Abstract
To establish a high-efficiency gamma interferon-specific enzyme-linked immunosorbent spot assay (IFN-γ ELISPOT assay) for detection of tuberculosis (TB), peptides (E6, E7, and C14) and peptide mixtures (E6 plus E7 and E6 plus E7 plus C14) were used to monitor peripheral blood (PBL) samples from patients with pulmonary TB (PTB), as well as control samples. The positive detection rates of the five IFN-γ ELISPOT assays were 78.38%, 74.86%, 55.83%, 90.43%, and 91.51%, respectively, and there were similar detection rates between the two combined peptide mixture IFN-γ ELISPOT assays and the tuberculin skin test (TST) (90.62% versus 95.59%). No significant difference was found between the detection rates of the two combined peptide mixture IFN-γ ELISPOT assays and the T-SPOT.TB assay for 86 patients with PTB (P > 0.05), but the median number of spot-forming cells/106 cells (SFP value) for positive results was higher by the former than by the latter assay (P < 0.05). In contrast, the 29.93% positive detection rate and median SFP value of 482 by the two combined peptide mixture IFN-γ ELISPOT assays were significantly higher than the corresponding values of 14.29% and 152 by T-SPOT.TB assay for the same 147 community donors (P < 0.05). For nine PTB patients tracked, the SFP value of 7 for the two peptide mixture IFN-γ ELISPOT assays began to decrease from the second month after regular treatment. A relatively low, almost normal, SFP level was reached and maintained after the third or fourth month. Two in-house IFN-γ ELISPOT assays and the T-SPOT.TB assay could reduce the false-positive and false-negative detection rates of TST and sputum acid-fast staining. Therefore, these two combined peptide mixture IFN-γ ELISPOT assays have a potential advantage, beyond their greater specificity and sensitivity, for use in screening and detection of active TB infection (TBI) and latent TB infection (LTBI) in China.
INTRODUCTION
Tuberculosis (TB) is an important global health problem. A report from the World Health Organization (WHO) showed that in 2010 there were still 8.8 million new cases of TB, 1.1 million deaths from TB among HIV-negative people, and an additional 0.35 million deaths from HIV-associated TB (27). About 20% of the world's population lives in China, and TB has become one of the major diseases in that country. The report of the 4th National Epidemiological Sampling Survey of TB in China (6) stated that the infection rate of TB in the country is 44.5% (about 550 million individuals). Rapid and effective diagnosis is the key to controlling the disease and preventing its emergence in a country with such widespread latent infection. In China, the tuberculin skin test (TST), which involves injecting people with purified protein derivative (PPD), is used widely for preliminary diagnosis of TB infection (TBI) and latent TB infection (LTBI) (5, 11, 18, 20). However, some compositions of PPD can also be found in Mycobacterium bovis bacillus Calmette-Guérin (BCG) and many kinds of nontuberculous mycobacteria (NTM) (12, 14). A TST cannot differentiate between BCG vaccination, environmental mycobacterial infection, or other situations that may give a positive test result (9, 26). Furthermore, about 80% of people in China are vaccinated with BCG before the age of 1 year. Thus, TST, which is based on PPD, has a low specificity for detecting TB, especially in China.
Recently, several researchers have focused on a new type of immunological assay for detection of TB—T-cell-based gamma interferon (IFN-γ) release assays (17). The principle of this technique is that T cells produce IFN-γ when they are incubated with Mycobacterium tuberculosis-specific protein antigens. Such antigens, including culture filtrate protein 10 (CFP-10) and an early secreted protein (ESAT-6), are used widely. These two proteins are encoded by a region of difference that is part of the M. tuberculosis genome but is not found in BCG and most NTM (14).
The QuantiFERON-TB Gold (Cellestis, Carnegie, Australia) and T-SPOT.TB (Oxford Immunotec, Oxford, United Kingdom) assays are assay methods based on this theory, and both are now available commercially in Europe and America. It has been shown that in contrast to individuals with TBI, those with LTBI never have clinical signs or symptoms of disease and have normal chest radiographs. In the past, TST was usually the reference standard for detection of latent infection, but its specificity was not reliable (24). Recently, it was proved that IFN-γ release assays are more effective than TST for rapid testing of TBI and LTBI, even in BCG-vaccinated individuals (10). However, none of these assays are used widely, and few data are available in China, mainly because of their high cost.
It has been reported that different populations respond differently to different peptide fragments owing to ethnic differences (3, 23). In our previous study, we obtained 10 T-cell-responsive peptides from 113 overlapping peptides spanning three entire M. tuberculosis proteins (ESAT-6, CFP-10, and Ag85B) by screening peripheral blood (PBL) and pleural fluid (PLF) samples from Chinese patients with TB, using an enzyme-linked immunosorbent spot (ELISPOT) assay. These 10 peptides included E6, E7, and C14, which had strong reactions for patients with confirmed TB. In this study, peptides E6, E7, and C14 and mixtures of two or three of these peptides, i.e., E6 plus E7 (E6+E7) and E6+E7+C14, were used as stimuli to establish the validity of an IFN-γ ELISPOT assay for monitoring a series of PBL samples from patients with confirmed pulmonary TB (PTB) during disease development and treatment.
MATERIALS AND METHODS
Participants.
In this study, diagnosis of active PTB was made on the basis of clinical, radiological, microbiological, and pathological information collected after recruitment (2). PBL samples (8 ml) were collected from in-patients with confirmed PTB who were untreated or in the initial stage of treatment. As shown in Table 1 and other related tables, a total of 731 patients with confirmed PTB were enrolled at Guangzhou Chest Hospital, Guangdong Province, People's Republic of China, between September 2005 and July 2009. Peripheral blood mononuclear cells (PBMCs) from PBL samples of 202 PTB patients were stimulated by all peptides. However, the rest were not stimulated by all peptides, since occasionally there were insufficient cells. The PBL samples from 75 adult donors who appeared to be negative based on clinical symptoms, sputum smear and culture results, TST results, and X-ray examination, collected from the Guangzhou Blood Center, were used as true-negative healthy controls. Twenty-one umbilical cord blood samples collected from the Women and Children's Hospital of Guangzhou were used as true-negative controls. The other control PBL samples were taken from one community population in Shenzhen City, Guangdong Province, from 147 adult donors who appeared to be negative based on clinical symptoms, sputum smear and culture results, and X-ray examination but were negative or positive by TST. Moreover, PBL samples from 9 outpatients with PTB, taken at months 1, 2, 3, 4, 5, and 6 during regular anti-TB therapy, were tested by a combined E6+E7 and E6+E7+C14 IFN-γ ELISPOT assay, and PBL samples from 19 in-patients with PTB and from 5 healthy donors were analyzed by intracellular cytokine staining (ICS).
Table 1.
Information on participants used for in-house IFN-γ ELISPOT assays in this study
| Donor group | No. of patients in age group (yr) |
Mean age (yr) ± SD | No. of males | No. of females | Total no. of patients | |||
|---|---|---|---|---|---|---|---|---|
| 15–44 | 45–59 | 60–74 | 75–90 | |||||
| Clinically confirmed (symptoms, antituberculosis therapy effect, sputum smear or culture result, TST result, and X-ray examination) pulmonary tuberculosis in-patientsa | 339 | 185 | 173 | 34 | 43.29 ± 18.86 | 421 | 310 | 731 |
| In-patients with pulmonary tuberculosis confirmed by symptoms, antituberculosis therapy effect, and sputum smear or culture resulta | ||||||||
| Positive | 21 | 12 | 11 | 17 | 46.46 ± 13.22 | 22 | 39 | 61 |
| Negative | 11 | 6 | 3 | 0 | 42.65 ± 19.21 | 14 | 6 | 20 |
| Total | 32 | 18 | 14 | 17 | 45.36 ± 18.13 | 36 | 45 | 81 |
| In-patients with pulmonary tuberculosis confirmed by symptoms, antituberculosis therapy effect, and TSTa | ||||||||
| 0 mm | 3 | 2 | 1 | 0 | 49.35 ± 9.27 | 1 | 5 | 6 |
| 5–9 mm | 2 | 1 | 1 | 1 | 47.14 ± 15.24 | 3 | 2 | 5 |
| 10–19 mm | 32 | 15 | 15 | 7 | 43.16 ± 14.56 | 42 | 27 | 69 |
| >20 mm | 16 | 21 | 6 | 3 | 48.33 ± 15.77 | 26 | 20 | 46 |
| Total | 53 | 39 | 23 | 11 | 44.57 ± 16.36 | 72 | 54 | 126 |
| True-negative adult donorsb | 35 | 13 | 17 | 10 | 46.17 ± 15.73 | 40 | 35 | 75 |
| Community donorsc | 49 | 39 | 35 | 24 | 49.51 ± 14.39 | 56 | 91 | 147 |
| Umbilical cord blood samples | 21 | |||||||
There are overlap cases in the first three groups.
All donors appeared negative based on clinical symptoms, sputum smear and culture results, TST results, and X-ray examination.
All donors appeared negative based on clinical symptoms, sputum smear and culture results, and X-ray examination but were negative or positive by TST.
The study was approved by the local ethics committee, and all patients and community donors enrolled gave written informed consent.
TST.
The subjects enrolled underwent a TST administered by the Mantoux method, with 0.1 ml (5 tuberculin units) of Biocinetest-PPD tuberculin RT23 (Chengdu Institute of Biological Products, China) injected intradermally into the volar surface of the forearm. Induration at 48 to 72 h was measured and recorded. Subjects with an induration of ≥5 mm were classified as positive. The TST responses were categorized as follows, based on the transverse diameter of induration: −, ≤4 mm; +, 5 to 9 mm; ++, 10 to 19 mm; and +++, ≥20 mm (2).
IFN-γ ELISPOT assay.
In our previous work, we obtained two M. tuberculosis-specific ESAT-6 peptides, E6 (amino acids [aa] 18 to 32; IQGNVTSIHSLLDEG [Sun Yat-Sen University, 29 December 2008, Chinese patent application CN 20111130364.8]) and E7 (aa 25 to 39; IHSLLDEGKQSLTKL [Sun Yat-Sen University, 19 May 2011, Chinese patent application CN 200810220523.1]) (19), and an M. tuberculosis-specific CFP-10 peptide, C14 (aa 52 to 65; AAVVRFQEAANKQK). E6, E7, C14, and the E6+E7 and E6+E7+C14 mixtures, with 10 μg/ml of each peptide, were used as antigenic stimuli for the IFN-γ ELISPOT assay. Briefly, PBMCs were obtained from PBL by Ficoll-Hypaque density gradient centrifugation (Ficoll-Paque Plus; Amersham Bioscience, Netherlands). Cells were then resuspended in RPMI 1640 containing (100 units/ml; penicillin and streptomycin sigma) and 10% fetal calf serum (FCS; HyClone). A total of 2.5 × 105 cells, resuspended in 150 μl culture medium, was plated in each well of a 96-well polyvinylidene-backed plate (MultiScreen-IP; Millipore, MA) which had been precoated with mouse anti-human IFN-γ antibody (5 μg/ml; eBioscience). Cells mixed with different antigenic stimuli were incubated for about 18 h at 37°C in 5% CO2. A phorbol myristate acetate (PMA) (25 ng/ml; Sigma, United Kingdom) and ionomycin (1 μg/ml; Sigma, United Kingdom) mixed stimulus was used as a positive control, a stimulus-free medium was used as a negative control, and a cell-free medium was used as a background control.
During incubation, peptide-responsive cells would release IFN-γ after they were stimulated by peptides. After washing with 0.05% Tween 20 in phosphate-buffered saline (PBST), biotin-conjugated mouse polyclonal anti-human IFN-γ antibody (0.5 μg/ml; eBioscience) was added and incubated for 2 h at room temperature. After washing, an alkaline phosphate-conjugated streptavidin (2 μg/ml; Thermo) was added and incubated for 2 to 2.5 h at room temperature. After a further washing step, nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (NBT/BCIP) substrate buffer was added to aid visualization. Finally, the plate was washed thoroughly with distilled water to stop the detection reaction. The individual spots were then counted using an automated image analysis system ELISPOT reader (Cellular Technology Ltd.). All readings were verified manually. An SFP value (number of spot-forming cells [SFC]/106 PBMCs) of ≥50 was used as the standard indicating positivity in this study.
T-SPOT.TB assay.
The T-SPOT.TB (Oxford Immunotec, United Kingdom) assay was performed using PBL according to the manufacturer's instructions. Four wells with a membrane precoated with mouse anti-human IFN-γ monoclonal antibody were used for each subject. The assay requires a total of 2.5 × 105 viable cells in each well. Cells in each well were stimulated with medium alone as a negative control, with phytohemagglutinin as a positive control, and with different peptide panels (ESAT-6 peptide mixture [panel A] and CFP-10 peptide mixture [panel B]). Finally, the individual spots were counted and verified manually as described above. Subjects were considered positive if there was a positive response to one or both antigen panels. The background number of spots in negative-control wells was always <5 spots per well. The response of stimulated cultures was considered negative if the test well contained <50 spots; in general, a positive response was defined as having an SFP value of ≥25 (SFC/106 viable cells) for either panel A, panel B, or both.
ICS.
A total of 2 × 105 to 5 × 105 PBMCs were resuspended in 200 μl RPMI 1640 and then plated in the wells of a 96-well plate (Costar; Corning Inc.). Anti-human CD28 antibody and anti-human CD49d antibody (Sigma), both at a final concentration of 1 μg/ml, were added to each of the wells containing PBMCs. Cells were then incubated with different peptides (E6, E7, or C14 at 10 μg/ml) for 72 h at 37°C in 5% CO2. Cells stimulated with medium alone and cells incubated with PMA and ionomycin were used as a negative control and a positive control, respectively. During the last 8 h of incubation time, brefeldin A (BFA; Sigma) was added to each well, to a final concentration of 10 μg/ml. After incubation, the cells from each well were collected, washed with 2% FCS-PBS, and then combined with anti-human CD3 monoclonal antibody (MAb)–fluorescein isothiocyanate (FITC) (Ancell), anti-human CD4 MAb–phycoerythrin (PE)–Cy5 (eBioscience), and anti-human CD8 MAb–allophycocyanin (APC) (eBioscience) in the dark at room temperature for 30 min. After further washing with 2% FCS-PBS, the cells were fixed, and Cytoperm was added and allowed to react at 4°C in the dark for 16 h. Phycoerythrin–IFN-γ MAb (Biolegend) was then included for 40 min at 4°C in the dark. Finally, the cells were fixed and detected by flow cytometric analysis.
HLA genotyping of patients with TB.
To investigate the alleles restricted to HLA-DR for the above peptides, including E6, E7, and C14, which respond mainly to CD4+ T cells of patients with TB, genotyping of the HLA-DRB alleles of the corresponding peptide IFN-γ ELISPOT assay-positive TB patient was performed by PCR–sequence-specific primers (PCR-SSP) according to the manufacturer's protocol (Invitrogen). Briefly, DNAs of PBL from patients with TB were extracted with QLAamp DNA kits (Invitrogen) and used as templates to perform PCR-SSP in a 96-well plate. The PCR products were identified by agarose gel electrophoresis, and Pel-Freez SSP HLA software analysis was used to determine the HLA-DR alleles.
Data analysis.
Data analyses were performed using the statistical software SPSS, version 16.0. Test results were considered statistically significant for P values of <0.05. GraphPad Prism, version 5.0, was used to construct the graphs and figures.
RESULTS
IFN-γ ELISPOT assay of PBL samples from patients with PTB, using different peptide stimuli.
The detection results for IFN-γ ELISPOT assays with different peptide stimuli for PBL samples from patients with PTB are shown in Table 2. The positive detection rates obtained with peptides E6, E7, C14, E6+E7, and E6+E7+C14 were 78.38% (377/481 cases), 74.86% (390/521 cases), 55.83% (268/480 cases), 90.43% (633/700 cases), and 91.51% (614/671 cases), respectively. The median SFP values for positive detection were 282, 221, 272, 474, and 433, respectively. These results suggested that the peptide mixtures (E6+E7 and E6+E7+C14) had much higher sensitivities for detecting patients with PTB than the single peptides.
Table 2.
IFN-γ ELISPOT examination with different peptides of PBL samples from patients with PTBb
| Peptide(s) | Total no. of samplesa | No. of positive samples | Positivity rate (%) | Median SFP value for positive results |
|---|---|---|---|---|
| E6 | 481 | 377 | 78.38 | 282 |
| E7 | 521 | 390 | 74.86 | 221 |
| C14 | 480 | 268 | 55.83 | 272 |
| E6+E7 | 700 | 633 | 90.43 | 474 |
| E6+E7+C14 | 671 | 614 | 91.51 | 433 |
A total of 731 patients with confirmed PTB were enrolled. Among them, PBMCs from PBL samples of 202 PTB patients were stimulated by all peptides, and the rest were not stimulated by all stimuli because there were not enough cells.
Among the five groups, the E6+E7 and E6+E7+C14 assays had a much higher sensitivity for detecting patients with PTB than the other peptide assays. A Wilcoxon test was used to analyze the differences between single-peptide and peptide mixture groups. It was found that the difference between E6 and E6+E7 was not significant (P > 0.05). Significant differences were found between the following pairs: C14 and E6+E7, E7 and E6+E7, C14 and E6+E7+C14, E6 and E6+E7+C14, E7 and E6+E7+C14, and E6+E7 and E6+E7+C14 (P < 0.05).
Evaluation of the specificities of E6+E7 IFN-γ ELISPOT assay and E6+E7+C14 IFN-γ ELISPOT assay.
To evaluate the specificities of the E6+E7 IFN-γ ELISPOT assay and the E6+E7+C14 IFN-γ ELISPOT assay, the PBL samples of 75 adult donors who appeared negative based on clinical symptoms, sputum smear and culture results, TST results, and X-ray examination, as well as 21 umbilical cord blood samples, were tested. The results (Table 3) showed that the specificities of the E6+E7 IFN-γ ELISPOT assay, the E6+E7+C14 IFN-γ ELISPOT assay, and both combined were 90.36%, 90.36%, and 86.21%, respectively, for the 75 true-negative samples and 100% for all 21 umbilical cord blood samples. Some of the adult donors for whom positive results were detected might not be truly negative; these results probably represent latent TB infection in immunosuppressed individuals.
Table 3.
Detection results for PBL samples from 75 true-negative adult donors and for 21 umbilical cord blood samples with E6+E7 IFN-γ ELISPOT assay and E6+E7+C14 IFN-γ ELISPOT assay
| Peptides | Sample group (n) | No. of positive samples | Positivity rate (%) | Specificity (%) of ELISPOT assaya | Median SFP value (SFC/106 PBMCs) |
|---|---|---|---|---|---|
| E6+E7 | True-negative PBL (75) | 8 | 10.67 | 90.36 | 4 |
| Umbilical cord blood (21) | 0 | 0 | 100 | 2 | |
| E6+E7+C14 | True-negative PBL (75) | 8 | 10.67 | 90.36 | 3 |
| Umbilical cord blood (21) | 0 | 0 | 100 | 2 | |
| Total of E6+E7 and E6+E7+C14 | True-negative PBL (75) | 12 | 16.00 | 86.21 | 4 |
| Umbilical cord blood (21) | 0 | 0 | 100 | 3 |
Specificity was calculated with the following formula: specificity (%) = number of true-negative samples/(number of true-negative samples + number of false-positive samples) × 100.
Comparison of IFN-γ ELISPOT assay with TST for patients with PTB.
To investigate the sensitivities of IFN-γ ELISPOT assays based on our peptides, PBL samples from patients with PTB who were monitored by TST were tested with the series of IFN-γ ELISPOT methods described above. The detection results for IFN-γ ELISPOT assays and TST were compared and analyzed statistically (Table 4). The two peptide mixtures, especially E6+E7+C14, had higher detection sensitivities than the three single peptides, with a similar sensitivity to that of TST (90.16% and 93.44% for E6+E7+C14 IFN-γ ELISPOT assay and TST, respectively; P > 0.05). Some level of positive detection by all of the IFN-γ ELISPOT assays was seen for both the positive and negative TST detection groups. These results suggest that all of the IFN-γ ELISPOT assays, with higher specificities than TST, might reduce the false-positive and false-negative rates of TST detection. The assays with the peptide mixtures (E6+E7 and E6+E7+C14) had much greater detection sensitivities than those with the single peptides, and the combination of the E6+E7 and E6+E7+C14 assays might be used to improve the rates of detection of TB disease and TBI.
Table 4.
Comparison of IFN-γ ELISPOT assay and TST for patients with PTB
| IFN-γ ELISPOT assay and result | No. (%) of samples with TST resulta |
P value | |||||
|---|---|---|---|---|---|---|---|
| Negative (0 mm) | Positive |
Total | |||||
| + (5–9 mm) | ++ (10–19 mm) | +++ (≥20 mm) | All positive samples | ||||
| E6 | |||||||
| Positive | 4 (5.71) | 3 (4.29) | 21 (30.00) | 15 (21.43) | 39 (55.71) | 43 (61.43) | 2.04 × 10−4 |
| Negative | 2 (2.86) | 2 (2.85) | 15 (21.43) | 8 (11.43) | 25 (35.71) | 27 (38.57) | |
| Total | 6 (8.57) | 5 (7.14) | 36 (51.43) | 23 (32.86) | 64 (91.43) | 70 | |
| E7 | |||||||
| Positive | 4 (4.00) | 2 (2.00) | 38 (38.00) | 20 (20.00) | 60 (60.00) | 64 (64.00) | 2.55 × 10−6 |
| Negative | 2 (2.00) | 2 (2.00) | 15 (15.00) | 17 (17.00) | 34 (34.00) | 36 (36.00) | |
| Total | 6 (6.00) | 4 (4.00) | 53 (53.00) | 37 (37.00) | 94 (94.00) | 100 | |
| C14 | |||||||
| Positive | 4 (5.88) | 2 (2.94) | 20 (29.41) | 11 (16.18) | 33 (48.53) | 37 (54.41) | 2.94 × 10−4 |
| Negative | 2 (2.94) | 3 (4.41) | 15 (22.06) | 11 (16.18) | 29 (42.65) | 31 (45.59) | |
| Total | 6 (8.82) | 5 (7.5) | 35 (51.47) | 22 (32.36) | 62 (91.18) | 68 | |
| E6+E7 | |||||||
| Positive | 6 (7.06) | 5 (5.88) | 37 (43.53) | 18 (21.18) | 60 (70.59) | 66 (77.65) | 0.02 |
| Negative | 1 (1.18) | 0 (0.00) | 10 (11.76) | 8 (9.41) | 18 (21.18) | 19 (22.35) | |
| Total | 7 (8.24) | 5 (5.88) | 47 (55.29) | 26 (30.59) | 78 (91.76) | 85 | |
| E6+E7+C14 | |||||||
| Positive | 6 (9.83) | 4 (6.56) | 35 (57.38) | 12 (19.67) | 51 (83.61) | 57 (93.44) | 0.75 |
| Negative | 0 (0.00) | 0 (0.00) | 2 (3.28) | 2 (3.28) | 4 (6.56) | 4 (6.56) | |
| Total | 6 (9.83) | 4 (6.56) | 37 (60.66) | 14 (22.95) | 55 (90.16) | 61 | |
No individual was found with a TST induration of 1 to 4 mm. The two peptide mixtures, particularly E6+E7+C14, had a higher detection sensitivity than the three single peptides, with a similar sensitivity to that of TST (93.44% and 90.16% for the E6+E7+C14 IFN-γ ELISPOT assay and TST, respectively; P > 0.05). Some level of positive detection was achieved by all IFN-γ ELISPOT assays for both the positive and negative TST detection groups.
Comparison of IFN-γ ELISPOT assays and T-SPOT.TB assay for patients with PTB.
The sensitivity of the IFN-γ ELISPOT assays was further investigated by comparing them with the T-SPOT.TB (Oxford Immunotec Limited, Abingdon, United Kingdom) assay, a mature, credible, and commercial IFN-γ ELISPOT assay-based test. The results of the comparison of our IFN-γ ELISPOT assays and the T-SPOT.TB assay for 86 PBL samples from patients with PTB are shown in Tables 5 and 6. The detection rates (median positive SFP values) for the E6+E7 panel, the E6+E7+C14 panel, and the combined E6+E7 and E6+E7+C14 panels in IFN-γ ELISPOT assays were 88.37% (754), 94.19% (733), and 96.51% (740), respectively, compared with 96.51% (154), 97.67% (271), and 98.84% (246) for panel A, panel B, and the total of panel A and panel B for the T-SPOT.TB assay, respectively. A Wilcoxon test was used to analyze the difference between IFN-γ ELISPOT assays and the T-SPOT.TB assay. The statistical results showed that the median SFP value with E6+E7 (or E6+E7+C14) was higher than that with panel A, and that for the total of E6+E7 and E6+E7+C14 was higher than that for the total of panel A and panel B (P < 0.05). No significant differences were found between E6+E7 (or E6+E7+C14) and panel B (P > 0.05); on the other hand, similar positive detection rates were found for E6+E7+C14 and panels A and B and for the total of E6+E7 and E6+E7+C14 and the total of panel A and panel B (P > 0.05), but the detection rate was lower with E6+E7 than with panel A and panel B (P < 0.05). Therefore, the E6+E7+C14 IFN-γ ELISPOT assay has a similar sensitivity to that of the T-SPOT.TB assay.
Table 5.
Comparison of IFN-γ ELISPOT assay and T-SPOT.TB assay with 86 PTB samplesb
| Assay and peptides | No. of positive samples | Positivity rate (%) | SFP value (SFC/106 PBMCs or viable cells)a |
||
|---|---|---|---|---|---|
| P25 | P50 | P75 | |||
| IFN-γ ELISPOT assay | |||||
| E6+E7 | 76 | 88.37 | 170 | 754 | 1450 |
| E6+E7+C14 | 81 | 94.19 | 160 | 733 | 1539 |
| Total of E6+E7 and E6+E7+C14 | 83 | 96.51 | 165 | 740 | 1456 |
| T-SPOT.TB assay | |||||
| Panel A | 83 | 96.51 | 66 | 154 | 344 |
| Panel B | 84 | 97.67 | 86 | 271 | 894 |
| Total of panel A and panel B | 85 | 98.84 | 66 | 246 | 801 |
P25, lower quartile SFP value; P50, median SFP value; P75, upper quartile SFP value.
All detection assays had a high sensitivity. A Wilcoxon test was used to analyze the difference between IFN-γ ELISPOT assay and T-SPOT.TB assay. It was found that the median SFP value for E6+E7 (or E6+E7+C14) was higher than that for panel A (P < 0.05), and that for the total of E6+E7 and E6+E7+C14 was higher than that for the total of panel A and panel B. No significant differences were found between E6+E7 (or E6+E7+C14) and panel B (P > 0.05); on the other hand, similar positive detection rates were found for E6+E7+C14 and panels A and B and for the total of E6+E7 and E6+E7+C14 and the total of panel A and panel B (P > 0.05), but the positive detection rate was lower with E6+E7 than with panel A and panel B (P < 0.05).
Table 6.
Agreement in rates of PTB detection between IFN-γ ELISPOT and T-SPOT.TB assaysa
| IFN-γ ELISPOT (E6+E7 and E6+E7+C14) assay result | No. of samples with T-SPOT.TB assay (panel A and panel B) result |
P value | ||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| Positive | 82 | 1 | 83 | 0.63 |
| Negative | 3 | 0 | 3 | |
| Total | 85 | 1 | 86 | |
Eighty-six PBL samples from patients with PTB were assayed at the same time by IFN-γ ELISPOT assay and T-SPOT.TB assay. The results were defined as positive if a spot appeared in the E6+E7 and/or E6+E7+C14 IFN-γ ELISPOT assay, as well as in panel A and/or panel B of the T-SPOT.TB assay. By χ2 test, no significant difference was found between the two methods (P > 0.05) (also see Table 5).
Comparison of IFN-γ ELISPOT assay and sputum acid-fast staining for patients with PTB.
Sputum acid-fast staining can be used to detect patients with active PTB (26). In this study, the patients with PTB fell into two categories, positive and negative, by sputum acid-fast staining. A comparison of the detection results for IFN-γ ELISPOT assay and sputum acid-fast staining for patients with TB is shown in Table 7. For some patients who appeared negative by sputum acid-fast staining, PTB could be detected by two IFN-γ ELISPOT assays. A higher detection sensitivity was found for the E6+E7 IFN-γ ELISPOT assay and the E6+E7+C14 IFN-γ ELISPOT assay than for sputum acid-fast staining (P < 0.05).
Table 7.
Comparison of two IFN-γ ELISPOT assays and sputum acid-fast staining for patients with PTB
| IFN-γ ELISPOT assay and result | No. of samples with sputum acid-fast staining resulta |
P valueb | ||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| E6+E7 | ||||
| Positive | 27 | 34 | 61 | 4.90 × 10−5 |
| Negative | 7 | 13 | 20 | |
| Total | 34 | 47 | 81 | |
| E6+E7+C14 | ||||
| Positive | 25 | 36 | 61 | 5.43 × 10−9 |
| Negative | 0 | 4 | 4 | |
| Total | 25 | 40 | 65 | |
Some patients who appeared to be negative by sputum acid-fast staining could be detected by the two IFN-γ ELISPOT assays. A higher detection sensitivity was found for the E6+E7 IFN-γ ELISPOT assay and the E6+E7+C14 IFN-γ ELISPOT assay than for sputum acid-fast staining (P < 0.05).
Calculated by χ2 test.
In vivo kinetics of peptide-specific and IFN-γ-productive T cells in PBL samples from PTB outpatients during TB development and treatment, assessed by IFN-γ ELISPOT assay.
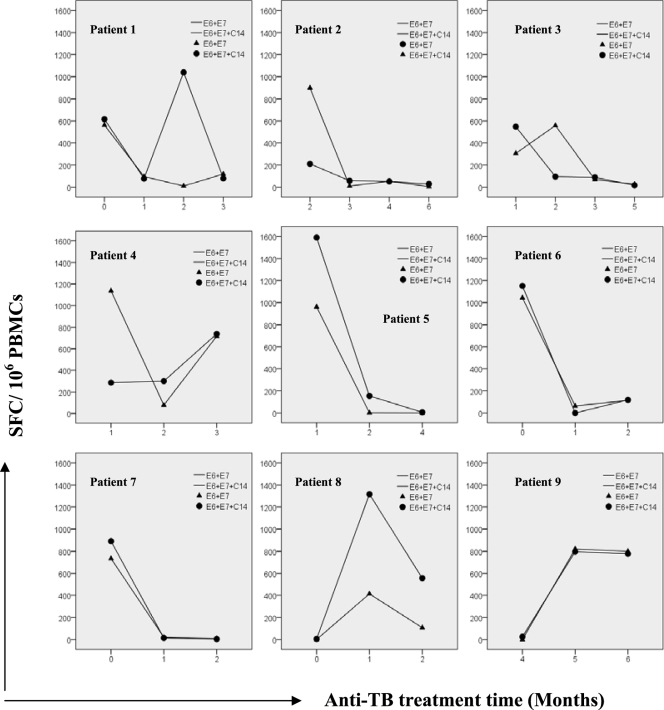
To track the in vivo kinetics of peptide-specific and IFN-γ-productive T cells in samples from patients with PTB during TB development and treatment, PBL samples from outpatients with PTB were collected at months 1, 2, 3, 4, 5, and 6, during regular anti-TB therapy, and examined by a combined E6+E7 and E6+E7+C14 IFN-γ ELISPOT assay. Nine patients' samples were monitored at three or more time points. These patients, initially diagnosed as having secondary PTB according to a positive TST, positive or negative sputum smear and sputum culture, positive X-ray examination, and previous TB history, began to improve clinically from month 2 after regular treatment. Figure 1 shows that with the exception of patients 4 and 9, the SFP value (SFC/106 PBMCs) began to decrease, with some variation, together with an amendment in TB symptoms, until a relatively low (almost normal) level was reached and maintained after 3 to 4 months.
Fig 1.
Dynamics of SFP values during the course of TB development and treatment, examined by E6+E7 and E6+E7+C14 IFN-γ ELISPOT assays. As shown in Table 6, PBL samples were collected at regular intervals from nine outpatients with PTB, during regular anti-TB treatment, and were monitored by E6+E7 and E6+E7+C14 IFN-γ ELISPOT assays. With the exception of patients 4 and 9, SFP values began to decrease, together with an amendment in TB symptoms, until a relatively low, almost normal, level was reached and maintained after 3 to 4 months. SFP, number of spot-forming cells/106 peripheral blood mononuclear cells.
Comparison of results for 147 community donors by combined IFN-γ ELISPOT assay, T-SPOT.TB assay, and TST.
One hundred forty-seven community donors from one community were monitored by a combined E6+E7 and E6+E7+C14 IFN-γ ELISPOT assay, T-SPOT.TB assay, and TST at the same time. As shown in Table 8, the positive detection rate of 14.29% and the median SFP value of 152 for the T-SPOT.TB assay were significantly lower than the 29.93% positive detection rate and median SFP value of 482 for the combined IFN-γ ELISPOT assay for the same 147 cases (P < 0.05); on the other hand, these positive detection rates were significantly lower than the rate of 82.99% for positive TST results (P < 0.05). As found for patients with PTB, some level of positive detection was achieved by the combined IFN-γ ELISPOT assay and the T-SPOT.TB assay for both the positive and negative TST detection groups.
Table 8.
Comparison of detection results for 147 community donors from one community, assayed by IFN-γ ELISPOT assay, T-SPOT.TB assay, and TSTc
| Assay and result | No. (%) of samples with TST resulta |
SFP value (SFC/106 PBMCs or viable cells)b |
||||
|---|---|---|---|---|---|---|
| Positive (≥5 mm) | Negative (0 mm) | Total | P25 | P50 | P75 | |
| IFN-γ ELISPOT assay (total of E6+E7 and E6+E7+C14 assays) | ||||||
| Positive | 35 (23.81) | 9 (6.12) | 44 (29.93) | 169 | 482 | 760 |
| Negative | 87 (59.18) | 16 (10.88) | 103 (70.07) | 0 | 0 | 0 |
| Total | 122 (82.99) | 25 (17.01) | 147 | 0 | 0 | 14 |
| T-SPOT.TB assay (total of panel A and panel B) | ||||||
| Positive | 15 (10.20) | 6 (4.08) | 21 (14.29) | 473 | 152 | 44 |
| Negative | 107 (72.79) | 19 (12.93) | 126 (85.71) | 1 | 3 | 0 |
| Total | 122 (82.99) | 25 (17.01) | 147 | 0 | 1 | 5 |
No individual was found with a TST induration of 1 to 4 mm.
P25, lower quartile SFP value; P50, median SFP value; P75, upper quartile SFP value.
For the T-SPOT.TB assay, a positive detection rate of 14.29% and a median positive SFP value of 152 were found, which were significantly lower than the 29.93% positive detection rate and median positive SFP value of 482 for the combined IFN-γ ELISPOT assay for the same 147 cases (P < 0.05); on the other hand, these positive detection rates were significantly lower than the rate of 82.99% for positive TST results (P < 0.05). Some level of positive detection was achieved by the two IFN-γ ELISPOT assays for both the positive and negative TST detection groups.
ICS results for PBL samples from patients with PTB and from healthy donors, using stimulation with E6, E7, and C14 peptides.
To determine the levels of peptide-specific and IFN-γ-productive cells in CD3+ CD4+ and CD3+ CD8+ T cells and to investigate the categories and characteristics of peptide-responsive T cells, PBL samples were obtained from 19 patients with PTB and from 5 healthy donors. PBMCs of the PBL samples were stimulated with peptides E6, E7, and C14 and then detected by ICS. Table 9 shows that the percentages of CD3+ CD4+ T cells producing IFN-γ in PTB samples after E6 and E7 stimulation were 0.69% ± 0.56% and 0.63% ± 0.67%, respectively, compared with 0.04% ± 0.05% and 0.05% ± 0.01%, respectively, in healthy control samples. These differences had greater significance (P = 0.007 and P = 0.004, respectively) than that found for CD3+ CD8+ T cells (0.59% ± 0.51% and 0.11% ± 0.02%, respectively, for E6 [P = 0.027] and 0.63% ± 0.59% and 0.14% ± 0.07%, respectively, for E7 [P = 0.049]). In contrast, no significant differences were found between PTB and healthy control samples (P > 0.05) for CD3+ CD4+ T cells or CD3+ CD8+ T cells producing IFN-γ after C14 stimulation. The results indicate that peptides E6 and E7 respond mainly to CD4+ T cells of patients with TB and are ideal CD4+ T-cell-responsive epitopes.
Table 9.
ICS results for PBL samples from patients with PTB and from healthy donors, using three peptidesa
| Peptide and cell type | Mean % ± SD |
P value | |
|---|---|---|---|
| PTB group (n = 19) | Healthy donor group (n = 5) | ||
| E6 | |||
| CD3+ CD4+ IFN-γ-producing T cells | 0.69 ± 0.56 | 0.04 ± 0.05 | 0.007 |
| CD3+ CD8+ IFN-γ-producing T cells | 0.59 ± 0.51 | 0.11 ± 0.02 | 0.027 |
| E7 | |||
| CD3+ CD4+ IFN-γ-producing T cells | 0.63 ± 0.67 | 0.05 ± 0.01 | 0.004 |
| CD3+ CD8+ IFN-γ-producing T cells | 0.63 ± 0.59 | 0.14 ± 0.07 | 0.049 |
| C14 | |||
| CD3+ CD4+ IFN-γ-producing T cells | 0.80 ± 0.76 | 0.21 ± 0.07 | >0.05 |
| CD3+ CD8+ IFN-γ-producing T cells | 0.59 ± 0.69 | 0.29 ± 0.09 | >0.05 |
The results suggest that peptides E6 and E7 respond mainly to CD4+ T cells of patients with TB and are ideal CD4+ T-cell-responsive epitopes.
HLA-DR genotyping of patients with PTB whose IFN-γ ELISPOT assay results showed a strong positive reaction.
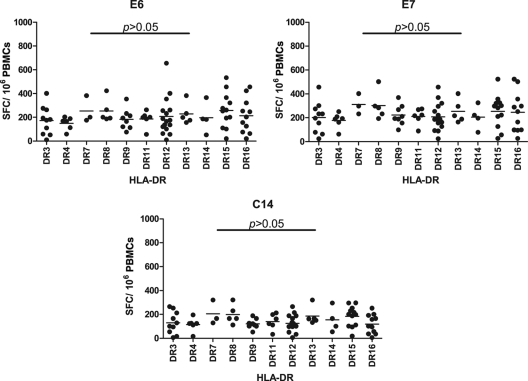
To investigate HLA-DR-restricted alleles of the peptides studied, including E6, E7, and C14, patients with PTB who had a strong positive response to peptides E6, E7, and C14 by IFN-γ ELISPOT assay were selected for detection of their HLA-DR alleles by PCR-SSP. Figure 2 shows that 11 types of HLA-DRB1 allele were detected, including HLA-DR3, -DR4, -DR7, -DR8, -DR9, -DR11, -DR12, -DR13, -DR14, -DR15, and -DR16. No significant differences were found among different HLA-DR alleles for the peptides E6, E7, and C14, suggesting that these peptides are widely HLA-DR allele-restricted epitopes.
Fig 2.
IFN-γ ELISPOT results for PBMCs of patients with TB whose HLA-DR genotype had been tested. Eleven types of HLA-DRB1 allele were detected. No significant differences were found among different HLA-DRB1 alleles for peptides E6, E7, and C14.
DISCUSSION
M. tuberculosis is phagocytized by macrophages after invading the body. The main anti-M. tuberculosis immune response is based on CD4+ T cells. Once infected by M. tuberculosis, the body generates an immune response, and memory T cells may be produced which remain in the body for a long time. When memory T cells are restimulated by specific antigens, they secrete cytokines, such as IFN-γ, tumor necrosis factor alpha, etc. (21). IFN-γ is related to the activation of macrophages and cytotoxic cells. The latter can kill the cells which phagocytize M. tuberculosis. Thus, IFN-γ is an important cytokine resulting from the immune reaction to M. tuberculosis (4, 15, 16). In this study, analysis by ICS demonstrated that peptides E6 and E7 stimulated T cells, especially CD4+ T cells, to release IFN-γ. The results for peptide C14 were not statistically significant, possibly owing to the limited test number. We used these M. tuberculosis-specific peptides to stimulate PBMCs in order to rapidly diagnose active TBI, and even LTBI. Many studies on the detection of TB by IFN-γELISPOT assay have used ESAT-6, CFP-10, or their fusion protein as a stimulus (1, 22). In contrast, we used short peptide sequences to stimulate PBMCs and achieved good results. Theoretically, an assay based on peptide sequences should have more effective stimulation (7).
All of the peptides used in our study were of high purity (>99%). Our previous study showed that the detection efficacies with E6, E7, and C14 were better than those with other selected peptides, and in this study, we continued our work on E6, E7, C14, and mixtures of these peptides (E6+E7 and E6+E7+C14). People in the Guangdong region, a large commercial region in the southern part of China, took part in the study. Guangdong has a moving population, so the prevention and treatment of TB are important but difficult.
Owing to the limited number of PBMCs that can obtained from one PBL sample, some cases could not be stimulated by all peptides and then detections performed. The detection results for patients with PTB showed that there were lower positive rates (55.83%, 78.38%, and 74.86%) for the three single-peptide groups than for the two peptide mixture groups (90.43% and 91.51%). Statistical differences were found between the peptide mixture groups and their component peptides, except for the difference between the E6+E7 mixture and the E6 peptide. Thus, use of a peptide mixture is advantageous. Both peptide mixture IFN-γ ELISPOT assays had a higher sensitivity for detecting patients with PTB than the single peptide groups, with a positive detection rate similar to that of TST (90.62% versus 95.59%) and greater than that of sputum acid-fast staining. On the other hand, the high specificity of the tests was also proved by detection with PBL samples from 75 true-negative adult donors who appeared negative based on clinical symptoms, sputum smear and culture results, TST results, and X-ray examination, as well as with 21 umbilical cord blood samples. Therefore, as seen in other reports (7, 8, 10, 13, 25), these IFN-γ ELISPOT assays could reduce the false-positive and false-negative detection rates of TST and the false-negative detection rate of sputum acid-fast staining.
All detected cases could be categorized into two pairs and four groups, as follows: (i) patients with PTB receiving initial treatment and patients with PTB who were retreated; and (ii) patients with PTB who were responsive to regular anti-TB drugs, including rifampin, isoniazid, pyrazinamide, and ethambutol, and patients resistant to these drugs. However, the detection results were similar between the two groups of each pair (data not shown).
In this study, 86 patients with PTB were selected randomly for testing by IFN-γ ELISPOT assay and T-SPOT.TB assay. The detection rates (median positive SFP values) for the E6+E7, E6+E7+C14, and combined E6+E7 and E6+E7+C14 IFN-γ ELISPOT assays were 88.37% (754), 94.19% (733), and 96.51% (740), respectively, compared with 96.51% (154), 97.67% (271), and 98.84 (246) for panel A, panel B, and the total of panel A and panel B, respectively, in the T-SPOT.TB assay. It was found that the median SFP value with E6+E7 (or E6+E7+C14) was higher than that with panel A (P < 0.05), and that for the total of E6+E7 and E6+E7+C14 was higher than that for the total of panel A and panel B (P < 0.05). No significant differences were found between E6+E7 (or E6+E7+C14) and panel B (P > 0.05); on the other hand, similar positive detection rates were found for E6+E7+C14 and panels A and B and for the total of E6+E7 and E6+E7+C14 and the total of panel A and panel B (P > 0.05), but the positive detection rate was lower for E6+E7 than for panel A and panel B (P < 0.05). Therefore, the E6+E7+C14 IFN-γ ELISPOT assay has a similar sensitivity to that of the T-SPOT.TB assay, and the addition of an E6+E7 assay may provide further improvement.
Since some peptides (for example, those originating from the proteins ESAT-6 and CFP-10) have been reported in other countries as showing a positive reaction for patients with TB by IFN-γ ELISPOT assay, we carried out a small-scale study of the use of these peptide mixtures in IFN-γ ELISPOT assays. The data showed that these peptides had low sensitivity when used for detection in Chinese people (data not shown). This result is in agreement with the study of Ravn et al., who reported that the specificity of ESAT-6 epitope recognition varies in some regions, possibly owing to differences in the HLA molecules frequently expressed in different populations (23). The results of HLA-DR genotyping of patients with PTB showed that E6, E7, and C14 responded to a wide range of different HLA-DRB1 alleles, further proving that these peptides are suitable for use in China. The mixture of these three peptides provides a powerful foundation for rapidly detecting TB disease in China.
Toward the end of our study, nine patients with PTB were tracked at various time points from before treatment to up to 6 months of treatment. PBL samples were obtained at three or more time points from all the patients with PTB; PBMCs were obtained from the PBL samples, and IFN-γ ELISPOT assays based on the E6+E7 mixture and the E6+E7+C14 mixture were carried out. Since samples were limited, only a general trend could be found from the results. After a period of anti-TB treatment, the patients' reactions to the peptides gradually weakened as time passed, apart from two patients, until after 3 to 4 months, a relatively low, almost normal, level was reached and maintained. Thus, this IFN-γ ELISPOT assay could be used to monitor the development of TBI, in agreement with the study of Adetifa et al. (1).
After the sensitivity and specificity of the peptide mixtures had been confirmed, 147 donors from one community were monitored by the combined E6+E7 and E6+E7+C14 IFN-γ ELISPOT assay, the T-SPOT.TB assay, and TST at the same time. A positive detection rate of 29.93% was obtained by the combined peptide mixture IFN-γ ELISPOT assay, which provided a better reflection of the >40.00% TB infection rate in China. Similarly, as found for patients with PTB, the results also suggested that the combined peptide mixture IFN-γ ELISPOT assay, like the T-SPOT.TB assay, reduced the false-positive and false-negative rates of TST detection in community people. This combined peptide IFN-γ ELISPOT assay has potential advantages beyond its greater sensitivity for use in screening the common population for LTBI.
In this study, we identified three M. tuberculosis-specific peptides and found that an ELISPOT assay based on a mixture of these three peptides could be used to detect TBI and LTBI in China. In a future study, we shall select some other ethnic TB patients to investigate the clinical utility of this test.
ACKNOWLEDGMENTS
This work was supported by the National Natural Sciences Foundation of China (30430660; to X.-M. Lai), the Serious Infectious Diseases Special Foundation of China (2008ZX-10003-007, 2008ZX10003-012, 2009ZX10003-018, and 2012ZX10004903-004-002; to X.-M. Lai), and the Guangdong Science and Technology Program (73107; to Y.-M. Fang).
We declare that we have no associations with commercial or other associations that might pose a conflict of interest.
Footnotes
Published ahead of print 11 January 2012
REFERENCES
- 1. Adetifa IM, et al. 2010. Decay kinetics of an interferon gamma release assay with anti-tuberculosis therapy in newly diagnosed tuberculosis cases. PLoS One 5: e12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Thoracic Society 2000. Diagnostic standards and classification of tuberculosis in adults and children. Am. J. Respir. Crit. Care Med. 161: 1376–1395 [DOI] [PubMed] [Google Scholar]
- 3. Arend SM, et al. 2000. Antigenic equivalence of human T-cell responses to Mycobacterium tuberculosis-specific RD1-encoded protein antigens ESAT-6 and culture filtrate protein 10 and to mixtures of synthetic peptides. Infect. Immun. 68: 3314–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arend SM, et al. 2000. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J. Infect. Dis. 181: 1850–1854 [DOI] [PubMed] [Google Scholar]
- 5. Dheda K, van Zyl Smit R, Badri M, Pai M. 2009. T-cell interferon-gamma release assays for the rapid immunodiagnosis of tuberculosis: clinical utility in high-burden vs. low-burden settings. Curr. Opin. Pulm. Med. 15: 188–200 [DOI] [PubMed] [Google Scholar]
- 6. Duanmu H. 2002. Report on the 4th national epidemiological sampling survey of tuberculosis. Chin. J. Tuberc. Respir. Dis. 25: 3–7 [PubMed] [Google Scholar]
- 7. Ertl HC, Xiang Z. 1996. Novel vaccine approach. J. Immunol. 156: 3579–3584 [PubMed] [Google Scholar]
- 8. Ewer K, et al. 2003. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet 361: 1168–1173 [DOI] [PubMed] [Google Scholar]
- 9. Farhat M, Greenaway C, Pai M, Menzies D. 2006. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int. J. Tuberc. Lung Dis. 10: 1192–1204 [PubMed] [Google Scholar]
- 10. Flynn JL, et al. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178: 2249–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hill PC, et al. 2008. Incidence of tuberculosis and the predictive value of ELISPOT and Mantoux tests in Gambian case contacts. PLoS One 3: e1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huebner RE, Schein MF, Bass JB. 1993. The tuberculin skin test. Clin. Infect. Dis. 17: 968–975 [DOI] [PubMed] [Google Scholar]
- 13. Jafari C, et al. 2009. Bronchoalveolar lavage enzyme-linked immunospot for a rapid diagnosis of tuberculosis: a Tuberculosis Network European Trials group study. Am. J. Respir. Crit. Care Med. 180: 666–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jasmer RM, Nahid P, Hopewell PC. 2002. Clinical practice. Latent tuberculosis infection. N. Engl. J. Med. 347: 1860–1866 [DOI] [PubMed] [Google Scholar]
- 15. Kaufmann SH, Cole ST, Mizrahi V, Rubin E, Nathan C. 2005. Mycobacterium tuberculosis and the host response. J. Exp. Med. 201: 1693–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lagrange PH, Simonney N, Herrmann JL. 2007. Philippe-Henri new immunological tests in the diagnosis of tuberculosis. Rev. Mal. Respir. 24: 453–472 [DOI] [PubMed] [Google Scholar]
- 17. Lalvani A, Pareek M. 2010. A 100 year update on the diagnosis of tuberculosis infection. Br. Med. Bull. 93: 69–84 [DOI] [PubMed] [Google Scholar]
- 18. Lee JY, et al. 2006. Comparison of two commercial interferon-gamma assays for diagnosing Mycobacterium tuberculosis infection. Eur. Respir. J. 28: 24–30 [DOI] [PubMed] [Google Scholar]
- 19. Li Y, et al. 2011. Use of HLA-DR*08032/E7 and HLA-DR*0818/E7 tetramers in tracking of epitope-specific CD4+ T cells in active and convalescent tuberculosis patients compared with control donors. Immunobiology 216: 947–960 [DOI] [PubMed] [Google Scholar]
- 20. Menzies D, Pai M, Comstock G. 2007. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann. Intern. Med. 146: 340–354 [DOI] [PubMed] [Google Scholar]
- 21. Ottenhoff TH, de Boer T, van Dissel JT, Verreck FA. 2003. Human deficiencies in type-1 cytokine receptors reveal the essential role of type-1 cytokines in immunity to intracellular bacteria. Adv. Exp. Med. Biol. 531: 279–294 [DOI] [PubMed] [Google Scholar]
- 22. Philip CH, et al. 2005. ESAT-6/CFP-10 fusion protein and peptides for optimal diagnosis of Mycobacterium tuberculosis infection by ex vivo enzyme-linked immunospot assay in the Gambia. J. Clin. Microbiol. 43: 2070–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ravn P, et al. 1999. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J. Infect. Dis. 179: 637–645 [DOI] [PubMed] [Google Scholar]
- 24. Schluger NW, Burzynski J. 2010. Recent advances in testing for latent TB. Chest 138: 1456–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thwaites GE, Chau TT, Farrar JJ. 2002. Improving the bacteriological diagnosis of tuberculous meningitis. J. Clin. Microbiol. 42: 378–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Von Reyn CF, et al. 2001. Skin test reactions to Mycobacterium tuberculosis purified protein derivative and Mycobacterium avium sensitin among health care workers and medical students in the United States. Int. J. Tuberc. Lung Dis. 5: 1122–1128 [PubMed] [Google Scholar]
- 27. WHO 2011. Global tuberculosis control 2011. WHO, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf Accessed 4 October 2011 [Google Scholar]