-limiting step of tryptophan (Trp) degradation in the kynurenine (Kyn) pathway. An increase in IDO activity determined by the serum Trp/Kyn ratio has been shown to be associated with poor prognosis in cancers and bacteremia. In TB, however, there are no studies measuring serum IDO activity to determine its clinical significance. We evaluated serum IDO activity with 174 pulmonary TB (PTB) patients and 85 controls, using liquid chromatography/electrospray ionization tandem mass spectrometry. IDO activity was estimated by calculating the serum Kyn-to-Trp ratio. PTB patients had significantly higher Kyn concentrations and IDO activity and significantly lower Trp concentrations (P < 0.0001, P < 0.0001, and P < 0.0001, respectively) than the controls. Of 174 PTB patients, 39 (22.4%) died. The patients who died had significantly higher concentrations of Kyn and significantly lower Trp concentrations, resulting in significantly higher IDO activity (P < 0.0001, P < 0.0001, and P < 0.0001, respectively). In a receiver operating characteristic (ROC) analysis, serum IDO activity had the highest area under the curve (0.850), and this activity was an independent prognostic factor in multivariate analysis. These results suggest that serum IDO activity can be used as a novel prognostic marker in PTB.
INTRODUCTION
Tuberculosis (TB) remains a worldwide health problem, with 8.8 million new cases per year (21, 24). Advanced diagnosis technique, such as PCR for TB DNA and gamma interferon (IFN-γ) release assays (IGRAs), have improved detection of TB, and progress in multidrug combination therapy and expansion of directly observed therapy (DOTS) has increased the treatment completion rate and reduced the relapse rate. However, TB still causes over 1.6 million deaths per year (21, 24). To assess the disease activity, therapeutic response, or risk for relapse, a large number of candidate biomarkers, such as sputum microbiologic examinations, serum neopterin, IGRAs, and urine tuberculous DNA, have been vigorously investigated (18, 21). However, there are few useful markers for predicting mortality in pulmonary TB (PTB).
Indoleamine 2, 3-dioxygenase (IDO) is the rate-limiting enzyme that catalyzes tryptophan (Trp) degradation along the kynurenine (Kyn) pathway (9, 11, 12, 19, 25). In infectious diseases, IDO was initially considered an antimicrobial molecule acting through local starvation of Trp, which is an essential amino acid for bacterial growth. Interestingly, recent studies have unveiled a novel immunoregulatory role of IDO. By reducing the local Trp concentration and producing immunomodulatory Trp metabolites, such as Kyn, IDO potently inhibits T-cell functions and generates regulatory T cells (Treg), leading to immune suppression or tolerance. More interestingly, this immunoregulatory role of IDO has been shown to be associated with the immune escape of cancers and pregnancy (2, 4, 7, 13, 16, 23). To date, several studies have shown that an increase in IDO activity is associated with poor prognosis in cancer patients, suggesting that IDO activity can be used as a prognostic factor (4, 7, 23). However, there have been no studies to measure serum IDO activity in TB patients and to determine its prognostic significance.
Recently, we developed a method for assessing IDO activity in human serum using liquid chromatography-electrospray ionization/tandem mass spectrometry (LC-ESI/MS-MS) (15, 16). This method enables the simultaneous measurement of Kyn and Trp in human serum. IDO activity can be estimated using the Kyn-to-Trp ratio, because Kyn is the first product formed through the catabolism of Trp, which is tightly regulated by IDO (11, 12, 14, 23). In the present study, we measured the serum concentrations of Kyn and Trp using LC-ESI/MS-MS and assessed the IDO activity in TB patients. In addition, we investigated the clinical significance of IDO and determined its ability to predict outcome.
MATERIALS AND METHODS
Subjects.
This prospective study was conducted in referral hospitals for treatment of TB in Shizuoka, Japan. From March 2010 to February 2011, 174 consecutive patients (112 men and 62 women, with a mean age of 70.1 years) with PTB who were admitted to our institutions were enrolled in the present study. PTB was diagnosed by the isolation of Mycobacterium tuberculosis in the presence of new radiographic pulmonary infiltration. The study also included 85 subjects, age and gender matched, recruited to our institutions as a control group (48 men and 37 women with a mean age of 69.9 years). This study was approved by the ethics committee of Hamamatsu University School of Medicine (approval number 21-142), and written informed consent was obtained in accordance with the institution's guidelines.
Laboratory examinations.
Blood samples were drawn at the time of admission before the start of antituberculosis drugs. Patient serum samples were frozen at −20°C until analysis, and routine laboratory examinations, such as blood cell counts and biochemical analysis, were subsequently performed. Of 174 PTB patients, procalcitonin (PCT) and serum amyloid A (SAA) were measured in 110 and 162 patients, respectively, by physician's decision.
Measurement of serum tryptophan and kynurenine.
l-Kynurenine and 3-nitro-tyrosine, used as an internal standard, were purchased from Sigma-Aldrich (St. Louis, MO), and l-tryptophan was purchased from Thermo Fisher Scientific (Waltham, MA). Ammonium formate and perchloric acid were obtained from Wako Pure Chemical Industries (Osaka, Japan). The serum concentrations of Kyn and Trp were determined by LC-ESI/MS-MS (TSQ 7000 LC-quadrupole mass spectrometer; Thermo Fisher, San Jose, CA) as described previously (15, 16). In brief, frozen serum samples were thawed at room temperature. The serum samples were then spiked with standards and deproteinized with 0.5 N perchloric acid for 10 min on ice. Then, the samples were centrifuged (15,000 × g; 10 min), and the supernatants were vortex mixed with an equal volume of 1 M ammonium formate. Analyses and detection were performed by LC-ESI/MS-MS. Chromatographic separation of the analytes was performed in isocratic mode on an Atrantis T3 analytical column (150 mm by 2.1 mm; particle size, 5 μm; Waters, Milford, MA). The mobile phase of 5 mM ammonium formate (0.01% trifluoroacetic acid [TFA])-methanol (80:20 [vol/vol]) was passed at a flow rate of 0.2 ml/min. Detection was performed using sheathless electrospray tandem mass spectrometry in the multiple-reaction-monitoring mode. IDO activity was determined by dividing the concentration of Kyn in serum by that of Trp.
Statistical analysis.
Discrete variables are expressed as counts (percentage), and continuous variables are described as the mean ± standard deviation (SD) unless otherwise specified. The Wilcoxon/Kruskal-Wallis test was used for continuous variables, and the analysis of variance (ANOVA) t test was used for multigroup comparisons. Categorical data were compared between the groups using the chi-square test for independence. Univariate and multivariate analyses were performed by Cox proportion-hazard regression analysis to predict mortality. A receiver operating characteristic (ROC) curve was used to evaluate the ability of IDO activity to discriminate between patients who survived and those who died during admission. The optimal cutoff value, i.e., that which ensured the best combination of sensitivity and specificity, was obtained. Cumulative survival probabilities were estimated using the Kaplan-Meier method. The log-rank test was used to compare survival among patients. Statistical analyses were performed using JMP Start Statistics (SAS Institute Inc., Cary, NC). P values of less than 0.05 were considered significant.
RESULTS
Clinical characteristics.
The clinical characteristics of 174 patients with PTB are summarized in Table 1. Of the 174 PTB patients, 31 (17.8%) had complicated tuberculous pleurisy and 15 (8.6%) had disseminated tuberculosis. Cavity lesions were found for 85 patients (48.9%) on chest radiographs. Multidrug-resistant TB (MDR-TB) was diagnosed in 3 cases. None of the patients had human immunodeficiency virus (HIV) infection. The most common comorbidity was cerebrovascular disease, followed by diabetes mellitus. Respiratory failure was present in 27 patients (15.5%), and impaired consciousness was found in 8 patients (4.6%).
Table 1.
Clinical characteristics of 174 patients with pulmonary tuberculosis
| Characteristica | Valueb |
P valuec | ||
|---|---|---|---|---|
| TB (n = 174) | Nonsurvivors (n = 39) | Survivors (n = 135) | ||
| Sex (M/F) | 112/62 | 22/17 | 45/90 | 0.2388 |
| Age (yr) | 70.1 ± 21.6 | 83.2 ± 12.3 | 66.3 ± 22.2 | <0.0001 |
| Body mass index | 18.7 ± 3.3 | 17.0 ± 3.3 | 19.2 ± 3.3 | 0.0038 |
| Observation period (mo) | 4.52 ± 3.29 | 1.74 ± 1.22 | 5.33 ± 3.26 | <0.0001 |
| Current smoker [no. (%)] | 29 (16.7) | 2 (5.1) | 27 (20.0) | 0.0282 |
| System involved | ||||
| PTB only [no. (%)] | 133 (76.4) | 25 (64.1) | 108 (80.0) | 0.0393 |
| Tuberculous pleurisy [no. (%)] | 31 (17.8) | 11 (28.2) | 20 (14.8) | 0.0542 |
| Disseminated TB [no. (%)] | 15 (8.6) | 7 (18.0) | 8 (5.9) | 0.0185 |
| Osteoarticular TB [no. (%)] | 5 (2.9) | 2 (5.1) | 3 (2.2) | 0.3386 |
| Bronchial TB [no. (%)] | 4 (2.3) | 0 (0) | 4 (3.0) | 0.2768 |
| Tuberculous colitis [no. (%)] | 3 (1.7) | 1 (2.6) | 2 (1.5) | 0.6473 |
| Radiographic findings | ||||
| Cavity [no. (%)] | 85 (48.9) | 21 (53.8) | 64 (47.4) | 0.4786 |
| Microbiological findings | ||||
| Sputum smear (0, 1+, 2+, 3+)d | 10, 69, 30, 65 | 2, 15, 5, 17 | 8, 54, 25, 48 | 0.2913 |
| MDR-TB [no. (%)] | 3 (1.7) | 0 (0) | 3 (2.2) | 0.3477 |
| Comorbidity [no. (%)] | ||||
| Congestive heart failure | 24 (13.8) | 15 (38.5) | 9 (6.7) | <0.0001 |
| Chronic pulmonary disease | 24 (13.8) | 3 (7.7) | 21 (15.6) | 0.2097 |
| Renal disease | 6 (3.4) | 5 (12.8) | 1 (0.7) | 0.0003 |
| Diabetes mellitus | 27 (15.5) | 6 (15.4) | 21 (15.6) | 0.9763 |
| Liver disease | 5 (2.9) | 2 (5.1) | 3 (2.2) | 0.3386 |
| Cerebrovascular disease | 33 (19.0) | 11 (28.2) | 22 (16.3) | 0.0947 |
| Neoplasm | 15 (8.6) | 8 (20.5) | 7 (5.2) | 0.0027 |
| Chronic corticosteroid treatment | 9 (5.2) | 3 (7.7) | 6 (4.4) | 0.4198 |
| Clinical characteristics on admission | ||||
| Body temp (°C) | 36.9 ± 0.8 | 37.1 ± 1.1 | 36.8 ± 0.7 | 0.1492 |
| Heart rate (beats/min) | 85.1 ± 16.5 | 88.5 ± 17.3 | 84.1 ± 16.2 | 0.2060 |
| Respiratory rate (breaths/min) | 19.6 ± 5.6 | 21.7 ± 5.6 | 19.0 ± 5.5 | 0.0713 |
| Respiratory failure (SaO2 < 90%) [no. (%)] | 27 (15.5) | 17 (43.6) | 10 (7.4) | <0.0001 |
| Impaired consciousness [no. (%)] | 8 (4. %) | 7 (17.9) | 1 (0.7) | <0.0001 |
| Laboratory findings | ||||
| BUN (mg/dl) | 17.5 ± 10.9 | 23.4 ± 15.0 | 15.8 ± 8.7 | 0.0041 |
| Alb (mg/dl) | 3.1 ± 0.9 | 2.4 ± 0.6 | 3.3 ± 0.8 | <0.0001 |
| Cre (mg/dl) | 0.80 ± 0.42 | 0.93 ± 0.70 | 0.76 ± 0.29 | 0.5380 |
| WBC/mm3 | 7550 ± 3590 | 7830 ± 4070 | 7470 ± 3460 | 0.9870 |
| ESR (mm/h) | 50.3 ± 33.4 | 50.4 ± 38.6 | 50.3 ± 31.8 | 0.7576 |
| CRP (mg/dl) | 5.1 ± 5.7 | 9.3 ± 6.5 | 3.8 ± 4.8 | <0.0001 |
| SAA (mg/dl) | 370 ± 548 | 571 ± 707 | 311 ± 479 | 0.0023 |
| PCT (mg/dl) | 0.30 ± 1.05 | 0.96 ± 1.99 | 0.09 ± 0.18 | <0.0001 |
M, male; F, female; SaO2, arterial oxygen saturation; Cre, creatinine.
Mean ± SD.
Nonsurvivors compared to survivors.
Sputum smear is defined by the Ziehl-Neelson method for acid-fast bacteria. Data given refer to the respective scale numbers.
Among the 174 PTB patients, 39 (22.4%) died during their observation periods. Comparing survivors with nonsurvivors, the latter had significantly higher age and lower body mass indexes. The proportions of combined disseminated tuberculosis were significantly higher in the nonsurvivors than in the survivors. Among the comorbidities, congestive heart failure, renal disease, and neoplasm were significantly more frequent in the nonsurvivors than in the survivors. Respiratory failure and impaired consciousness were significantly more common in the nonsurvivors than in the survivors. The levels of blood urea nitrogen (BUN) and C-reactive protein (CRP) were significantly higher, and albumin (Alb) concentrations were significantly lower in the nonsurvivors. In addition, the levels of PCT and SAA were significantly elevated in the nonsurvivors, while white blood cell counts (WBC) and erythrocyte sedimentation rates (ESR) did not significantly differ in the two groups.
Serum concentrations of Kyn and Trp and serum IDO activity.
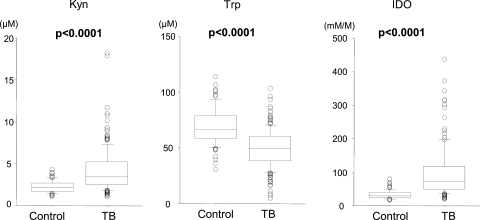
The serum concentrations of Kyn and Trp and the IDO activity are presented in Fig. 1 and 2. No significant differences were found in age or gender between the patients and healthy controls. The PTB patients had significantly higher levels of Kyn (4.23 ± 2.60 μM versus 2.28 ± 0.71 μM; P < 0.0001), together with significantly lower levels of Trp (49.7 ± 17.6 μM versus 69.4 ± 16.3 μM; P < 0.0001), than the controls, resulting in significantly higher IDO activity (112.9 ± 157.9 mM/M versus 33.7 ± 12.4 mM/M; P < 0.0001).
Fig 1.
Serum concentrations of Kyn and Trp and IDO activity in patients with PTB and control subjects. The P values were determined by the Wilcoxon/Kruskal-Wallis test. The upper and lower portions of the boxes indicate 25th and 75th percentiles, respectively, and the horizontal lines within the boxes indicate the 50th percentile (median). The horizontal lines above and below the boxes indicate the 1.5 interquartile range of the lower and upper quartiles, respectively.
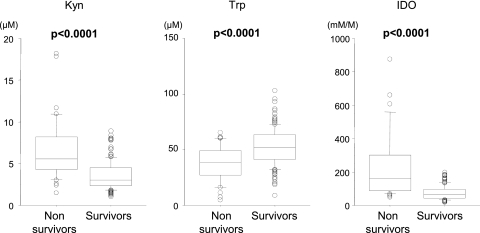
Fig 2.
Serum concentrations of Kyn and Trp and IDO activity in nonsurvivors and survivors. The P values were determined by the Wilcoxon/Kruskal-Wallis test. The upper and lower portions of the boxes indicate 25th and 75th percentiles, respectively, and the horizontal lines within the boxes indicate the 50th percentile (median). The horizontal lines above and below the boxes indicate the 1.5 interquartile range of the lower and upper quartiles, respectively.
Among the PTB patients, the nonsurvivors had significantly higher Kyn concentrations and significantly lower Trp concentrations than the survivors (Kyn, 6.50 ± 3.69 μM versus 3.57 ± 1.71 μM [P < 0.0001]; Trp, 37.6 ± 15.9 μM versus 53.3 ± 16.4 μM [P < 0.0001]). As a result, significantly higher IDO activity was found in the nonsurvivors than in the survivors (250.0 ± 288.1 mM/M versus 73.2 ± 40.2 mM/M; P < 0.0001).
Prognostic values of serum Kyn and Trp and IDO activity.
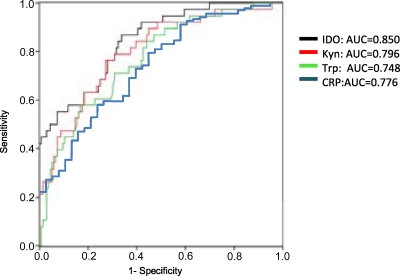
To evaluate the potential of serum Kyn and Trp and IDO activity to predict deaths in PTB, a ROC analysis was performed. In the ROC analysis, the area under the ROC curve (AUC) was 0.850 for IDO activity, which was superior to those of Kyn, Trp, and CRP (AUC, 0.796, 0.748, and 0.776, respectively) (Fig. 3). Regarding other biomarkers, the AUCs of PCT and SAA were 0.834 and 0.665, respectively, with limited cases. Using several cutoff points, the sensitivity and specificity to predict mortality and the positive (PPV) and negative (NPV) predictive values are shown in Table 2. With an optimal calculated IDO activity threshold of 76.7 mM/M, the sensitivity and specificity were 87.2% and 66.7%, with PPV and NPV of 43.0% and 94.7%.
Fig 3.
Receiver operator curve analysis of serum concentrations of Kyn, Trp, and CRP and IDO activity for predicting mortality in patients with PTB.
Table 2.
Diagnostic value of Kyn, Trp, and IDO activity to predict mortality in PTB patients
| Predictor | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| IDO (mM/M) | ||||
| 50 | 97.4 | 32.6 | 29.5 | 97.8 |
| 76.7 | 87.2 | 66.7 | 43.0 | 94.7 |
| 100 | 64.1 | 78.5 | 46.3 | 88.3 |
| 150 | 51.3 | 93.3 | 69.0 | 86.9 |
| 200 | 41.0 | 100 | 100 | 85.4 |
| Kyn (μM) | ||||
| 3.0 | 92.3 | 48.9 | 34.3 | 95.7 |
| 3.75 | 82.1 | 63.0 | 39.0 | 92.4 |
| 5.0 | 56.4 | 81.5 | 46.8 | 86.6 |
| 6.0 | 46.2 | 91.1 | 60.0 | 85.4 |
| 7.0 | 30.8 | 94.8 | 63.2 | 82.6 |
| Trp (μM) | ||||
| 30 | 38.5 | 94.1 | 65.2 | 84.1 |
| 40 | 56.4 | 78.5 | 43.1 | 86.2 |
| 49 | 76.9 | 58.5 | 34.9 | 89.8 |
| 60 | 89.7 | 31.9 | 27.6 | 91.5 |
| 70 | 100 | 14.8 | 25.3 | 100 |
| CRP (mg/dl) | ||||
| 2.58 | 84.6 | 56.3 | 35.9 | 92.7 |
| 5.0 | 64.1 | 70.4 | 38.5 | 87.2 |
| 7.5 | 59.0 | 83.7 | 51.1 | 87.6 |
| 10 | 41.0 | 91.1 | 57.1 | 84.2 |
| 15 | 23.1 | 97.0 | 69.2 | 81.4 |
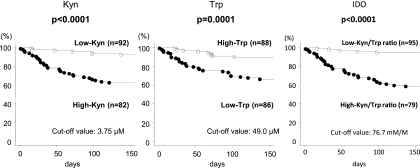
Next we performed Kaplan-Meier analysis using optimal cutoff values, including Kyn and Trp (3.75 μM and 49.0 μM, respectively). The high-Kyn (n = 82), low-Trp (n = 86), and high-IDO-activity (n = 79) groups showed significantly worse survival than the low-Kyn (n = 92), high-Trp (n = 88), and low-IDO-activity (n = 95) groups (P < 0.0001, P = 0.0001, and P < 0.0001, respectively) (Fig. 4).
Fig 4.
Kaplan-Meier curves of patients with tuberculosis produced according to Kyn and Trp concentrations and IDO activity. The P values were determined by the log rank test.
Finally, to determine the prognostic values of serum Kyn and Trp and IDO activity with regard to outcome, we performed Cox proportion-hazard regression analyses (Table 3). In univariate analysis, various factors, including Kyn, Trp, and IDO activity, were significant predictors of mortality. In multivariate analysis, we analyzed the serum Kyn and Trp and the IDO activity separately, because the IDO activity was strongly affected by the Kyn and Trp concentrations. In multivariate analyses, IDO activity, as well as age, was found to be an independent predictor of death in PTB. When entering Kyn and Trp, Kyn, as well as age, was a significant predictor of mortality (P = 0.0158 and P = 0.0267, respectively) (data not shown).
Table 3.
Prediction of mortality with PTB patients: univariate and multivariate analyses
| Predictor | HRa | 95% CIb | P value |
|---|---|---|---|
| Univariate analysis | |||
| Age (yr) | 1.062 | 1.029–1.096 | 0.0002 |
| Sex, female | 1.565 | 0.825–2.967 | 0.1701 |
| Body mass index (kg/m2) | 0.843 | 0.753–0.944 | 0.0030 |
| PTB only | 0.446 | 0.231–0.864 | 0.0167 |
| Disseminated TB | 2.545 | 1.119–5.787 | 0.0258 |
| Congestive heart failure | 5.557 | 2.881–10.716 | <0.0001 |
| Neoplasm | 2.881 | 1.320–6.286 | 0.0079 |
| Respiratory failure | 5.551 | 2.924–10.541 | <0.0001 |
| Impaired consciousness | 7.955 | 3.432–18.441 | <0.0001 |
| BUN (mg/dl) | 1.044 | 1.025–1.063 | <0.0001 |
| Alb (mg/dl) | 0.295 | 0.190–0.459 | <0.0001 |
| CRP (mg/dl) | 1.096 | 1.057–1.137 | <0.0001 |
| Kyn (μM) | 1.273 | 1.189–1.362 | <0.0001 |
| Trp (μM) | 0.951 | 0.932–0.971 | <0.0001 |
| IDO (log10 mM/M) | 26.080 | 12.097–56.226 | <0.0001 |
| Multivariate analysis | |||
| Age (yr) | 1.052 | 1.006–1.099 | 0.0246 |
| Sex, female | 0.728 | 0.286–1.854 | 0.5049 |
| Body mass index (kg/m2) | 0.964 | 0.832–1.117 | 0.6247 |
| PTB only | 1.078 | 0.464–2.504 | 0.8613 |
| Disseminated TB | 0.674 | 0.191–2.374 | 0.5387 |
| Congestive heart failure | 1.670 | 0.634–4.395 | 0.2993 |
| Neoplasm | 1.616 | 0.597–4.374 | 0.3452 |
| Respiratory failure | 2.059 | 0.870–4.877 | 0.1005 |
| Impaired consciousness | 1.757 | 0.421–7.326 | 0.4392 |
| BUN (mg/dl) | 0.976 | 0.931–1.023 | 0.3062 |
| Alb (mg/dl) | 0.731 | 0.303–1.765 | 0.4865 |
| CRP (mg/dl) | 1.046 | 0.972–1.126 | 0.2277 |
| IDO (log10 mM/M) | 9.620 | 1.910–48.443 | 0.0060 |
HR, hazard ratio.
CI, confidence interval.
Serum concentrations of Kyn and Trp and serum IDO activity in PTB patients who were under 65 years old and had no significant comorbidities.
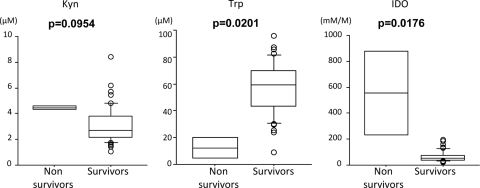
It is known that age and comorbidities affect the prognosis of infectious diseases. To examine whether those factors in our PTB patients critically affected our results, we performed a subset analysis in 49 PTB patients who were under 65 years old without significant comorbidities. Among these PTB patients, although serum Kyn concentrations did not significantly differ between survivors and nonsurvivors, serum Trp concentrations were significantly lower in nonsurvivors than in survivors (P = 0.0201). As a result, serum IDO activity was significantly higher in nonsurvivors than in survivors (P = 0.0176) (Fig. 5).
Fig 5.
Serum concentrations of Kyn and Trp and IDO activity in patients who were under 65 years old and had no significant comorbidities. The P values were determined by the Wilcoxon/Kruskal-Wallis test. The upper and lower portions of the boxes indicate 25th and 75th percentiles, respectively, and the horizontal lines within the boxes indicate the 50th percentile (median). The horizontal lines above and below the boxes indicate the 1.5 interquartile range of the lower and upper quartiles, respectively.
DISCUSSION
The present study measured serum concentrations of Kyn and Trp and assessed IDO activity in PTB patients. The PTB patients showed significant increases in Kyn concentrations and IDO activity and significant decreases in Trp concentrations compared to control subjects. Interestingly, among the PTB patients, nonsurvivors had significantly higher Kyn concentrations and significantly lower Trp concentrations, resulting in a significant increase in IDO activity over that in survivors. Most importantly, multivariate analysis showed that the IDO activity was a significant independent predictor of death in PTB.
The immunoregulatory molecule IDO, which catalyzes Trp along the Kyn pathway and induces immune tolerance, has been shown to play a critical role in various pathological conditions, including pregnancy, cancer, and infectious diseases (2, 4, 7, 9, 11, 12, 13, 15, 16, 19, 23, 25). Overexpression of IDO by cancer cells is itself a fundamental immune escape mechanism, and higher IDO activity is a novel prognostic factor in several types of cancer patients (4, 7, 9, 11, 12, 19, 23, 25). Similar to neoplasm, higher serum IDO activity was recently found to be a prognostic factor in patients with bacteremia and sepsis (6, 17). However, there have been only two studies focusing on IDO in TB. Almeida et al. reported that mRNA expression of IDO in induced sputum cells was increased in PTB patients and decreased after anti-TB treatment (1). Local immune status in the pleural fluid in TB pleurisy has been shown to be immunologically downregulated. In this regard, Li et al. demonstrated that an inhibitor of IDO restored decreased T-cell-derived cytokine production in the pleural fluid of patients with TB pleurisy, suggesting that IDO is responsible for the impairment of T-cell functions in TB pleurisy (10). Collectively, these data imply that IDO activity is involved in the immunological processes of TB through its immunosuppressive effect. However, there have been no data about serum IDO activity and its clinical significance in TB. In the present study, we demonstrated for the first time that serum IDO activity is elevated in PTB patients and is associated with its prognosis.
For patients with TB, there are a variety of recently developed biomarkers for assessing disease activity, response to treatment, and risk of relapse (20, 21). However, for individual TB patients and physicians, one of important concerns is predicting outcome, but there have been few studies focused on a biomarker for mortality in TB. In this regard, we clearly showed that serum IDO activity is a prognostic marker in PTB. With an IDO activity threshold of 76.7 mM/M, sensitivity and NPV for death were quite high (87.2% and 94.7%, respectively), and the high-IDO group showed significantly worse survival than the low-IDO group. We also compared the predictive values for mortality with those of other inflammatory markers, CRP, PCT, and SAA, in ROC analysis, and IDO activity showed the highest AUC among these inflammatory markers. In addition, multivariate analysis revealed that IDO activity was a significant independent predictor of death in PTB. These results suggest that assessment of serum IDO activity is useful in management of PTB to predict outcome.
In infectious diseases, the precise role of IDO is not yet fully determined. Originally, IDO was considered to function as an antimicrobial molecule, but it also exerts a potent immunosuppressive effect. Thus, it is unclear whether increased IDO activity in infections is beneficial for host defense. To date, several studies have examined the effect of blockading IDO in animal models of infection (3, 5, 8, 25). Bozza et al. demonstrated that IDO inhibition exacerbated Candida albicans infection, while Jung et al. demonstrated that blockade of IDO activity protected mice against lipopolysaccharide (LPS)-induced endotoxin shock (3, 8). In Mycobacterium avium infection, blockading IDO reduced the antimycobacterial effect of immunostimulatory oligodeoxynucleotide (ISS-ODN) analogs in vitro (5). These discrepancies might be due to differences between infectious pathogens and/or the severity of infection. In regard to TB, no data are available about the effect of inhibition of IDO in vivo. Further studies are needed to elucidate the precise role of IDO in infections, especially in TB.
Because our PTB patients had a high incidence of comorbidities with relatively high age, it is unclear whether our results also apply to younger PTB patients without comorbidities. To examine this, we performed a subset analysis in younger PTB patients (<65 years old) who had no significant morbidities. Even among the younger patients, we found that serum IDO activities were significantly higher in nonsurvivors than in survivors. However, we could not perform univariate or multivariate prognostic analysis, because the mortality of those TB patients was low (<5%). Thus, to confirm our results in younger PTB patients without comorbidities, further study is needed in larger series of such PTB patients, including more nonsurvivors.
There are several limitations to this study. First, the mortality of our PTB population was higher than that reported in Western countries. The prevalence of TB in Japan is not similar to that in Western countries. The estimated incidence of TB in Japan has gradually decreased but is still far higher than that in the European and North American regions (24). In Japan, over 24,000 new cases of TB per year, including 10,000 cases of smear-positive TB, and over 2,000 deaths caused by TB have been recently reported (18). Thus, the mortality of the smear-positive TB patients is approximately 20%, which is comparable to that of our study population (22.4%). Second, although our subjects included relatively large number of PTB patients, none of them were HIV positive, and most had drug-susceptible pulmonary tuberculosis. The proportions of HIV-positive and/or MDR-TB patients in Japan were reported to be 0.2% and 0.5%, respectively (18). In addition, the PTB patients in the present study were relatively old with a high incidence of comorbidities. Thus, the prognostic value of IDO activity remains to be determined in HIV-positive and/or MDR-TB patients, as well as younger PTB patients without comorbidities. Third, we examined serum IDO activity, determined by the serum Kyn/Trp ratio, but this study did not clarify the mechanisms or origin of increased IDO activity in the sera of PTB patients. Previous studies have shown that inflammatory cytokines, such as IFN-γ, and bacterial products can induce IDO expression in a variety of cell types, including monocytes/macrophages, dendritic cells, and endothelial cells (11, 22, 25). Likewise, in PTB, functional IDO expression was induced in these types of cells under such conditions. Future studies will be required to clarify these issues.
In summary, the present study showed that serum IDO activity was increased in PTB patients and that this activity was an independent predictor of mortality. These results suggest that serum IDO activity can be used as a prognostic marker of PTB.
ACKNOWLEDGMENTS
We declare that no competing interests exist.
Author contributions: Yuzo Suzuki, conception and design, data collection, data analysis and interpretation, and manuscript writing; Takafumi Suda, conception and design, data analysis and interpretation, manuscript writing, and final approval of the manuscript; Kazuhiro Asada, data collection; Seiichi Miwa, data collection; Masako Suzuki, data analysis; Michio Fujie, data analysis; Kazuki Furuhashi, data collection; Yutaro Nakamura, data collection; Naoki Inui, data collection; Toshihiro Shirai, data collection; Hiroshi Hayakawa, data collection; Hirotoshi Nakamura, conception, design, and interpretation; Kingo Chida, conception and design, administrative support, and data analysis and interpretation.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 4 January 2012
REFERENCES
- 1. Almeida AS, et al. 2009. Tuberculosis is associated with a down-modulatory lung immune response that impairs Th1-type immunity. J. Immunol. 183: 718–731 [DOI] [PubMed] [Google Scholar]
- 2. Aluvihare VR, Kallikourdis M, Betz AG. 2004. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 5: 266–271 [DOI] [PubMed] [Google Scholar]
- 3. Bozza S, et al. 2005. A crucial role for tryptophan catabolism at the host/Candida albicans interface. J. Immunol. 174: 2910–2918 [DOI] [PubMed] [Google Scholar]
- 4. Brandacher G, et al. 2006. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin. Cancer Res. 12: 1144–1151 [DOI] [PubMed] [Google Scholar]
- 5. Hayashi T, et al. 2001. Enhancement of innate immunity against Mycobacterium avium infection by immunostimulatory DNA is mediated by indoleamine 2,3-dioxygenase. Infect. Immun. 69: 6156–6164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huttunen R, et al. 2010. High activity of indoleamine 2,3-dioxygenase enzyme predicts disease severity and case fatality in bacteremic patients. Shock 33: 149–154 [DOI] [PubMed] [Google Scholar]
- 7. Ino K, et al. 2006. Indoleamine 2,3-dioxygenase is a novel prognostic indicator for endometrial cancer. Br. J. Cancer 95: 1555–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jung ID, et al. 2009. Blockade of indoleamine 2,3-dioxygenase protects mice against lipopolysaccharide-induced endotoxin shock. J. Immunol. 182: 3146–3154 [DOI] [PubMed] [Google Scholar]
- 9. Katz JB, Muller AJ, Prendergast GC. 2008. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol. Rev. 222: 206–221 [DOI] [PubMed] [Google Scholar]
- 10. Li Q, et al. 2011. Pleural fluid from tuberculous pleurisy inhibits the function of T cells and differentiation of Th1 cells via immunosuppressive factors. Cell. Mol. Immunol. 8: 172–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mellor AL, Munn DH. 2004. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 4: 762–774 [DOI] [PubMed] [Google Scholar]
- 12. Munn DH, Mellor AL. 2007. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J. Clin. Invest. 117: 1147–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Munn DH, et al. 1998. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281: 1191–1193 [DOI] [PubMed] [Google Scholar]
- 14. Schröcksnadel K, Wirleitner B, Winkler C, Fuchs D. 2006. Monitoring tryptophan metabolism in chronic immune activation. Clin. Chim Acta 364: 82–90 [DOI] [PubMed] [Google Scholar]
- 15. Suzuki Y, et al. 2011. Serum activity of indoleamine 2,3-dioxygenase predicts prognosis of community-acquired pneumonia. J. Infect. 63: 215–222 [DOI] [PubMed] [Google Scholar]
- 16. Suzuki Y, et al. 2010. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer 67: 361–365 [DOI] [PubMed] [Google Scholar]
- 17. Tattevin P, et al. 2010. Enhanced indoleamine 2,3-dioxygenase activity in patients with severe sepsis and septic shock. J. Infect. Dis. 201: 956–966 [DOI] [PubMed] [Google Scholar]
- 18. Tuberculosis Surveillance Center RIT JATA 2011. Tuberculosis annual report 2009. Series 1. Summary of TB notification statistics in 2009. Kekkaku 86: 127–130 [PubMed] [Google Scholar]
- 19. Uyttenhove C, et al. 2003. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 9: 1269–1274 [DOI] [PubMed] [Google Scholar]
- 20. Wallis RS, et al. 2010. Biomarkers and diagnosis for tuberculosis: progress, needs, and translation into practice. Lancet 375: 1920–1937 [DOI] [PubMed] [Google Scholar]
- 21. Wallis RS, et al. 2009. Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect. Dis. 9: 162–172 [DOI] [PubMed] [Google Scholar]
- 22. Wang Y, et al. 2010. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat. Med. 16: 279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weinlich G, Murr C, Richardsen L, Winkler C, Fuchs D. 2007. Decreased serum tryptophan concentration predicts poor prognosis in malignant melanoma patients. Dermatology 214: 8–14 [DOI] [PubMed] [Google Scholar]
- 24. WHO 2008. Global tuberculosis control—surveillance, planning financing. WHO/HTM/TB 2008.393. World Health Organization, Geneva, Switzerland [Google Scholar]
- 25. Zelante T, Fallarino F, Bistoni F, Puccetti P, Romani L. 2009. Indoleamine 2,3 dioxygenase in infection: the paradox of an evasive strategy that benefits the host. Microbes Infect. 11: 133–141 [DOI] [PubMed] [Google Scholar]