Abstract
After WHO declared H1N1 pandemic, global vaccination was carried out immediately after much research. However, the data on long-term immunogenicity were lacking. We aimed to investigate the long-term immunogenicity of different H1N1 vaccine dosage groups 24 weeks after vaccination by a randomized clinical trial. A total of 218 participants were stratified into adult (≤60 years old) and elderly (>60 years old) groups. The adults were randomized in a 1:1:1 ratio. The first group received a single dose of vaccine with 15 μg hemagglutination antigen (HA). The other two groups received two doses with 15 μg or 30 μg HA triweekly. The elderly were randomized 1:1 for two doses of 15 or 30 μg HA. We evaluated serologic responses at prevaccination and weeks 3, 6, and 24. We also examined possible associated factors of immunogenicity by multivariate logistic regression analyses. At week 24, seroprotection (anti-HA antibody level ≥ 1:40) remained at 76.8% and 46.2% in the adult and elderly groups, respectively. The adult group had a higher seroprotection rate (odds ratio of 2.98, 95% confidence interval [CI]: 1.21 to 7.36) than the elderly group. There was no statistical difference in seroprotection and seroconversion rates between different adult and elderly dosage groups. Lower immunogenicity in the elderly than in the adults 24 weeks after the vaccination was observed. However, there was no statistically significant difference among different dose groups. Therefore, we suggest only a single vaccination dose of 15 μg HA for adults and two doses of 15 μg HA for the elderly in the future.
INTRODUCTION
In March 2009, a novel strain of reassorted influenza virus A H1N1 caused human infection in Mexico, with worldwide spread in the next 3 months (13, 21). On 11 June 2009, the World Health Organization (WHO) declared the influenza virus A H1N1 pandemic (24). Global H1N1 vaccination was carried out after much research on immunogenicity and safety (5, 7, 14, 16, 17, 19, 20, 30). However, data on the long-term immunity conferred by and clinical outcomes of vaccination are lacking (9).
In Taiwan, a randomized clinical trial was conducted to assess the immunogenicity of influenza virus vaccine AdimFlu-S (A/H1N1) in healthy volunteers. Age, gender, and diabetes were statistically significant factors affecting the seroprotection rate (12). We followed up this clinical trial cohort for long-term immunogenicity and clinical outcomes.
MATERIALS AND METHODS
Study design and subjects.
From September 2009 to November 2009, we enrolled a total of 218 subjects from National Taiwan University Hospital (NTUH) in Taipei City, Taiwan. The study was to evaluate long-term immunogenicity and clinical outcomes of H1N1 vaccine.
The subjects were men or nonpregnant women who were at least 18 years old, in good physical health, and willing to collaborate with the study design. All subjects signed the informed consent agreement. The exclusion criteria included having influenza vaccine shots within the previous 6 months, history of hypersensitivity to eggs or vaccine ingredients, personal or family history of Guillain-Barré syndrome (11), acute febrile illness within the 72 h prior to vaccination, and any coagulation disorder posing a contraindication for intramuscular injection. In the adult cohort (≤60 years old), all volunteers were randomized in a 1:1:1 ratio to receive 2 doses of triweekly vaccine with 15 μg hemagglutination antigen, 2 doses of triweekly vaccine with 30 μg hemagglutination antigen, or 1 dose of vaccine with 15 μg hemagglutination antigen. In the elderly cohort (>60 years old), all volunteers were randomized in a 1:1 ratio to receive two doses triweekly of 15 or 30 μg hemagglutination antigen. The randomization scheme was generated by the biostatistician through the computer software program with a standard procedure for generating random numbers.
The procedures of the study were in accordance with the ethical standards of the research ethics committee of National Taiwan University Hospital, the principles of the Declaration of Helsinki, the standards of Good Clinical Practice, and Taiwanese regulatory requirements. A signed informed consent was obtained from each subject. The study was conducted and the data were gathered by nonindustry investigators and analyzed by National Taiwan University Hospital.
The vaccine was administered according to different dose groups randomly (single dose of 15 μg hemagglutination antigen, two doses of 15 μg, and two doses of 30 μg). The second dose was administered at week 3, after blood samples had been collected from the subjects. Serum samples were obtained prior to vaccination and also 3 weeks and 6 weeks after vaccination. At week 24, we collected serum samples of those with seroprotection at week 3.
Vaccine.
The monovalent, unadjuvanted H1N1 vaccine, produced by Adimmune Corporation (Taipei, Taiwan), was an antigen of the influenza virus A/California/7/2009 NYMC X-179A strain (H1N1) inactivated by formalin and purified by zonal centrifugation. The vaccine strain in pandemic vaccines worldwide is based on the initial isolate of influenza virus A/California/7/2009 (H1N1) or a faster-growing influenza virus A (H1N1) strain (PR8) named influenza virus A/California/7/2009 (H1N1)v-like. Since the initial virus isolation in April 2009, there was no significant antigenic drift (11). The split-virus vaccine was prepared in embryonated chicken eggs using standard techniques for the seasonal influenza vaccines. The vaccine contained 30 μg hemagglutinin antigen per ml, 0.1 mg/ml of thimerosal, 0.1 μl/ml of formalin, and 0.1 μl/ml of polysorbate.
Assays.
Serum samples were tested for antihemagglutinin (anti-HA) antibodies by hemagglutination inhibition (HAI) assay using turkey erythrocytes with an international standard at the QC laboratory of Adimmune Corporation. The HAI assay validation involved an evaluation of the accuracy, specificity, intermediate precision, and repeatability of the method. Reference antiserum to A/California/7/2009 was obtained from the National Institute for Biological Standards and Control (NIBSC). Influence of nonspecific inhibitors was eliminated with receptor-destroying enzymes. The sera were titrated by 2-fold serial dilutions, starting with a serum dilution of 1:10. HAI assays were performed in duplicate for each sample, and the different-stage sera were examined nonsequentially.
Immunogenicity and clinical outcomes.
The two most common parameters for measuring immune response are rate of seroprotection and rate of seroconversion (3). Seroconversion was considered achieved if seronegative prevaccination (HAI antibody tiiter < 1:10) showed a postvaccination serum HAI titer more than or equal to 1:40 or when there was a 4-fold or greater increase in HAI titers for seropositive prevaccination serum (HAI antibody titer ≧ 1:10). Seroprotection was defined as the condition with an antibody level equal to or higher than 1:40 on HAI assay. Geometric mean titers (GMT) and geometric mean fold rise (GMFR) of the HAI antibody titer were also analyzed by transforming the mean of the log titer with the antilog. For statistical calculation, the negative samples, with HAI titers of less than 1:10, were assigned a titer of 1:5. The antiserum to A/California/7/2009 from NIBSC was used as the reference.
Influenza-like illness was surveyed by monthly telephone calls until 24 weeks. Symptoms, including fever, chills, malaise, dry cough, loss of appetite, nausea, and body aches, were recorded monthly until 24 weeks.
Statistical analysis.
To compare the baseline characteristics of different dose groups, Student's t test and one-way analysis of variance were applied for continuous variables and the chi-square test or Fisher's exact test was used for discrete variables. The 95% confidence intervals (CIs) of GMT and GMFR were obtained using an analysis of covariance (ANCOVA) model. The natural-log-transformed HAI antibody titers at week 3, week 6, and week 24 were response variables, and the prevaccination titer was covariate. In order to avert the effect of dropout, we also calculated the seroprotection rate at week 24 according to intention-to-treat (ITT) analysis.
Multivariate analyses were conducted to identify the independent factors associated with seroprotection at week 24. Statistical significance was defined as a P value of <0.05. All statistical analyses were performed by SPSS software (version 11).
RESULTS
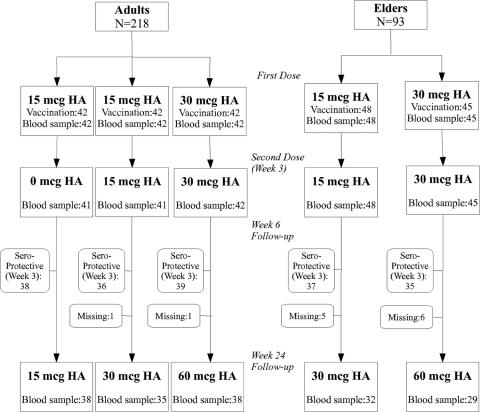
Figure 1 shows the detailed enrollment and follow-up data. A total of 218 subjects between 21 and 86 years of age received the first dose of vaccine, and 176 (80.7%) of them received the second dose 3 weeks later. Table 1 shows the demographic characteristics and prevaccination antibody titers. At prevaccination, 9 of 218 subjects (4.1%) had antibody titers of 1: 40 or more on HAI assay. The proportions with HAI titers of 1:40 or more were 0 to 7.1% in the adult group and 4.2 to 4.4% in the elderly group. There was no significant difference in gender (P = 0.318 and 0.084 for adult and elderly groups, respectively) and body mass index (BMI) (P = 0.877 and 0.254 for adult and elderly groups) between dose groups (Table 1). The BMI groups were categorized according to Asia-Pacific perspective (27, 29). The cutoffs for overweight, obese I, and obese II are 23.0, 25.0, and 30 kg/m2, respectively.
Fig 1.
Enrollment and outcome. mcg, μg.
Table 1.
Demographic characteristics and prevaccination antibody titer
| Parametera | Value for: |
||||||
|---|---|---|---|---|---|---|---|
| Adults (n = 125) |
Elderly (n = 93) |
||||||
| 15 μg, 1 dose (n = 42) | 15 μg, 2 doses (n = 41) | 30 μg, 2 doses (n = 42) | Pb | 15 μg, 2 doses (n = 48) | 30 μg, 2 doses (n = 45) | Pb | |
| No. (%) of males | 13 (31.7) | 17 (41.5) | 11 (26.8) | 0.318 | 22 (64.7) | 12 (35.3) | 0.084* |
| Age (yr) ± SD | 37.0 ± 8.4 | 37.8 ± 9.6 | 38.8 ± 8.0 | 0.647 | 68.9 ± 6.2 | 68.6 ± 5.5 | 0.835 |
| BMI (kg/m2) | 24.0 ± 3.6 | 24.2 ± 4.2 | 23.8 ± 4.6 | 0.877 | 24.7 ± 3.0 | 25.7 ± 5.1 | 0.254 |
| No. (%) with T2DM | 0 | 1 (2.4) | 2 (4.8) | 0.362 | 9 (18.8) | 10 (22.2) | 0.798* |
| No. (%) with HAI titer ≥1:40 | 2 (4.8) | 0 | 3 (7.1) | 0.240 | 2 (4.2) | 2 (4.4) | 1.000* |
| No. (%) with HAI titer ≥1:10 (%) | 9 (21.4) | 4 (9.8) | 8 (19.0) | 0.325 | 18 (37.5) | 13 (28.9) | 0.379 |
| GMT (95% CI) | 6.7 (5.5–8.2) | 5.4 (5.0–5.9) | 7.2 (5.4–9.7) | 0.149 | 7.4 (6.2–8.8) | 6.8 (5.7–8.1) | 0.496 |
BMI, body mass index; T2DM, type 2 diabetes mellitus; GMT, geometric mean titer; HAI, hemagglutination inhibition.
Values were determined by the chi-square test except for those marked with an asterisk, which were calculated by Fisher's exact test.
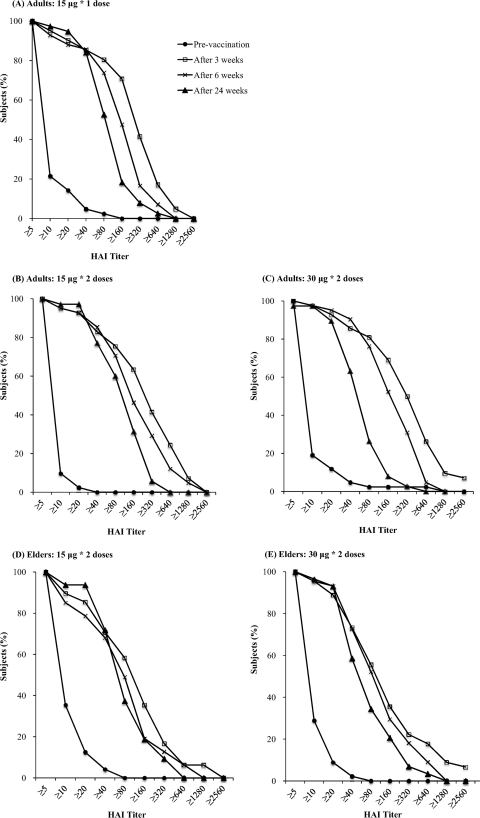
Immunogenicity data at week 3, week 6, and week 24 are individually shown in Table 2 and Fig. 2 by reverse cumulative distribution curves. A single dose of 15 μg HA in the AdimFlu-S (A/H1N1) vaccine resulted in antibody titers of 1:40 or more on HAI assay in 87.8 to 92.7% of the adult group and 77.1% of elderly subjects at week 3. The seroprotection rates provided by a single dose of 30 μg of the vaccine were 92.9% in the adults and 77.8% in the elderly group. At week 6, two doses of 15 μg and 30 μg of the vaccine provided seroprotection rates of 90.2% to 97.6% in the adults and 72.3% to 77.3% in the elderly, respectively. A single dose of 15 μg of the vaccine provided a seroprotection rate of 90.5% in the adults. There were no statistically significant differences of seroprotection rates and seroconversion rates among the different dose groups.
Table 2.
Antibody titers and immunogenicity after vaccination
| Wk | Parameter | Value for: |
||||
|---|---|---|---|---|---|---|
| Adults |
Elderly |
|||||
| 15 μg, 1 dose | 15 μg, 2 doses | 30 μg, 2 doses | 15 μg, 2 doses | 30 μg, 2 doses | ||
| 3 | No. of subjects | 41 | 41 | 42 | 48 | 45 |
| No. (% [95% CI]) with seroprotection (titer ≥ 1:40) | 38 (92.7 [80.0–98.2]) | 36 (87.8 [74–95.1]) | 39 (92.9 [80.3–98.2]) | 37 (77.1 [63.3–86.9]) | 35 (77.8 [63.6–87.6]) | |
| GMT (95% CI) | 191.8 (128.8–285.5) | 188.2 (125.9–281.2) | 242.8 (162.8–362.1) | 79.6 (52.1–121.8) | 111.1 (71.6–172.3) | |
| GMFR (95% CI) | 29.8 (20–44.3) | 29.2 (19.5–43.6) | 37.7 (25.3–56.2) | 11.2 (7.3–17.2) | 15.7 (10.1–24.3) | |
| No. (% [95% CI]) with seroconversion | 38 (92.7 [80–98.2]) | 36 (87.8 [74–95.1]) | 38 (90.5 [77.4–96.8]) | 35 (72.9 [58.9–83.5]) | 35 (77.8 [63.6–87.6]) | |
| 6 | No. of subjects | 42 | 41 | 42 | 47 | 44 |
| No. (% [95% CI]) with seroprotection (titer ≥ 1:40) | 38 (90.5 [77.4–96.8]) | 37 (90.2 [76.9–96.7]) | 41 (97.6 [86.6–100]) | 34 (72.3 [58.1–83.1]) | 34 (77.3 [62.8–87.3]) | |
| GMT (95% CI) | 105.1 (75.3–146.6) | 123.5 (87.9–173.5) | 135.8 (96.8–190.4) | 51.1 (35.4–73.9) | 86.3 (59–126.3) | |
| GMFR (95% CI) | 16.3 (11.7–22.8) | 19.2 (13.7–27) | 21.1 (15.1–29.6) | 7.3 (5–10.5) | 12.3 (8.4–17.9) | |
| No. (% [95% CI]) with seroconversion | 37 (88.1 [74.5–95.3]) | 37 (90.2 [76.9–96.7]) | 38 (90.5 [77.4–96.8]) | 33 (70.2 [55.9–81.4]) | 34 (77.3 [62.8–87.3]) | |
| 24 | Total no. of subjectsa | 38 | 35 | 38 | 32 | 29 |
| Seroprotection (titer ≥ 1:40) | ||||||
| No. protected | 33 | 28 | 35 | 25 | 18 | |
| % protected (95% CI) relative to: | ||||||
| All wk 24 subjects | 86.8 (72.2–94.7) | 80.0 (63.8–90.3) | 92.1 (78.5–98) | 78.1 (61–89.3) | 62.1 (44–77.4) | |
| Total (%) | 96/111 (86.5) | 43/61 (70.5) | ||||
| All wk 3 subjects | 78.6 (63.9–88.5) | 68.3 (52.9–80.5) | 83.3 (69.1–92) | 52.1 (38.3–65.5) | 40 (27–54.6) | |
| Total (%) | 96/125 (76.8) | 43/93 (46.2) | ||||
| GMT (95% CI) | 66.8 (51.9–86.1) | 76.7 (58.7–100) | 81.5 (63–105.4) | 52.1 (37.8–71.9) | 47 (33.6–65.9) | |
| GMFR (95% CI) | 10.4 (8.1–13.4) | 11.9 (9.1–15.6) | 12.7 (9.8–16.4) | 6.9 (5–9.5) | 6.2 (4.5–8.8) | |
| No. (% [95% CI]) with seroconversion | 31 (81.6 [66.3–91.1]) | 28 (80.0 [63.8–90.3]) | 34 (89.5 [75.3–96.4]) | 24 (75 [57.7–87]) | 18 (62.1 [44–77.4]) | |
| Total (%) | 93/111 (83.8) | 42/61 (68.9) | ||||
The participants with seroprotection at week 3 were recruited at week 24.
Fig 2.
Reverse cumulative distribution curves of antibody titers before and 3 weeks, 6 weeks, and 24 weeks after the first dose of vaccine, according to the age and dosage groups.
The follow-up of immunogenicity half a year later showed no statistically significant difference of seroconversion rates and seroprotection rates among different dose groups. According to the subtotal at week 24, 86.5% of the adults had seroprotection compared to 70.5% of the elderly (P = 0.015). In the adult group, the seroprotection rate (92.1%) of those receiving two doses of 30 μg at week 24 was the highest. However, the seroprotection rates among different dose groups showed no statistical significance in the adults and elderly (P = 0.318 and P = 0.17, respectively). According to ITT analysis, 76.8% of the adults and 46.2% of the elderly had seroprotection 24 weeks after the first vaccination. Despite ITT analysis, the seroprotection rates among different dosage groups of the adults and elderly were still not statistically significant (P = 0.25 and P = 0.24, respectively).
Using multivariate logistic regression analyses adjusting for gender and vaccination dose, we found that the odds ratio for remaining seroprotected at week 24 in adults was 2.98 (95% CI: 1.21 to 7.36) compared to the elderly (Table 3). There was no statistical difference between different dose groups. The odds ratios for remaining seroprotected in different BMI groups (27, 29) were 1.85 (95% CI: 0.51 to 6.75), 1.05 (95% CI: 0.39 to 2.82), and 0.61 (95% CI: 0.16 to 2.32) in overweight (BMI, 23 to 24.9 kg/m2), obese I (BMI, 25 to 29.9 kg/m2), and obese II (BMI ≥ 30 kg/m2) groups, respectively.
Table 3.
Predictors associated with seroprotection at week 24 using multivariate logistic regression analysis
| Predictor | Odds ratioa | 95% confidence interval |
|---|---|---|
| Age group | ||
| >60 yr | Reference | |
| ≤60 yr | 2.98 | 1.21–7.36 |
| Gender | ||
| Male | Reference | |
| Female | 2.16 | 0.94–4.94 |
| Dose group | ||
| 15 μg, single dose | Reference | |
| 15 μg, two doses | 0.85 | 0.23–3.21 |
| 30 μg, two doses | 0.83 | 0.22–3.16 |
| BMI (kg/m2) group | ||
| Normal range (18.5–22.9) | Reference | |
| Overweight (23–24.9) | 1.85 | 0.51–6.75 |
| Obese I (25–29.9) | 1.05 | 0.39–2.82 |
| Obese II (≥30) | 0.61 | 0.16–2.32 |
“Reference” indicates the reference group, for which the odds ratio is equal to 1.
In subgroup analysis, multivariate logistic regression analyses showed that age was still a significant factor for remaining seroprotected in the adult group (95% CI: 0.86 to 0.99). In the elderly group, diabetic people had a nonstatistically significantly lower odds ratio, 0.47, for remaining seroprotected than nondiabetic ones (95% CI: 0.12 to 1.78).
The subjects did not receive seasonal influenza vaccination during the study period. There were no symptoms of influenza reported until 24 weeks.
DISCUSSION
Our study showed the persistence of immunogenicity at 24 weeks after H1N1 influenza vaccination. By ITT analysis, the single 15-μg vaccination produced the seroprotection rate of 78.6% in adults 24 weeks after vaccination, and a booster dose at week 3 or higher doses (30 μg) did not provide a higher immune response (seroprotection rates were 68.3% and 83.3%, respectively; P = 0.25). In the elderly, two 15-μg doses produced a seroprotection rate of 52.1% 24 weeks after vaccination, and a higher dose (30 μg) did not provide a better immune response (seroprotection rate was 40%; P = 0.24). Only a relatively small portion of our study population had antibody titers of 1:40 or more before vaccination (4.0% in adult group and 4.3% in elderly group). This was similar to the study in China (30), which found lower initial titers than those in Western countries (1, 5). Differences in prevaccination antibody titers might be associated with different levels of pandemic H1N1 activity geographically (25). Our study also showed a rapid decline of HAI titers in the elderly although there were no flu-like symptoms among the participants during the 24-week period.
Most studies on the immunogenicity of H1N1 vaccine were conducted 42 days postvaccination (5, 14, 30). In China, Zhu et al. showed that subjects 18 years old or older who received 15 μg of nonadjuvant vaccine exhibited seroprotection rates of 79.1 to 97.1% on day 21 after the first dose; rates increased to 93.3 to 97.1% on day 35, 14 days after the second dose (30). In Australia, Greenberg et al. showed that a single dose of 15 μg of unadjuvanted H1N1 vaccine resulted in a seroprotection rate of 95.0% in those 18 to 64 years old by day 21. After the second dose, seroprotection increased only to 98.3% (5). Another study also showed that neither 2 doses of 15 μg nor 2 doses of 30 μg produced greater immunity than a single 15-μg dose (14). Likewise, we found that the seroprotection rates with 15 μg HA on day 21 were 77.1% in the elderly and 87.8 to 92.7% in adults 3 weeks after vaccination; these rates changed to 72.3% in the elderly and 90.2% in the adults 3 weeks after the second vaccination. In the adult and elderly groups, there was no statistically significant difference of seroprotection rates and seroconversion rates among the different dosage groups 3 weeks, 6 weeks, and 24 weeks after vaccination.
Participants less than 60 years old had a greater odds ratio for seroprotection (2.98; 95% CI = 1.21 to 7.36) than those over 60 years old (Table 3). A study showed that a single dose of 15 μg or 30 μg provided no statistical difference in seroprotection at week 3 (12). However, other studies showed similar results at longer follow-up (5, 6, 15, 30). An age of over 60 years was an important predictor for less immunogenicity at week 24, consistent with other studies with cut points of age of 50 (5) and 60 (30) years. The elderly had more comorbidities, and H1N1-infected elders were at higher risk of death (8). In addition, age was associated with early decline of HAI titers, falling below seroprotective levels around 6 months after seasonal influenza vaccination (23). It may be necessary to recommend a booster, especially for the elderly. In fact, the World Health Organization (WHO) has announced that H1N1 virus is one of the viruses recommended for seasonal influenza vaccines in the Northern Hemisphere in 2011 to 2012 (28). A recent study also indicated that a booster dose may confer additional benefits for the elderly (9).
There was no participant with flu symptoms, as determined by follow-up monthly surveillance phone calls. Based on the relationship between HAI antibody titer and clinical protection against seasonal influenza proposed by Coudeville et al. using a meta-analytical approach (2), the mean GMT (46.2 to 90) at week 24 in all subjects (Table 2) approximated a probability of protection of 0.8 to 0.9. Our results may support their prediction models for protective efficacy based on the immunological profile. However, this efficacy of protection may be caused by other protective strategies since the rates of flu symptoms also highly depend on whether or not an epidemic is present.
In a French study, obesity and diabetes mellitus could lead to a higher risk of H1N1-associated mortality (8). In addition, morbid obesity elevated the possibility for comorbidities in patients hospitalized for influenza virus A H1N1 infection (4, 10, 18, 26). Interestingly, our study showed that obese people (BMI ≥ 30 kg/m2) had a lower seroprotection rate than the normal weight group at week 24, although without significant difference (odds ratio: 0.61; 95% CI = 0.16 to 2.32), as shown in Table 3. In the elderly group, those with diabetes mellitus did not have statistically significantly lower seroprotection than the nondiabetic group (odds ratio: 0.47; 95% CI = 0.12 to 1.78). Taken together, these results imply that obese and diabetic subjects showed relatively lower seroprotection rates without statistical significance.
Our study, however, had some limitations. First, the study was not placebo controlled. Therefore, whether the vaccine contributed to the zero clinical symptoms of influenza in all subjects was not conclusively proven. Also, we did not explore the H1N1-specific cell-mediated immunity (CMI) in addition to humoral immunity. The humoral immunity, the antibody titer, is still the main marker most vaccine research uses (22). Second, the previous records of seasonal influenza vaccination were not obtained from the subjects. It has been reported that the history of seasonal influenza virus vaccination could be an important factor for immunogenicity and persistency of pandemic H1N1 influenza vaccine. Third, this study did not include the other components of seasonal influenza vaccine. Therefore, it was unlikely to explore whether a cross-reaction between pandemic H1N1 virus and other seasonal influenza viruses exists. Fourth, the ratio of loss in follow-ups, 1.8% in the adult group and 15.3% in the elderly group, may cause some bias to the seroprotection rate. Finally, our sample size was smaller than those in other studies (25, 30), and this may affect the statistical significance of obesity and diabetes mellitus in the multivariate regression analysis.
In conclusion, seroprotection was still observed in 76.8% and 46.2% of the subjects at week 24 in adult and elderly groups, respectively. Obesity and diabetes mellitus seemed to be associated with lower seroprotection rates than in those lacking these conditions. However, no statistical difference existed among different dosage groups of adults and elderly. Therefore, we suggest only a single vaccination dose of 15 μg HA for the adults and two doses of 15 μg for the elderly in the future.
ACKNOWLEDGMENTS
Yi-Chun Lai was in charge of data analysis and writing of the draft. Szu-Min Hsieh, Chien-An Yao, and Long-Teng Lee participated in the data collection and study design. Kuen-Cheh Yang helped with the data management and interpretation of results. K.-C.H. synthesized the analyses and headed the writing of the manuscript. All authors read and approved the final manuscript.
We thank Wen-Chao Weng and Yi-Ru Chen for their technical support.
All authors do not have commercial or other associations that might pose a conflict of interest.
The Adimmune Corporation supported the vaccines used in the study and lab analyses.
Footnotes
Published ahead of print 18 January 2012
REFERENCES
- 1. Clark TW, et al. 2009. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N. Engl. J. Med. 361: 2424–2435 [DOI] [PubMed] [Google Scholar]
- 2. Coudeville L, et al. 2010. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a Bayesian random-effects model. BMC Med. Res. Methodol. 10: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deans GD, Stiver HG, McElhaney JE. 2010. Influenza vaccines provide diminished protection but are cost-saving in older adults. J. Intern. Med. 267: 220–227 [DOI] [PubMed] [Google Scholar]
- 4. Dee S, Jayathissa S. 2010. Clinical and epidemiological characteristics of the hospitalised patients due to pandemic H1N1 2009 viral infection: experience at Hutt Hospital, New Zealand. N. Z. Med. J. 123: 45–53 [PubMed] [Google Scholar]
- 5. Greenberg ME, et al. 2009. Response to a monovalent 2009 influenza A (H1N1) vaccine. N. Engl. J. Med. 361: 2405–2413 [DOI] [PubMed] [Google Scholar]
- 6. Gross PA, et al. 1987. Immunization of elderly people with two doses of influenza vaccine. J. Clin. Microbiol. 25: 1763–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grotto I, Engelhard D. 2009. The safety and immunogenicity of the vaccines against pandemic (H1N1) 2009 influenza. Harefuah 148: 799–803, 857 [PubMed] [Google Scholar]
- 8. Hanslik T, Boelle PY, Flahault A. 2010. Preliminary estimation of risk factors for admission to intensive care units and for death in patients infected with A(H1N1)2009 influenza virus, France, 2009–2010. PLoS Curr. 2: RRN1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huijskens E, et al. 2011. Immunogenicity, boostability, and sustainability of the immune response after vaccination against influenza A virus (H1N1) 2009 in a healthy population. Clin. Vaccine Immunol. 18: 1401–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jain S, et al. 2009. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N. Engl. J. Med. 361: 1935–1944 [DOI] [PubMed] [Google Scholar]
- 11. Johansen K, Nicoll A, Ciancio BC, Kramarz P. 2009. Pandemic influenza A(H1N1) 2009 vaccines in the European Union. Euro Surveill. 14: 19361. [PubMed] [Google Scholar]
- 12. Kao TM, et al. 2010. Immune response of single dose vaccination against 2009 pandemic influenza A (H1N1) in the Taiwanese elderly. Vaccine 28: 6159–6163 [DOI] [PubMed] [Google Scholar]
- 13. Khanna M, Gupta N, Gupta A, Vijayan VK. 2009. Influenza A (H1N1) 2009: a pandemic alarm. J. Biosci. 34: 481–489 [DOI] [PubMed] [Google Scholar]
- 14. Kung HC, et al. 2010. A clinical study to assess the immunogenicity and safety of a monovalent 2009 influenza A (H1N1) vaccine in an area with low-level epidemics of pandemic influenza. Vaccine 28: 7337–7343 [DOI] [PubMed] [Google Scholar]
- 15. Levine M, Beattie BL, McLean DM. 1987. Comparison of one- and two-dose regimens of influenza vaccine for elderly men. CMAJ 137: 722–726 [PMC free article] [PubMed] [Google Scholar]
- 16. Liang XF, et al. 2010. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 375: 56–66 [DOI] [PubMed] [Google Scholar]
- 17. Lu W, Tambyah PA. 2010. Safety and immunogenicity of influenza A H1N1 vaccines. Expert Rev. Vaccines 9: 365–369 [DOI] [PubMed] [Google Scholar]
- 18. Morgan OW, et al. 2010. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS One 5: e9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nolan T, et al. 2010. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA 303: 37–46 [DOI] [PubMed] [Google Scholar]
- 20. Plennevaux E, Sheldon E, Blatter M, Reeves-Hoche MK, Denis M. 2010. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet 375: 41–48 [DOI] [PubMed] [Google Scholar]
- 21. Rothberg MB, Haessler SD. 2010. Complications of seasonal and pandemic influenza. Crit. Care Med. 38: e91–e97 [DOI] [PubMed] [Google Scholar]
- 22. Skowronski DM, et al. 2011. Immuno-epidemiologic correlates of pandemic H1N1 surveillance observations: higher antibody and lower cell-mediated immune responses with advanced age. J. Infect. Dis. 203: 158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song JY, et al. 2010. Long-term immunogenicity of influenza vaccine among the elderly: risk factors for poor immune response and persistence. Vaccine 28: 3929–3935 [DOI] [PubMed] [Google Scholar]
- 24. Sullivan SJ, Jacobson RM, Dowdle WR, Poland GA. 2010. 2009 H1N1 influenza. Mayo Clin. Proc. 85: 64–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vajo Z, Tamas F, Sinka L, Jankovics I. 2010. Safety and immunogenicity of a 2009 pandemic influenza A H1N1 vaccine when administered alone or simultaneously with the seasonal influenza vaccine for the 2009–10 influenza season: a multicentre, randomised controlled trial. Lancet 375: 49–55 [DOI] [PubMed] [Google Scholar]
- 26. van't Klooster TM, et al. 2010. Surveillance of hospitalisations for 2009 pandemic influenza A(H1N1) in the Netherlands, 5 June–31 December 2009. Euro Surveill. 15: pii–19461. [DOI] [PubMed] [Google Scholar]
- 27. Weisell RC. 2002. Body mass index as an indicator of obesity. Asia Pac. J. Clin. Nutr. 11(Suppl. 8): S681–S68412534690 [Google Scholar]
- 28. WHO February 2011Recommended composition of influenza virus vaccines for use in the 2011–2012 Northern Hemisphere influenza season. http://www.who.int/influenza/vaccines/2011_02_recommendation.pdf WHO, Geneva, Switzerland [Google Scholar]
- 29. WHO/IASO/IOTF 2000. The Asia-Pacific perspective: redefining obesity and its treatment, ISBN 0-9577082-1-1. Health Communications Australia, Melbourne, Australia [Google Scholar]
- 30. Zhu FC, et al. 2009. A novel influenza A (H1N1) vaccine in various age groups. N. Engl. J. Med. 361: 2414–2423 [DOI] [PubMed] [Google Scholar]