Abstract
Gamma interferon (IFN-γ)-induced protein 10 (IP-10) has recently shown promise as a diagnostic biomarker of Mycobacterium tuberculosis infection of humans. The aim of the current study was to compare IP-10 and IFN-γ responses upon Mycobacterium bovis infection in cattle by using archived samples from two aerosol inoculation studies. In the first study (104 CFU M. bovis by aerosol, n = 7), M. bovis purified protein derivative (PPDb)-specific IP-10 and IFN-γ gene expression was detected as early as 29 days after challenge. PPDb-specific IP-10 and IFN-γ mRNA responses followed a similar pattern of expression over the course of this study and were highly correlated (r = 0.87). In the second study (105 CFU M. bovis by aerosol, n = 5), IP-10 and IFN-γ (protein) responses to mycobacterial antigens were compared following challenge. IFN-γ responses to mycobacterial antigens were detected at 29 days after challenge and were sustained during the remainder of the study. IFN-γ responses to mycobacterial antigens exceeded corresponding responses in nonstimulated cultures. IP-10 responses to mycobacterial antigens exceeded preinfection responses at 7, 29, and 63 days after challenge. In contrast to IFN-γ responses, IP-10 responses to mycobacterial antigens generally did not exceed the respective responses in nonstimulated cultures. IP-10 responses to medium alone and to mycobacterial antigens followed a similar pattern of response. Correlations between IP-10 and IFN-γ (protein) responses were modest (r ≈ 0.50 to 0.65). Taken together, these findings do not support the use of IP-10 protein as a biomarker for bovine tuberculosis using the current testing protocol and reagents; however, mRNA-based assays may be considered for further analysis.
INTRODUCTION
Tuberculosis (TB) in humans and animals may result from exposure to bacilli within the Mycobacterium tuberculosis complex (i.e., M. tuberculosis, M. bovis, M. africanum, M. pinnipedi, M. microti, M. caprae, or M. canetti [7]). Mycobacterium bovis is the species most often isolated from tuberculous cattle. In addition to traditional skin test procedures, gamma interferon (IFN-γ) release assays (IGRAs) have been incorporated into TB testing algorithms for both humans and cattle (22, 23). Such tests include the Quantiferon-TB Gold in-tube test (Cellestis Limited, Melbourne, Australia) and T-SPOT.TB (Oxford Immunotech, Oxfordshire, United Kingdom) for humans and Bovigam (Prionics Ag, Schlieren, Switzerland) for cattle. Additional biomarkers have recently emerged as potential candidates for evaluation in antemortem TB tests for humans (reviewed by Walzl et al. [31]) and cattle (e.g., interleukin-1β [IL-1β], tumor necrosis factor alpha [TNF-α], nitric oxide, and IL-17 [5, 11, 30, 32]). Of these candidates, the monocyte-derived chemokine IFN-γ-induced protein 10 (IP-10) (also termed CXCL10) has shown particular promise (18–20).
IP-10 is a chemokine that is detected within tuberculous granulomas in humans (9) and is involved in delayed-type hypersensitivity responses to mycobacterial antigens (14). IP-10 attracts activated T cells and monocytes to inflammatory foci (8), promotes Th1 responses (21), and has antimicrobial functions (6). In relation to diagnosis, IP-10 is detected in pleural effusions and plasma of TB-infected humans, with potential as a biomarker for both treatment monitoring and disease progression (4, 12). As with TB, IP-10 levels are elevated within tissues and sera of leprosy patients (25, 26). Type 1 reactions, a systemic inflammatory syndrome of borderline leprosy patients, are associated with a significant increase in serum IP-10 but not IFN-γ (25). In a study to develop diagnostic approaches for subclinical/early-stage leprosy, IP-10 was shown to augment the diagnostic potential of IFN-γ/M. leprae peptide-based tests (10). Most recently, a multicenter evaluation demonstrated that a Luminex-based IP-10 assay performed similarly to commercial IGRAs (17) for the diagnosis of TB in humans. The conclusions were that antigen-specific IP-10 is produced in response to TB infection, the IP-10-based test has accuracy comparable to that of commercial IGRAs, IP-10 appears to be less affected by non-TB bacterial or viral infections than IFN-γ, and IP-10 is detected in more patients with relevant TB risk factors than IFN-γ. The last finding, however, may indicate either a higher sensitivity or lower specificity of the IP-10 assay. Other studies (1, 13) have demonstrated that HIV infection does not impair TB-specific IP-10 responses observed with IFN-γ-based tests. Additionally, IP-10 is a potential biomarker of TB exposure in children; however, like IFN-γ, IP-10 cannot be used to discriminate between active and latent TB in this population (34).
With cattle, IP-10 gene expression is increased in monocytes/macrophages from Eimeria bovis-infected animals stimulated with E. bovis sporozoites or antigen, likely contributing to the attraction of mononuclear cells to infection sites and a Th1-dominated adaptive response to this parasite (27). IP-10 levels are also elevated in SCID-bovine chimeric mice in response to M. bovis infection, and treatment with anti-bovine WC1 (a subset of γδ T cells in cattle) reduces plasma IP-10 (3). The diagnostic potential of bovine IP-10 for detection of tuberculous cattle, however, has not been evaluated. The objective of the present study was to compare antigen-specific IP-10 and IFN-γ responses in cattle infected with M. bovis and to determine the potential usefulness of IP-10 as a biomarker of infection.
MATERIALS AND METHODS
Calves, aerosol challenge, and necropsy.
Twelve male age-matched, TB-free Holstein-Friesian calves were housed in a biosafety level 3 facility at the National Animal Disease Center (NADC). All animal care and use procedures were reviewed and approved by the NADC Animal Care and Use Committee. Calves received 105 CFU (n = 5) or 104 CFU (n = 7) M. bovis strain 95-1315 by aerosol at 6 months of age as described previously (16). Enumeration of M. bovis for challenge inoculum, postmortem (∼4.5 months after challenge) and histopathology procedures, and culture of M. bovis from tissues were as previously described (16, 33) using standard techniques.
Cell culture, RNA isolation, and reverse transcription of leukocyte RNA.
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation of peripheral blood buffy coat fractions collected into 2× acid citrate dextrose (32). PBMC were seeded into 96-well round-bottom microtiter plates (Falcon; Becton Dickinson, Lincoln Park, NJ) at 1 × 106 cells in a total volume of 200 μl of complete RPMI (RPMI 1640 with 2 mM l-glutamine, 25 mM HEPES buffer, 100 units/ml penicillin, 0.1 mg/ml streptomycin, 1% nonessential amino acids [Sigma, St. Louis, MO], 2% essential amino acids [Sigma], 1% sodium pyruvate [Sigma], 50 μM 2-mercaptoethanol [Sigma], and 10% [vol/vol] fetal bovine serum [FBS]). Wells contained medium alone (nonstimulated) or 10 μg/ml M. bovis purified protein derivative (PPD) (10 μg/ml; Prionics Ag, Schlieren, Switzerland). Cultures were incubated at 39°C with 5% CO2 for 16 h.
Isolation and reverse transcription of PBMC RNA were performed as previously described (28, 29). Briefly, PBMC were harvested by centrifugation and lysed with 150 μl/well buffer RLT (Qiagen, Valencia, CA) according to the manufacturer's directions. The contents of replicate wells were combined and samples stored at −80°C. RNA was isolated using an RNeasy minikit (Qiagen) according to the manufacturer's directions and eluted from the column with 50 μl RNase-free water (Ambion, Austin, TX). Contaminating DNA was enzymatically removed by treating RNA with DNA-free (Ambion). One microgram of RNA was reverse transcribed in a 50-μl reaction mixture using SuperScript II (Invitrogen, Carlsbad, CA) with 0.5 μg of oligo(dT)12-18 and 40 units of RNaseOut (Invitrogen) according to the manufacturer's directions. Samples were heated at 70°C for 5 min and then reverse transcribed at 42°C for 60 min. The resulting cDNA was stored at −80°C until used in real-time PCRs.
Analysis of cytokine gene expression by real-time PCR.
Real-time PCR was performed using Applied Biosystems TaqMan gene expression assays for IFN-γ (Bt03212723_m1) and IP-10 (Bt03235839_m1) with the eukaryotic 18S rRNA endogenous control (4352930E). Assays were performed according to the manufacturer's directions. Briefly, 2 μl of cDNA was added to 10 μl TaqMan Master Mix, 1 μl primer/probe, and 7 μl water. The real-time PCR was carried out in a ABI Prism 7900HT according to the manufacturer's directions.
Whole-blood stimulation.
Duplicate 250-μl heparinized whole-blood aliquots were distributed in 96-well plates with antigen (Table 1), pokeweed mitogen (PWM) (10 μg/ml), or no antigen and incubated at 39°C with 5% CO2 for 18 h. The normal body temperature of cattle is 39°C, and incubation of human blood at 39°C, compared to 37°C, augments IP-10 and IFN-γ responses (2). Stimulated plasma was harvested and IFN-γ and IP-10 protein concentrations determined by enzyme-linked immunosorbent assay (ELISA).
Table 1.
Antigens
| Antigen (designation) | Concn (μg/ml) | Source(s) |
|---|---|---|
| M. bovis purified protein derivative (PPDb) | 0.1, 1.0, 10 | CSL,a Lelystad,b Perugia,c Weybridge,d AsureQualitye |
| M. avium purified protein derivative (PPDa) | 0.1, 1.0, 10 | CSL, Lelystad, Perugia, Weybridge, AsureQuality |
| Early secretory antigenic target-6:culture Filtrate protein 10 (E:C) | 1.0, 5.0 | Iowa State University,f Lionex,g AHVLAh |
| Outer membrane protein A of M. tuberculosis (OmpATB) (24) | 5.0 | Proteixi |
| TB10.4 | 5.0 | Proteix |
| TB27.4 | 5.0 | Proteix |
| Mobility protein of M. bovis 83 (MPB83) | 1.0, 5.0, 10.0 | NADC,j Lionex |
Prionics Ag, Schlieren, Switzerland.
Lelystad Biologicals, Lelystad, Netherlands.
Istituto Zooprofilatico, Perugia, Spain.
Tuberculin Production Unit, VLA, Weybridge, United Kingdom.
AsureQuality, Auckland, New Zealand.
Chris Minion, Veterinary Microbiology and Preventive Medicine, Iowa State University Ames, Iowa.
Lionex Diagnostics and Therapeutics GmbH, Braunschweig, Germany.
Animal Health and Veterinary Laboratory Agencies, Weybridge, United Kingdom.
Proteix Biotechnologies, Prague, Czech Republic.
John Bannantine, National Animal Disease Center, Ames, IA.
IFN-γ and IP-10 assays.
IFN-γ and IP-10 (CXCL10) concentrations in stimulated plasma were determined using commercial ELISA-based kits (Bovigam [Prionics Ag, Schieren, Switzerland] and human IP-10 immunoassay [PeproTech Inc., Rocky Hill, NJ]) according to the manufacturers' instructions. Absorbances of standards (recombinant bovine IFN-γ [Endogen, Rockford, IL] and recombinant human IP-10 [PeproTech]) and test samples were read at 450 nm using an ELISA plate reader (Molecular Devices, Menlo Park, CA). Duplicate samples for individual treatments were analyzed and both IP-10 and IFN-γ data presented as ng of protein/ml of plasma.
Statistics.
Data were analyzed as a completely randomized design using Statview software (version 5.0; SAS Institute, Inc., Cary, NC). The experimental unit was each individual animal (i.e., calf) for the analysis of all data. Longitudinal changes in gene expression (IP-10 and IFN-γ mRNA) and the concentration (ng/ml) of IP-10 and IFN-γ in stimulated plasma were analyzed as a split plot with repeated-measures analysis of variance (ANOVA). Spearman's rank correlation coefficients between IP-10 and IFN-γ responses were computed using GraphPad Prism (La Jolla, CA) to determine associations.
RESULTS AND DISCUSSION
Infection status.
Two independent M. bovis infection trials were performed (104 CFU [n = 7] and 105 CFU [n = 5]). With both trials, M. bovis was isolated from granulomatous lesions collected from lung and lung-associated lymph nodes of each animal. In general, lesions were more extensive (with increased lesion severity and distribution) in animals receiving 105 then in those receiving 104 CFU M. bovis. Samples from these two trials were used to evaluate M. bovis-induced IP-10 and IFN-γ gene transcription (mRNA responses) and protein responses.
IP-10 and IFN-γ mRNA responses to M. bovis infection.
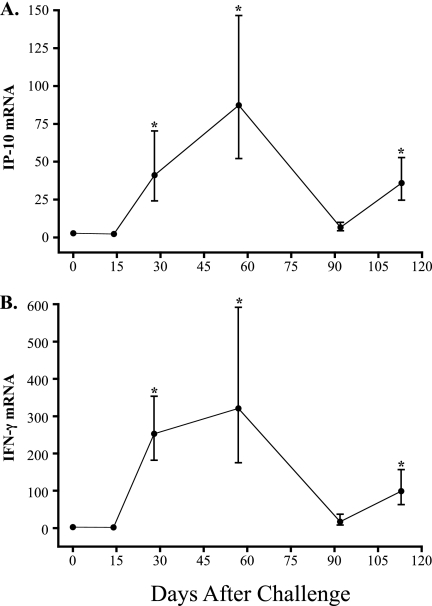
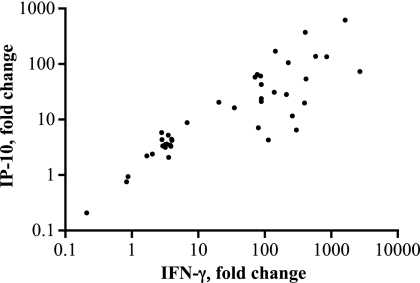
At sequential time points relative to M. bovis challenge (104 CFU, n = 7), PBMC were analyzed for IP-10 and IFN-γ gene expression in response to M. bovis PPD stimulation (Fig. 1). mRNA responses were detected as early as 28 days after infection. Upon infection, IP-10 mRNA responses to M. bovis PPD followed kinetics similar to those of IFN-γ responses (Fig. 1). IP-10 and IFN-γ mRNA responses were highly correlated (r = 0.87) (Fig. 2). These findings demonstrate the strong correlation of bovine IP-10 and IFN-γ mRNA responses and provide justification for further evaluation of IP-10 as a diagnostic biomarker for bovine TB.
Fig 1.
Kinetics of IP-10 and IFN-γ responses (mRNA) to M. bovis PPD. PBMC were isolated from cattle receiving M. bovis (104 CFU) by aerosol and stimulated with M. bovis PPD or medium alone (i.e., no stimulation), and RNA was harvested for measurement of IP-10 (A) and IFN-γ (B) gene expression using the 2−ΔΔCT method with nonstimulated cells as the calibrator. Data are presented as relative fold change mRNA expression (mean ± standard error of the mean [SEM], n = 7). *, differs (P < 0.05) from preinfection (time zero) values.
Fig 2.
Correlation between IP-10 and IFN-γ responses (mRNA) to M. bovis PPD. PBMC were isolated from cattle receiving M. bovis (104 CFU) by aerosol at 0, 14, 28, 57, 92, and 113 days relative to challenge and stimulated with M. bovis PPD or medium alone (i.e., no stimulation), and RNA was harvested for measurement of IP-10 and IFN-γ gene expression using the 2−ΔΔCT method with nonstimulated cells as the calibrator. Data represent IP-10 (y axis) versus IFN-γ (x axis) responses for each individual animal at each time point as relative fold change in mRNA expression (n = 42 [7 animals × 6 time points]; results from one animal missing for the 57-day time point) (r = 0.87, P < 0.001).
IP-10 and IFN-γ responses (protein) to M. bovis infection.
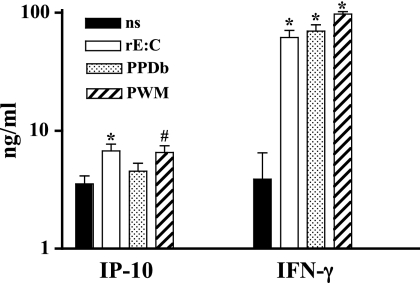
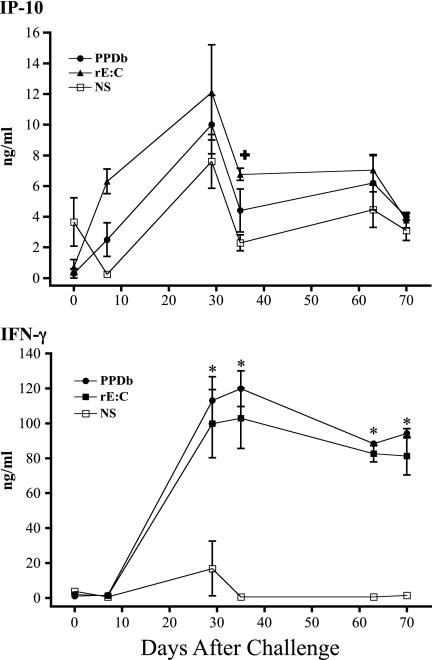
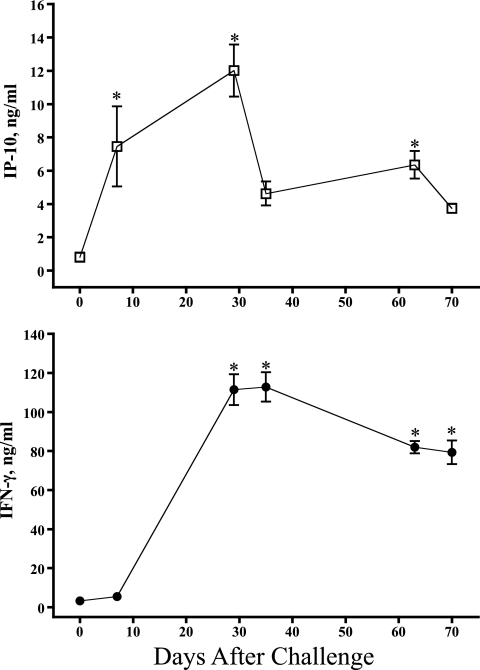
The evaluation of IP-10 and IFN-γ protein responses was performed retrospectively on archived samples, as previously described for similar studies with humans (13, 17, 34). Archived RNA samples were not available from these animals for a direct comparison of mRNA and protein responses. The effects of in vitro antigen stimulation on levels of IP-10 and IFN-γ proteins over the course of an infection trial (105 CFU, n = 5 × 6 time points combined) are shown in Fig. 3. IP-10 and IFN-γ responses to rESAT-6:CFP10 and pokeweed mitogen exceeded responses to medium alone. Additionally, IFN-γ responses to M. bovis PPD exceeded responses to medium alone. Over the course of the study, IP-10 responses to M. bovis PPD did not exceed the respective responses to medium alone at any time point (Fig. 4). IP-10 responses to rESAT-6:CFP10 exceeded the respective responses to medium alone at 35 days after challenge but not at other time points (Fig. 4). In contrast, IFN-γ responses to M. bovis PPD and rESAT-6:CFP10 (Fig. 4) exceeded the respective responses to medium alone beginning 29 days after challenge and continuing throughout the remainder of the study. In general, IP-10 responses detected in nonstimulated cultures followed a kinetics of response over the course of infection that was similar to that in antigen-stimulated cultures, indicative of an in vivo-initiated response detectable without antigen restimulation. Levels of IP-10 production by cattle were similar to those previously published for humans using a similar assay (34).
Fig 3.
Overall IP-10 and IFN-γ responses (protein). Whole blood from M. bovis-infected cattle (105 CFU by aerosol) was stimulated with recombinant ESAT-6:CFP10 (rE:C), M. bovis PPD (PPDb), pokeweed mitogen (PWM), or medium alone (no stimulation [ns]) for 18 h. Stimulated plasma was harvested and IP-10/IFN-γ protein levels determined by ELISA. Values represent mean ± SEM for responses over the course of the study (n = 30 [5 animals × 6 time points]). *, P < 0.05; #, P = 0.07 (differs from nonstimulated values).
Fig 4.
Kinetics of IP-10 and IFN-γ responses (protein) to M. bovis PPD or ESAT-6:CFP10. Whole blood from M. bovis-infected cattle (105 CFU by aerosol) was stimulated with M. bovis PPD (PPDb), recombinant ESAT-6:CFP10 (rE:C), or medium alone (no stimulation [NS]) for 18 h. Stimulated plasma was harvested and IP-10/IFN-γ protein levels determined by ELISA (mean ± SEM, n = 5). *, differs (P < 0.05) from nonstimulated values at the respective time points after challenge for both rEC and PPDb responses. +, differs (P < 0.05) from nonstimulated values at the respective time points after challenge for rEC responses only.
While IP-10 responses to antigen and no stimulation rarely differed, IP-10 responses to antigen stimulation did increase over time (Fig. 5, showing combined responses to M. bovis PPD, M. avium PPD, and rESAT-6:CFP). At 7, 29, and 63 days after challenge, IP-10 responses to mycobacterial antigens exceeded preinfection responses (Fig. 5). At 29 days after challenge and continuing throughout the study, IFN-γ responses exceeded preinfection responses (Fig. 5). As with M. tuberculosis infection in humans (20), these findings demonstrate that M. bovis infection elicits an IP-10 response in cattle.
Fig 5.
Kinetics of IP-10 and IFN-γ responses (protein) to antigen. Whole blood from M. bovis-infected cattle (105 CFU by aerosol) was stimulated with M. bovis PPD (PPDb), recombinant ESAT-6:CFP10 (rE:C), or M. avium PPD (PPDa) for 18 h. Stimulated plasma was harvested and IP-10/IFN-γ protein levels determined by ELISA. Responses to PPDb, rE:C, and PPDa followed similar kinetics; thus, data were combined to demonstrate a time effect on antigen responses (mean ± SEM, n = 15 [5 animals × 3 antigens]). *, differs (P < 0.05) from preinfection (day 0) responses.
One discrepancy in this study is the lack of correlation between antigen-specific IP-10 mRNA responses (Fig. 1) and protein responses (Fig. 4). The primary difference in these two responses is the increasing IP-10 protein response in nonstimulated cultures after infection, which was not evident with mRNA responses. As demonstrated in Fig. 5, the IP-10 protein response to M. bovis antigens did increase upon M. bovis infection; however, it was matched by a similar increase in IP-10 in nonstimulated cultures.
Responses to additional antigens and antigen concentration effects.
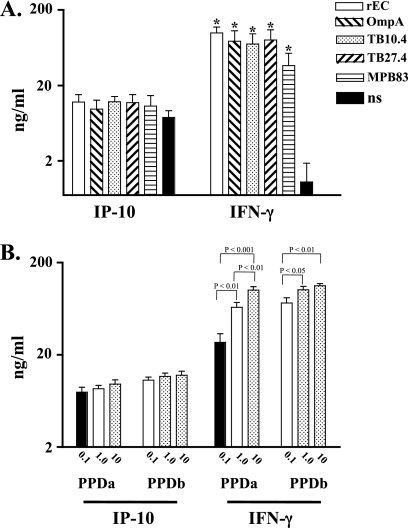
IP-10 and IFN-γ responses to additional M. bovis antigens are shown in Fig. 6A. At 29 days after challenge, IFN-γ responses to rESAT-6:CFP10, OmpA, TB10.4, TB27.4, and MPB83 all exceeded the respective responses to medium alone (no stimulation), whereas IP-10 responses to antigens did not differ from responses to medium alone. As with the IP-10 response to M. bovis PPD and rESAT-6:CFP10 (Fig. 4), IP-10 responses to specific antigens (e.g., MPB83, various sources of ESAT-6:CFP10, and combinations of OmpA, TB10.4, TB27.4, and MPB83 with ESAT-6:CFP10) increased from 7 to 29 days postchallenge (data not shown), indicating an infection-induced IP-10 response. Source (AHVLA, ISU, or Lionex) and dose (1 or 5 μg/ml) effects on ESAT-6:CFP10-elicited responses were not detected (data not shown).
Fig 6.
Responses to M. tuberculosis complex antigens (A) and purified protein derivatives (B). Whole blood from M. bovis-infected cattle (105 CFU by aerosol, n = 5) was collected at 29 days after challenge and stimulated with 5 μg/ml recombinant ESAT-6:CFP10 (rE:C), OmpA, TB10.4, TB27.4, MPB83, M. bovis PPD (PPDb) (0.1, 1.0, or 10 μg/ml), M. avium PPD (PPDa) (0.1, 1.0, or 10 μg/ml), or medium alone (no stimulation [ns]) for 18 h. Five sources of PPDs were included for evaluation of responses to M. avium and M. bovis PPDs at each concentration (n = 25 [5 sources × 5 animals]). Stimulated plasma was harvested and IP-10/IFN-γ protein levels determined by ELISA (mean ± SEM). *, differs (P < 0.05) from nonstimulated values (A) or significant differences are noted on the graph (B).
IP-10 and IFN-γ responses to various concentrations of PPDs are shown in Fig. 6B. Antigen concentration effects (0.1 μg/ml < 1 μg/ml < 10 μg/ml) were detected with IFN-γ but not IP-10 responses with both M. avium and M. bovis PPDs. With a few exceptions, effects of PPD source on PPD-elicited responses were not detected (data not shown). The greater discriminatory potential of IFN-γ than of IP-10 responses may be due to technical (i.e., assay sensitivity) or biological reasons.
Correlations between IP-10 and IFN-γ (protein) responses elicited by various antigens were also evaluated. Comparisons included the following: global (all treatments and all time points), PPDs at day 29 postchallenge, and selected antigens over time (PPDa, PPDb, r ESAT-6:CFP10, PWM, and medium alone at each time point). Correlation coefficients were weak (r ≈ 0.50 to 0.65); however, a significant correlation (P < 0.05; r = 0.65) was detected with responses to M. bovis PPD over the course of the study. Variable levels of IP-10 production (ranging from 0 to 25 ng/ml) were detected at the high range of IFN-γ production (∼130 ng/ml, the maximum detectable level for this assay). These findings indicate that bovine IP-10 and IFN-γ (protein) responses are not closely associated.
Conclusions.
IP-10 is an emerging biomarker for the diagnosis of human TB (17). The present findings demonstrate that M. bovis infection of cattle elicits an IP-10 response. Antigen-specific IP-10 and IFN-γ mRNA responses were detected as early as 30 days after M. bovis challenge and followed similar kinetics. Recently, a whole-blood quantitative reverse transcription-PCR (RT-PCR) approach has been described for the detection of TB-, HIV-, and cytomegalovirus (CMV)-infected humans (15). For the TB assay, MIG (monokine-induced by IFN-γ) and IP-10 mRNA responses to ESAT-6 and CFP10 peptide pools correlated with IFN-γ (protein) responses as detected by an enzyme-linked immunosorbent spot (ELISPOT) assay (15). The present findings indicate that a similar approach may be useful for the detection of tuberculous cattle.
There are two important limitations of the current study. First, archived mRNA samples were not available to directly compare protein and mRNA responses at the same time points and from the same animals. Second, IP-10 protein responses were evaluated with archived samples obtained with protocols designed for optimal antigen-specific IFN-γ responses. In particular, the stimulation period (i.e., 18 h) may not be optimal for IP-10 responses. With that said, this same approach has been used for the comparison of IP-10 and IFN-γ responses with samples from TB-infected humans (13, 17, 34); thus, while not optimal, this approach may be valid for initial proof-of-concept studies. Obviously, further studies are warranted to optimize protocols for the detection of antigen-specific bovine IP-10, both protein and mRNA. Additionally, it may be useful to evaluate IP-10 mRNA and protein responses in noninfected animals (i.e., in addition to preinfection responses in the current study) to better characterize baseline levels of this chemokine and potential interactions with inflammatory disease (i.e., as indicated by increasing protein responses in nonstimulated samples upon M. bovis infection in the current study).
Despite significant responses to mycobacterial antigens upon infection, IP-10 concentrations in nonstimulated whole-blood cultures were similar to those in antigen-stimulated cultures. High levels of IP-10 are also detected in nonstimulated whole-blood samples from children with TB (latently infected > active TB) (34) and in plasma samples from adults with active TB, possibly due to chronic TB-induced inflammation (4). Additional studies are warranted to evaluate IP-10 concentrations in sera from TB-infected cattle to determine diagnostic potential and associations with disease progression and vaccine efficacy. The present findings do not support the use of IP-10 protein as a biomarker for bovine TB using the current testing protocol and reagents.
ACKNOWLEDGMENTS
We thank Jessica Pollock, Kristin Bass, Shelly Zimmerman, and the animal care staff at NADC for excellent technical support and animal care.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Footnotes
Published ahead of print 11 January 2012
REFERENCES
- 1. Aabye MG, et al. 2010. Potential of interferon-γ-inducible protein 10 in improving tuberculosis diagnosis in HIV-infected patients. Eur. Respir. J. 36:1488–1490 [DOI] [PubMed] [Google Scholar]
- 2. Aabye MG, Ravn P, Johansen IS, Eugen-Olsen J, Ruhwald M. 2011. Incubation of whole blood at 39°C augments gamma interferon (IFN-γ)-induced protein 10 and IFN-γ responses to Mycobacterium tuberculosis antigens. Clin. Vaccine Immunol. 18:1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alvarez A, Endsley JJ, Werling D, Estes DM. 2009. WC1(+) gammadelta T cells indirectly regulate chemokine production during Mycobacterium bovis infection in SCID-bo mice. Transbound. Emerg. Dis. 56:275–284 [DOI] [PubMed] [Google Scholar]
- 4. Azzurri A, et al. 2005. IFN-gamma-inducible protein 10 and pentraxin 3 plasma levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect. 7:1–8 [DOI] [PubMed] [Google Scholar]
- 5. Blanco FC, et al. 2011. Increased IL-17 expression is associated with pathology in a bovine model of tuberculosis. Tuberculosis (Edinb.) 91:57–63 [DOI] [PubMed] [Google Scholar]
- 6. Cole AM, et al. 2001. IFN-inducible ELR-CXC chemokines display defensin-like antimicrobial activity. J. Immunol. 167:623–627 [DOI] [PubMed] [Google Scholar]
- 7. Cousins DV, et al. 2003. Tuberculosis in seals caused by a novel member of the Mycobacterium tuberculosis complex: Mycobacterium pinnipedii sp. nov. Int. J. Syst. Evol. Microbiol. 53:1305–1314 [DOI] [PubMed] [Google Scholar]
- 8. Farber JM. 1997. Mig and IP-10: CXC chemokines that target lymphocytes. J. Leukoc. Biol. 61:246–257 [PubMed] [Google Scholar]
- 9. Ferrero E, et al. 2003. Macrophages exposed to Mycobacterium tuberculosis release chemokines able to recruit selected leucocyte subpopulations: focus on gammadelta cells. Immunology 108:365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geluk A, et al. 2010. Enhancing sensitivity of detection of immune responses to Mycobacterium leprae peptides in whole-blood assays. Clin. Vaccine Immunol. 17:993–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones GJ, Pirson C, Hewinson RG, Vordermeier HM. 2010. Simultaneous measurement of antigen-stimulated interleukin-1 beta and gamma interferon production enhances test sensitivity for the detection of Mycobacterium bovis infection in cattle. Clin. Vaccine Immunol. 17:1946–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Juffermans NP, et al. 1999. Elevated chemokine concentrations in sera of human immunodeficiency virus (HIV)-seropositive and HIV-seronegative patients with tuberculosis: a possible role for mycobacterial lipoarabinomannan. Infect. Immun. 67:4295–4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kabeer BS, Sikhamani R, Raja A. 2010. Comparison of interferon gamma and interferon gamma-inducible protein-10 secretion in HIV-tuberculosis patients. AIDS 24:323–325 [DOI] [PubMed] [Google Scholar]
- 14. Kaplan G, Luster AD, Hancock G, Cohn ZA. 1987. The expression of a gamma interferon-induced protein (IP-10) in delayed immune responses in human skin. J. Exp. Med. 166:1098–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kasprowicz VO, et al. 2011. A molecular assay for sensitive detection of pathogen-specific T-cells. PLoS One 6:e20606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palmer MV, Waters WR, Whipple DL. 2003. Aerosol delivery of virulent Mycobacterium bovis to cattle. Tuberculosis (Edinb.) 82:275–282 [DOI] [PubMed] [Google Scholar]
- 17. Ruhwald M, et al. 2011. A multicentre evaluation of the accuracy and performance of IP-10 for the diagnosis of infection with M. tuberculosis. Tuberculosis (Edinb.) 91:260–267 [DOI] [PubMed] [Google Scholar]
- 18. Ruhwald M, Bjerregaard-Andersen M, Rabna P, Eugen-Olsen J, Ravn P. 2009. IP-10, MCP-1, MCP-2, MCP-3, and IL-1RA hold promise as biomarkers for infection with M. tuberculosis in a whole blood based T-cell assay. BMC Res. Notes 2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ruhwald M, et al. 2008. Evaluating the potential of IP-10 and MCP-2 as biomarkers for the diagnosis of tuberculosis. Eur. Respir. J. 32:1607–1615 [DOI] [PubMed] [Google Scholar]
- 20. Ruhwald M, et al. 2007. CXCL10/IP-10 release is induced by incubation of whole blood from tuberculosis patients with ESAT-6, CFP10 and TB7.7. Microbes Infect. 9:806–812 [DOI] [PubMed] [Google Scholar]
- 21. Sauty A, et al. 1999. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J. Immunol. 162:3549–3558 [PubMed] [Google Scholar]
- 22. Schiller I., et al. 2011. Bovine tuberculosis in Europe from the perspective of an OTF country: trade, surveillance and diagnostics. Vet. Microbiol. 151:153–159 [DOI] [PubMed] [Google Scholar]
- 23. Schiller I, et al. 2010. Bovine tuberculosis: a review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound. Emerg. Dis. 57:205–220 [DOI] [PubMed] [Google Scholar]
- 24. Schiller I, et al. 2009. Assessment of OmpA as a novel antigen for the diagnosis of bovine tuberculosis. Clin. Vaccine Immunol. 16:1314–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scollard DM, et al. 2011. Increased CXC ligand 10 levels and gene expression in type 1 leprosy reactions. Clin. Vaccine Immunol. 18:947–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stefani MM, et al. 2009. Potential plasma markers of type 1 and type 2 leprosy reactions: a preliminary report. BMC Infect. Dis. 9:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taubert A, Behrendt JH, Sühwold A, Zahner H, Hermosilla C. 2009. Monocyte- and macrophage-mediated immune reactions against Eimeria bovis. Vet. Parasitol. 164:141–153 [DOI] [PubMed] [Google Scholar]
- 28. Thacker TC, Palmer MV, Waters WR. 2006. Correlation of cytokine gene expression with pathology in white-tailed deer (Odocoileus virginianus) infected with Mycobacterium bovis. Clin. Vaccine Immunol. 13:640–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thacker TC, Palmer MV, Waters WR. 2007. Associations between cytokine gene expression and pathology in Mycobacterium bovis infected cattle. Vet. Immunol. Immunopathol. 119:204–213 [DOI] [PubMed] [Google Scholar]
- 30. Vordermeier HM, et al. 2009. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect. Immun. 77:3364–3373 (Erratum, 79:2134, 2011.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. 2011. Immunological biomarkers of tuberculosis. Nat. Rev. Immunol. 11:343–354 [DOI] [PubMed] [Google Scholar]
- 32. Waters WR, Palmer MV, Whipple DL, Carlson MC, Nonnecke BJ. 2003. Diagnostic implications of antigen-induced IFN-γ, nitric oxide, and TNF-α production by blood mononuclear cells from Mycobacterium bovis-infected cattle. Clin. Diagn. Lab. Immunol. 10:960–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Waters WR, et al. 2010. Immune responses in cattle inoculated with Mycobacterium bovis, Mycobacterium tuberculosis, or Mycobacterium kansasii. Clin. Vaccine Immunol. 17:247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Whittaker E, Gordon A, Kampmann B. 2008. Is IP-10 a better biomarker for active and latent tuberculosis in children than IFN gamma? PLoS One 3:e3901. [DOI] [PMC free article] [PubMed] [Google Scholar]