Abstract
Enzyme-linked immunosorbent assay (ELISA) is normally used to quantify the amount of serum IgG antibodies against measles, mumps, rubella, and varicella-zoster virus (MMRV). However, this method is time- and material-consuming. Therefore, a multiplex immunoassay for the simultaneous quantitative detection of antibodies against MMRV was developed. In-house as well as commercially available antigens can be used, making the assay available for all laboratories. The multiplex assay is much more sensitive than the separate ELISAs and has a high specificity, and only 5 μl of serum is needed. Heterologous inhibition did not exceed 11.5%, while homologous inhibition varied between 91.3 and 97.9%. Good correlations with the in-house ELISAs for measles (R2 = 0.98), mumps (R2 = 0.97), and rubella (R2 = 0.97) virus as well as with the ELISA kit for varicella-zoster virus (R2 = 0.95) were obtained. In conclusion, the MMRV multiplex assay is a good alternative to the conventional ELISAs and suitable for use in serosurveillance and vaccine studies.
INTRODUCTION
The combination vaccine of measles, mumps, and rubella (MMR) has been part of the Dutch national immunization program (NIP) since 1987 and is routinely administered at the age of 14 months, with a second dose administered at the age of 9 years. Monovalent vaccines of rubella and measles have been available in the NIP since 1974 and 1976, respectively. Until 1987, the rubella vaccine was administered only to 11-year-old girls and the measles vaccine was administered to all infants. Despite a high vaccination coverage (96% and 93% for, respectively, the first and second doses of MMR vaccine) (20), recent outbreaks have occurred in The Netherlands for measles in 2000 (17), for mumps in 2007-2008 (11), and for rubella in 2004-2005 (9). Although those outbreaks were limited to communities with relatively low vaccination coverage, they raised concern about the effectiveness of the vaccine.
To obtain insight into the long-term protection of the population and to assess the effect of changes in the NIP over time, it is necessary to monitor the immune status of the population in general. For this purpose, two large cross-sectional population-based serosurveillance studies were performed, one in 1995-1996 (3) and one in 2006-2007 (18), in which, respectively, 9,948 and 7,904 serum samples were collected from persons aged 0 to 79 years. The detection of IgG serum antibodies against measles, mumps, rubella, and varicella-zoster virus (MMRV) within the first serum bank was performed using separate enzyme-linked immunosorbent assays (ELISAs) (2, 10, 15, 16). However, these assays are too material- and time-consuming if large serosurveillance and vaccine studies have to be performed. The bead-based multiplex immunoassay (MIA) using Luminex technology (19) can combine the measurement of antibody levels against several antigens, which saves time, and in addition, the MIA uses very little biological material. Here we describe the development and validation of a MIA for the simultaneous quantitative detection of IgG antibodies against MMRV and its correlation with the separate ELISAs.
MATERIALS AND METHODS
Serum samples.
The international rubella standard serum RUBI-1-94 (1,600 IU/ml; NIBSC, Potters Bar, United Kingdom) was used as a reference for the MMRV MIA and was calibrated against the international standard serum for measles (66/202; 5 IU/ml; WHO, NIBSC) and varicella-zoster (W1044; 50 IU/ml; WHO, NIBSC) and the in-house standard for mumps (770 RIVM units [RU])/ml) with 30 independent dilutions divided over 3 to 5 days. Several in-house serum samples originating from Dutch vaccinated adults and intravenous Ig (IVIg; Baxter SA, Lessines, Belgium) were used as low, medium, or high controls.
For the development of the MMRV MIA, a panel of 70 serum samples with a broad range of concentrations determined in ELISA was composed. The panel originated from the first serum bank and contained serum samples from vaccinated as well as unvaccinated people aged 0 to 79 years (3). The panel for the comparison of the MIA with the ELISA using commercially obtained antigens originated from the second serum bank and had similar characteristics as the development panel (18).
MMRV MIA.
Each of the four antigens used in the MIA was coupled to differently labeled carboxylated beads that were chosen so that they were not directly adjacent to each other. Beads (12.5 × 106/ml; Luminex, Austin, TX) were activated as described earlier (14) and resuspended in 500 μl phosphate-buffered saline (PBS; pH 7.2) containing 240 μg of mumps virus (strain Jeryl Lynn [in-house] or strain Enders [GenWay, San Diego, CA]), 430 μg of measles virus (strain Edmonston [in-house] or strain Edmonston [GenWay]), 15 μg of rubella virus (strain HPV-77; GenWay), or 55 μg of varicella-zoster virus (strain VZ-10; GenWay) purified antigen. The in-house measles and mumps virus preparations were cultured, purified, and freeze-dried in stabilization buffer containing hydrolyzed gelatin (10, 16). The measles virus preparation was dissolved in PBS containing 0.1% Tween 20, incubated at room temperature (RT) for 8 h, and freeze-thawed before use. Beads were incubated for 2 h at RT in the dark under constant rotation at 25 rpm and subsequently washed 3 times with PBS. After the final washing step, beads were stored in PBS containing 0.05% (wt/vol) sodium azide and 1% (wt/vol) bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO) at 4°C in the dark until used.
The assessment of the antibody concentrations in the serum samples was performed essentially as previously described (19). The reference serum (RUBI-1-94), serum samples, and control sera were prediluted in PBS containing 0.1% (vol/vol) Tween 20 and 3% (wt/vol) BSA. The reference was 3-fold serially diluted over 10 wells, and serum samples were measured in two dilutions (1/200 and 1/4,000). The reference, controls, and blanks were included on each plate. After the final washing step, beads were resuspended in 100 μl PBS and shaken before analysis in a Bio-Plex 200 instrument (Bio-Rad Laboratories, Hercules, CA) using Bio-Plex Manager (version 4.1.1) software (Bio-Rad) with a 5-parameter fit. For the commercially obtained measles virus preparation, samples needed to be preincubated before analysis with the cell material used for culturing the virus.
ELISAs.
The antigens used for the measles, mumps, and rubella virus ELISAs were identical to those used for the MIA. The ELISAs have been performed as described earlier (2, 10, 15, 16). Briefly, antigens were individually coated to high-binding 96-well microtiter plates in a concentration of 2 μg/ml in carbonate buffer (pH 9.6). Serum samples and controls were prediluted in PBS containing 0.05% Tween 20 and 0.5% BSA. For each serum sample, including the reference, a 2-fold serial dilution over 8 wells was tested. Each plate contained an in-house reference serum, calibrated against the specific international or in-house standard. Serum samples were tested with a starting dilution of 1/25. Results for measles and rubella viruses were expressed in international units (IU) per milliliter, and those for mumps virus were expressed in RU per milliliter (15).
Quantitation of antibodies directed against varicella-zoster virus was performed with a VZV IgG ELISA kit (Human, Wiesbaden, Germany) according to the manufacturer's protocol, with the following minor adaptation. When the antibody concentration of a sample was ≤0.66 IU/ml or ≥2 IU/ml, the sample was retested in either a lower (1/10, 1/25, or 1/50) or a higher (1/400 or 1/1,000) dilution.
Specificity, sensitivity, reproducibility, and robustness of the MIA.
The specificity of the MIA was tested by inhibition experiments. Serum samples (n = 9) with known high IgG antibody concentrations against all four antigens were diluted 1/2,000 in PBS containing 0.1% (vol/vol) Tween 20 and 3% (wt/vol) BSA. Four aliquots were prepared from each serum sample. Each aliquot was incubated with an equal volume of one of the four antigens in an antigen-dependent concentration for 1 h at RT. For measles virus, the antigen concentration used was 50 μg/ml; for mumps virus, 25 μg/ml; for rubella virus, 25 μg/ml; and for varicella-zoster virus, 50 μg/ml. A 1/4,000 dilution of the serum was used as a control. The (inhibited) samples were assayed as described above. Homologous and heterologous inhibition of the mean fluorescence intensity (MFI) compared to the control was expressed in percentages after subtraction of the background.
To calculate the sensitivity of the assay, the mean and standard deviation (SD) were determined from 32 individual wells containing 50% antibody-depleted human serum (ADHS). The lower limit of detection (LLOD) was obtained by interpolation of the mean + 2 SDs in the standard curve (3-fold serial dilutions over 10 wells, 1/400 to 1/7,873,200) (12) and the lower limit of quantitation (LLOQ) was calculated as 3 times the LLOD (7).
The reproducibility of the assay was determined for each antigen via measurement of both intra-assay and interassay variation. For the intra-assay variation within a plate, 12 to 13 individual samples were analyzed in 3-fold on the same plate. For the intra-assay variation between plates, dependent dilutions of samples (n = 43 to 49) were analyzed on different plates on the same day. For the interassay variation, samples (n = 70) were tested in three separate assay runs. In all cases, the percent coefficient of variation (CV) for each sample was calculated and averaged. The robustness of the assay was determined by calculating and averaging the CVs of 200 samples analyzed on different days by a single operator as well as by different operators.
For all antigens, the reproducibility of different coupled bead batches was determined by calculating the correlation coefficient between the batches using 60 individual samples.
RESULTS
Single reference serum sample to quantify antibody concentrations against measles, mumps, rubella, and varicella-zoster virus.
In pilot experiments, we found that the international rubella standard not only contained high IgG levels against rubella virus but also high IgG levels against measles, mumps, and varicella-zoster virus. Therefore, we tested whether the rubella virus standard serum would be suitable to serve as a single reference serum for the simultaneous quantitation of antibody levels against MMRV in serum samples. After calibration against the international standards for measles and varicella-zoster virus and our in-house standard for mumps virus, IgG levels could be set to 63 ± 6 IU/ml for measles virus, 4,385 ± 235 RU/ml for mumps virus, and 22 ± 3 IU/ml for varicella-zoster virus. Corresponding CVs were 10% for measles and mumps virus and 12% for varicella-zoster virus.
Development of an MMRV tetraplex immunoassay (MIA).
For each virus, a range of antigen concentrations varying between 10 and 1,000 μg/12.5 × 106 beads was tested to obtain the optimal conjugation concentration. We found the optimal concentration to be 430 μg of measles virus antigen, 240 μg of mumps virus antigen, 15 μg of rubella virus antigen, and 55 μg of varicella-zoster virus antigen per 12.5 × 106 beads.
Various assay buffers were tested to further improve the performance of the MIA. The optimal serum dilution buffer consisted of PBS containing 0.1% (vol/vol) Tween 20 and 3% (wt/vol) BSA. The addition of 50% (vol/vol) antibody-depleted human serum in PBS (4) or 0.5% (wt/vol) polyvinyl alcohol and 0.8% (wt/vol) polyvinylpyrrolidone (21) to our assay buffer did not improve the correlation observed between the MIA and the 4 separate ELISAs.
High specificity, sensitivity, robustness, and reproducibility of MIA.
The correlations (R2) between MIAs performed in monoplex and tetraplex forms varied between 0.982 and 0.996, indicating that there was no interference between the different antigens. To determine the assay specificity, inhibition experiments using sera with known high IgG antibody concentrations against all four antigens were performed. After the absorption, the serum samples were analyzed, and this revealed that heterologous inhibition did not exceed 11.5%, while homologous inhibition varied between 91.3% and 99.4%, indicating a high specificity (Table 1).
Table 1.
Specificity of MMRV MIA with homologous and heterologous inhibitorsa
| Analyte virus | Mean % inhibition with the following virus: |
|||
|---|---|---|---|---|
| Measles | Mumps | Rubella | Varicella-zoster | |
| Measles | 96.9 | 0 | 1.9 | 4.8 |
| Mumps | 6.6 | 97.9 | 4.2 | 5.8 |
| Rubella | 10.4 | 8.5 | 99.4 | 11.5 |
| Varicella-zoster | 0 | 0 | 0 | 91.3 |
n = 9 serum samples. The antigen concentrations used were 25 μg/ml for mumps and rubella virus and 50 μg/ml for measles and varicella-zoster virus. Results with homologous inhibition are shown in boldface.
By collecting the MFI values of 59 blank wells from independent assays, the LLOD and LLOQ for each analyte were assessed and compared with those obtained in ELISA (Table 2). The MIA proved to be much more sensitive than the conventional ELISAs, especially for varicella-zoster virus, where the LLOQ of the MIA exceeded that of the ELISA by a factor of 1,000.
Table 2.
Sensitivity of MIA compared to in-house ELISAs
| Analyte virus | MIA LLOD (mIU/ml) | MIA LLOQ (mIU/ml) | ELISA LLOQ (mIU/ml) |
|---|---|---|---|
| Measles | 0.24 | 0.72 | 20 |
| Rubella | 1.29 | 3.86 | 3,000 |
| Varicella-zoster | 0.24 | 0.71 | 660 |
| Mumpsa | 53 | 160 | 4,000 |
Units for mumps virus are in milli-RIVM units (mRU/ml).
Intra-assay variation within a plate varied between 6% and 8%, and the intra-assay variation between plates varied between 5 and 9%, with exception of that for mumps virus, which had a slightly higher intra-assay variation of 14% (Table 3). The mean CV for the interassay variation ranged from 12 to 16% for all 4 analytes. The robustness of the assay was high. The mean CV for the assay performed by one operator on different days ranged from 10 to 14%, while the CV ranged from 8 to 15% when performed by different operators (Table 4).
Table 3.
Reproducibility of MMRV MIA by intra-assay and interassay variations
| Analyte virus | Mean % CV (n) |
||
|---|---|---|---|
| Intra-assay (within plate) | Intra-assay (between plates) | Interassay (between assays) | |
| Measles | 6 (13) | 6 (46) | 16 (70) |
| Mumps | 8 (12) | 14 (44) | 16 (70) |
| Rubella | 6 (13) | 5 (47) | 12 (70) |
| Varicella-zoster | 8 (13) | 9 (47) | 12 (70) |
Table 4.
Robustness of MMRV MIA for 200 serum samples assayed on different days or by different operators
| Analyte virus | Mean % CV by: |
|
|---|---|---|
| Day | Operator | |
| Measles | 10 | 15 |
| Mumps | 14 | 14 |
| Rubella | 11 | 8 |
| Varicella-zoster | 11 | 13 |
The correlation between different bead batches for all 4 antigens was routinely determined when new batches were made using a panel of serum samples. High correlation coefficients were found, with R2 values ranging from 0.941 to 0.994. Moreover, the coupled beads proved to be stable for at least 1 year at 4°C.
Strong correlation between MIA and ELISA.
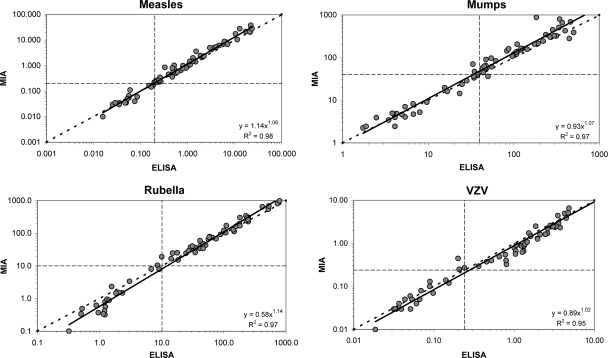
Figure 1 shows that the correlations (R2 values) between antibody concentrations found in the MIA and in the separate ELISAs varied between 0.953 and 0.983 for all 4 antigens. The trend lines in the correlation graphs were almost identical to the ideal correlation lines for measles, mumps, and varicella-zoster virus. Only for rubella virus, a small discrepancy between both techniques could be observed in the lower-concentration region below the cutoff level. In this region, ELISA results were generally higher than the MIA values.
Fig 1.
Comparison of results obtained by the MIA with results from the in-house measles, mumps, and rubella virus ELISAs and the varicella-zoster virus ELISA kit. Cutoff levels are indicated by the horizontal and vertical dashed lines, and the ideal line is represented by the diagonal dashed line.
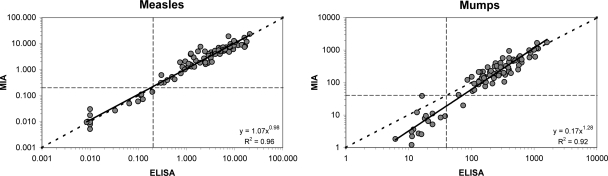
For measles and mumps virus, a comparison between the MIA and ELISA using commercially available antigens was also performed (Fig. 2). Correlations of 0.96 and 0.92 for, respectively, measles and mumps virus were observed. As with the in-house antigen, the trend line for measles virus was again nearly identical to the ideal line. However, the trend line for mumps virus was somewhat tilted due to the fact that for mumps virus results below the cutoff level were generally higher in the ELISA than in the MIA. MFI values of the reference curve from the commercially obtained measles virus preparation did not reach the maximum because of an excess of cell material present in this preparation (result not shown).
Fig 2.
Comparison of results obtained by the MIA with results from measles and mumps virus ELISAs using commercially obtained antigens. Cutoff levels are indicated by the horizontal and vertical dashed lines, and the ideal line is represented by the diagonal dashed line.
DISCUSSION
Here we describe the development of a tetravalent MMRV immunoassay. In line with other multiplex assays (1, 4, 6, 12, 19), the assay showed a high specificity and a great reproducibility and turned out to be much more sensitive than the conventional ELISAs, and the conjugated beads were stable for at least 1 year.
Another great advantage of the MIA is that it considerably reduces the costs for determining the concentrations of IgG against all 4 antigens. It was estimated earlier that the breakeven point in costs for the MIA compared with the separate ELISAs is reached if more than two different antigens are measured simultaneously (19). Also, with our assay, unlike the commercially available MMRV IgG AtheNA Multi-Lyte assay (Zeus Scientific, Raritan, NJ) (5) and the BiopPlex 2200 MMRV IgG assay, we were able to generate quantitative results.
Optimal antigen concentrations for conjugation were found to be antigen dependent and ranged from 15 μg (rubella virus) to 430 μg (measles virus) per 12.5 × 106 activated beads. The amount of antigen needed for the commercially obtained rubella and varicella-zoster virus antigens was comparable to that needed for other MIAs (13, 19), but a larger amount was needed for our in-house mumps virus and especially measles virus antigens. The requirement to use larger amounts may be caused by the freeze-drying stabilization buffer that was used for our mumps and measles virus antigen preparation. This buffer contained hydrolyzed gelatin, which resulted in a high protein concentration of the virus preparation. The actual virus antigen concentration in the preparation is much lower. For the commercially obtained measles virus antigen, a concentration up to 1,500 μg per 12.5 × 106 activated beads was not sufficient to give optimal conjugation results. The MFI values of the reference curve did not reach the maximum, thereby narrowing the detection range of the assay. The excess of cell material present in this preparation is the most likely explanation for the nonoptimal conjugation, and it also caused the nonspecific signal in the MIA for some serum samples. By preincubation of those samples with Vero cell material, we were able to inhibit this nonspecific signal, which was not necessary for any of the other in-house and commercial antigens.
For the in-house measles virus preparation (16), treatment with 0.1% Tween 20 and a subsequent single freeze-thaw cycle at −80°C appeared to be critical steps for proper conjugation to the beads. The measles virus antigen probably had to be well disintegrated for the conjugation in order to obtain a degree of coupling that is similar to the coating obtained for the ELISA. Coating of the measles virus antigen in ELISA plates is performed in carbonate buffer (pH 9.6), which disintegrates the virus as well. In the in-house mumps virus preparation and the commercial measles and mumps virus preparations, the viruses were probably already disintegrated. A possible explanation for the need to disintegrate the virus is that a considerable part of the antibody response against the virus is directed not only against the viral glycoproteins in the envelope but also against the viral nucleus/core proteins (8).
A great advantage of our assay is that just one reference serum sample can be used for all 4 antigens. We found that the international rubella reference (RUBI) contained not only very high anti-rubella virus IgG levels but also high measles, mumps, and varicella-zoster virus IgG levels, so that it could function as a multivalent reference serum, which leaves more wells on a plate available for the measurement of more samples in one run. The dilution series of the reference was chosen in such a way that it spanned 3.5 to 4 log10 units of MFI. As a result, two dilutions (1/200 and 1/4,000) of the serum samples were found to be sufficient for the measurement of a broad range of antibody concentrations covering 99.8% of the antibody determinations up until now.
The optimal buffer for this assay proved to be PBS containing Tween 20 and BSA. Since the Tween-BSA buffer is also used for the diphtheria, tetanus, and pertussis MIA (19), it might enable us in the future to simultaneously quantitate the antibodies against 10 vaccine antigens (diphtheria, tetanus, and acellular pertussis vaccine [DTaP4] and MMRV) used in the Dutch national immunization program in a single serum sample.
Comparison of the MIA with our in-house ELISAs revealed a high correlation between the two techniques (R2 ≥ 0.95). Higher values were observed in the ELISA in the lower concentration range below the cutoff only for the commercially available rubella and mumps virus antigens. A possible explanation is that all material present in the antigen preparation (e.g., lipids) can bind to the surface of the ELISA plate, which may increase nonspecific reactions. In the MIA, only proteins with free amino groups can bind to the activated bead surface, which will contribute to a reduction in the nonspecific binding and increase the specificity.
In conclusion, the MMRV MIA is a specific, sensitive, reproducible, sample- and antigen-saving quantitative method to determine IgG antibodies directed against measles, mumps, rubella, and varicella-zoster virus in one serum sample simultaneously. Above all, it is a fast method compared to the conventional assays. The correlation of this technique with ELISA (in-house or commercial) was high, and therefore, it is a good alternative method for the detection of MMRV antibodies in large-scale immunosurveillance studies.
ACKNOWLEDGMENT
We have no conflict of interest to state.
Footnotes
Published ahead of print 11 January 2012
REFERENCES
- 1. Biagini RE, et al. 2003. Method for simultaneous measurement of antibodies to 23 pneumococcal capsular polysaccharides. Clin. Diagn. Lab. Immunol. 10: 744–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Haas R, van den Hof S, Berbers GA, de Melker HE, Conyn-van Spaendonck MA. 1999. Prevalence of antibodies against rubella virus in The Netherlands 9 years after changing from selective to mass vaccination. Epidemiol. Infect. 123: 263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Melker HE, Conyn-van Spaendonck MA. 1998. Immunosurveillance and the evaluation of national immunization programmes: a population-based approach. Epidemiol. Infect. 121: 637–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Voer RM, et al. 2008. Development of a fluorescent-bead-based multiplex immunoassay to determine immunoglobulin G subclass responses to Neisseria meningitidis serogroup A and C polysaccharides. Clin. Vaccine Immunol. 15: 1188–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dhiman N, et al. 2010. Detection of IgG-class antibodies to measles, mumps, rubella, and varicella-zoster virus using a multiplex bead immunoassay. Diagn. Microbiol. Infect. Dis. 67: 346–349 [DOI] [PubMed] [Google Scholar]
- 6. Elberse KE, Tcherniaeva I, Berbers GA, Schouls LM. 2010. Optimization and application of a multiplex bead-based assay to quantify serotype-specific IgG against Streptococcus pneumoniae polysaccharides: response to the booster vaccine after immunization with the pneumococcal 7-valent conjugate vaccine. Clin. Vaccine Immunol. 17: 674–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. EMEA 1995. ICH topic Q2 (R1) validation of analytical procedures: text and methodology. Note for guidance on validation of analytical procedures: text and methodology. Report CGMP/ICH/381/95 EMEA, London, United Kingdom [Google Scholar]
- 8. Gans HA, et al. 2004. T cell immunity to measles viral proteins in infants and adults after measles immunization. Viral Immunol. 17: 298–307 [DOI] [PubMed] [Google Scholar]
- 9. Hahne S, et al. 2009. Rubella outbreak in The Netherlands, 2004-2005: high burden of congenital infection and spread to Canada. Pediatr. Infect. Dis. J. 28: 795–800 [DOI] [PubMed] [Google Scholar]
- 10. Harmsen T, et al. 1992. Comparison of a neutralization enzyme immunoassay and an enzyme-linked immunosorbent assay for evaluation of immune status of children vaccinated for mumps. J. Clin. Microbiol. 30: 2139–2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karagiannis I, et al. 2008. Mumps in a community with low vaccination coverage in the Netherlands. Euro Surveill. 13(25): pii=18906 [PubMed] [Google Scholar]
- 12. Lal G, et al. 2005. Development and validation of a nonaplex assay for the simultaneous quantitation of antibodies to nine Streptococcus pneumoniae serotypes. J. Immunol. Methods 296: 135–147 [DOI] [PubMed] [Google Scholar]
- 13. Opalka D, et al. 2003. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed Luminex assay. Clin. Diagn. Lab. Immunol. 10: 108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Staros JV, Wright RW, Swingle DM. 1986. Enhancement by N-hydroxy-sulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal. Biochem. 156: 220–222 [DOI] [PubMed] [Google Scholar]
- 15. van den Hof S, Beaumont MT, Berbers GA, de Melker HE. 2003. Antibodies against mumps in The Netherlands as assessed by indirect ELISA and virus neutralization assay. Epidemiol. Infect. 131: 703–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van den Hof S, Berbers GA, de Melker HE, Conyn-van Spaendonck MA. 1999. Sero-epidemiology of measles antibodies in The Netherlands, a cross-sectional study in a national sample and in communities with low vaccine coverage. Vaccine 18: 931–940 [DOI] [PubMed] [Google Scholar]
- 17. van den Hof S, et al. 2001. Measles outbreak in a community with very low vaccine coverage, The Netherlands. Emerg. Infect. Dis. 7: 593–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Klis FR, Mollema L, Berbers GA, de Melker HE, Coutinho RA. 2009. Second national serum bank for population-based seroprevalence studies in The Netherlands. Neth. J. Med. 67: 301–308 [PubMed] [Google Scholar]
- 19. van Gageldonk PG, van Schaijk FG, van der Klis FR, Berbers GA. 2008. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J. Immunol. Methods 335: 79–89 [DOI] [PubMed] [Google Scholar]
- 20. van Lier EA, et al. 2009. High vaccination coverage of the National Immunization Programme in The Netherlands. Ned. Tijdschr. Geneeskd. 153: 950–957 (In Dutch.) [PubMed] [Google Scholar]
- 21. Waterboer T, Sehr P, Pawlita M. 2006. Suppression of non-specific binding in serological Luminex assays. J. Immunol. Methods 309: 200–204 [DOI] [PubMed] [Google Scholar]