Abstract
Francisella tularensis, a category A bioterrorism agent, is a highly infectious organism that is passed on via skin contact and inhalation routes. A live attenuated vaccine strain (LVS) has been developed, but it has not been licensed for public use by the FDA due to safety concerns. Thus, there exists a need for a safer and improved vaccine. In this study, we have constructed a replication-incompetent adenovirus, Ad/opt-Tul4, carrying a codon-optimized gene for expression of a membrane protein, Tul4, of F. tularensis LVS. Its ability to protect against lethal challenge and its immunogenicity were evaluated in a murine model. An intramuscular injection of a single dose (1 × 107 PFU) of Ad/opt-Tul4 elicited a robust Tul4-specific antibody response. Assays suggest a Th1-driven response. A single dose elicited 20% protection against challenge with 100 × 50% lethal dose (LD50) F. tularensis LVS; two additional booster shots resulted in 60% protection. In comparison, three doses of 5 μg recombinant Tul4 protein did not elicit significant protection against challenge. Therefore, the Ad/opt-Tul4 vaccine was more effective than the protein vaccine, and protection was dose dependent. Compared to LVS, the protection rate is lower, but an adenovirus-vectored vaccine may be more attractive due to its enhanced safety profile and mucosal route of delivery. Furthermore, simple genetic modification of the vaccine may potentially produce antibodies protective against a fully virulent strain of F. tularensis. Our data support the development and further research of an adenovirus-vectored vaccine against Tul4 of F. tularensis LVS.
INTRODUCTION
Tularemia is an extremely infectious zoonotic bacterial disease caused by Francisella tularensis. The CDC has classified it as a category A bioterrorism agent with high potential to be used by bioterrorists (4, 5, 7). A live attenuated vaccine, derived from the multiple passage of a fully virulent strain of F. tularensis subspecies holarctica, was previously used as an investigational new drug in the United States. However, this vaccine is not fully licensed and does not offer a high level of protection against respiratory challenge. In addition, cultures of live vaccine strain (LVS) under some conditions can result in poorly immunogenic variants (8), and there have been reports of human tularemia arising from incorrect administration. Therefore, there is a need to move forward with developing a safe and improved vaccine against F. tularensis.
Strategies for development of a new generation of tularemia vaccines include identification of individual components of F. tularensis, such as the lipopolysaccharide or various other outer membrane proteins, as potential vaccine antigens. A 17-kDa membrane protein, Tul4 (of unknown function), has been previously characterized as a potential immunogen (16). Although Tul4 protein immunization has been shown to be ineffective at protecting against low-virulence strains, it reduces liver and spleen colonization by F. tularensis (13) and elicits strong humoral and T-cell proliferative activity (25, 26). Therefore, Tul4 may warrant use of another approach as a vaccine target. In this study, Tul4 was delivered as an adenovirus-vectored genetic vaccine.
Replication-incompetent adenoviruses are currently available as efficient gene transfer vehicles for both in vitro and in vivo approaches (2, 24, 31). A replication-incompetent adenovirus is ideal as a vaccine vector because it can infect a broad range of human cells, causes only mild symptoms in humans, and can accommodate up to 7.5 kbp of DNA (29). Adenovirus-vectored recombinant vaccines expressing a wide array of antigens have previously been constructed, and protective immunity against different pathogens has been demonstrated in animal models (19, 28, 30, 33, 34). In this study, we constructed a replication-incompetent adenovirus carrying the Tul4 gene, Ad/opt-Tul4, and demonstrated the efficacy of using Ad/opt-Tul4 for genetic vaccination against tularemia in a murine model.
MATERIALS AND METHODS
Construction of adenoviral vector encoding codon-optimized Tul4 of F. tularensis.
The adenoviral vector used in this study was derived from human adenovirus serotype 5 that was rendered replication incompetent by deletion of the E1 and E3 regions. Replication-incompetent recombinant adenoviral vectors were constructed using the AdEasy System (Stratagene, La Jolla, CA). The codon-optimized gene encoding Tul4 of F. tularensis was synthesized by GenScript (Piscataway, NJ). The signal peptide of human tissue plasminogen activator (PLAT) (amino acids 1 to 25; GenBank accession no. BC002795) plus two serine residues were added upstream of the Tul4 sequence. The human codon-optimized sequence of tul4, along with the native gene and protein sequences of Tul4, are shown in Table 1.
Table 1.
Native and codon-optimized tul4 DNA sequences along with the protein sequencesa
Nucleotide changes are in red.
The synthesized DNA was subsequently cloned into a shuttle vector, pShuttle-CMV (Stratagene, La Jolla, CA), at its SalI site. The DNA sequence of the synthesized gene was further confirmed by DNA sequencing analysis. The adenoviral vector was then constructed according to the standard procedures as described previously (15, 32). Similarly, the Ad/Null vector without transgene was also constructed as a negative control. Adenoviruses isolated from single plaques were then propagated in AD293 cells (Stratagene, La Jolla, CA), purified by CsCl gradient purification, and dialyzed with adenovirus storage buffer (10 mM Tris, pH 7.5, 135 mM NaCl, 5 mM KCl, and 1 mM MgCl2). The purified adenovirus was stored in 1.0 M sucrose at −86°C until use. Viral titers (PFU/ml) were determined by plaque assay.
Animal vaccination, sample collection, and challenge.
Six- to 8-week-old female BALB/c mice were purchased from Taconic Farms (Hudson, NY) and housed with 5 animals per cage. They were maintained in a controlled environment (22 ± 2°C; cycles of 12 h light and 12 h dark) in accordance with the U.S. Public Health Service and the Guide for the Care and Use of Laboratory Animals (20). The animals were provided Laboratory Rodent Diet 5001 with access to food and water ad libitum. The research was conducted in compliance with the Animal Welfare Act and other federal and state statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals (20).
Mice were allotted into different groups comprised of 5 to 10 mice per group. They were vaccinated on week 0 as follows: with the Ad/opt-Tul4 vaccine or the control vector Ad/Null via injection intramuscularly (i.m.; 105 PFU/mouse) to the hind-leg quadriceps or intradermally (i.d.; 107 PFU/mouse), with recombinant Tul4 protein (i.m.; 5 μg/mouse), or with F. tularensis LVS (i.d.; 103 CFU). Various mouse strains have been shown to survive i.d. challenge with LVS ≥ 105 CFU (23); thus, we deemed an i.d. dose of 103 CFU appropriate as a positive control for our vaccination studies. Some groups of Ad/opt-Tul4-, Ad/Null-, or recombinant Tul4-vaccinated mice were administered booster doses on week 2 and week 4, while other groups were not. Mice vaccinated once with F. tularensis LVS did not receive additional doses.
Animal sera were obtained by retro-orbital bleeding every 2 weeks (week 0, 2, 4, and 6) and stored at −20°C. All animals were challenged via intraperitoneal (i.p.) injection of F. tularensis LVS (210 to 400 CFU) after 7 weeks of vaccination. It has been previously shown that 100% of BALB/c mice perish following i.p. administration of 100 CFU LVS by 7 days postinoculation (12). The challenged mice in this study were monitored for 10 days. They were observed four times a day for 1 week and then twice a day thereafter. The number of deaths for each group was recorded as the endpoint.
ELISA for determination of antibody concentration.
Serum anti-Tul4 IgG and subtypes IgG1 and IgG2a antibody concentrations were determined using an enzyme-linked immunosorbent assay (ELISA) quantization kit (Bethyl Laboratories, Inc., Montgomery, TX) using a modified procedure. Ninety-six-well flat-bottom immunoplates (Nunc) were coated with 100 ng/well of His-tagged Tul4 recombinant protein (produced in BL21 Star DE3 Escherichia coli) at 4°C overnight. For the standard curve, plates were coated with capture antibodies as follows: goat anti-mouse IgG, IgG1, or IgG2a affinity purified (Bethyl) in 100 μl coating buffer (0.05 M carbonate-bicarbonate buffer, pH 9.6). Plates were washed 5 times with phosphate-buffered saline (PBS) buffer containing 0.05% Tween and then blocked with 200 μl PBS (pH 7.4) containing 1% bovine serum albumin (BSA) for 1 h at room temperature. After the plates were washed, 1:100 dilutions of mouse serum samples in PBS (pH 7.4) containing 0.05% Tween 20 and 1% BSA were added and incubated at 37°C for 2 h. For the standard curve, 100-μl serial dilutions of reference serum containing given amounts of mouse antibodies were added. The plates were washed five times and incubated with 100 μl/well of 1:1,000 dilution of goat anti-mouse IgG, IgG1, or IgG2a conjugated to alkaline phosphatase for 1 h at room temperature. After the plates were washed 5 times, the bound antibody was developed with p-nitrophenyl phosphate phosphatase substrate system (KPL, Gaithersburg, MD) for 20 min. The color reaction was stopped by adding 100 μl 0.5 M EDTA, and the absorbance values were obtained using a Dynatech MR4000 model microplate reader at 405 nm. A standard curve was generated for each set of samples, and serum antibody concentrations were calculated in accordance with the standard curve.
Statistical analyses.
All the statistical analyses were performed on GraphPad Prism version 5.00 (GraphPad Software, San Diego, CA). Serum antibody titers from different time points in vaccinated groups were compared and analyzed using a two-tailed, unpaired t test; those with P values of <0.05 and <0.01 were considered to be significant and very significant, respectively. A paired t test was used to analyze the subclass of IgG titer within the same group. The log-rank (Mantel-Cox) survival test was used to compare the survival statistics between two groups.
Nucleotide sequence accession number.
The nucleotide sequence of the synthesized Tul4 gene has been deposited in GenBank under accession number JQ629934.
RESULTS
Protective immunity elicited by Ad/opt-Tul4.
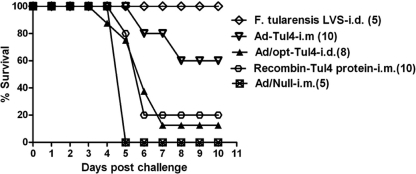
To explore whether a Tul4-based adenovirus-vectored vaccine, Ad/opt-Tul4, could protect against tularemia disease, vaccinated mice were i.p. challenged with 210 CFU of F. tularensis LVS 3 weeks after the third dose of vaccination. The results showed that i.m. vaccination with Ad/opt-Tul4 protected 60% of the mice against challenge, while the negative control Ad/Null was not protective (Fig. 1). Additionally, the positive-control mouse group, vaccinated i.d. with F. tularensis LVS, resulted in 100% protection. Finally, a vaccination with recombinant Tul4 protein and adjuvant conferred only 20% protection against challenge (Fig. 1). The log-rank (Mantel-Cox) survival test shows a significant difference between the Ad/opt-Tul4 group and the recombinant Tul4 protein group (P < 0.05).
Fig 1.
Survival of Ad/opt-Tul4 given intramuscularly (i.m.) and intradermally (i.d.) and recombinant-Tul4 protein and Ad/Null-immunized mice challenged intraperitoneally (i.p.) with 210 CFU of F. tularensis LVS. The positive-control group, F. tularensis LVS (i.d.) immunized mice, were challenged i.p. with 400 CFU of F. tularensis LVS. The difference in survival percentage of i.m. Ad/opt-Tul4 is significantly different from that of recombinant Tul4 protein (P = 0.02).
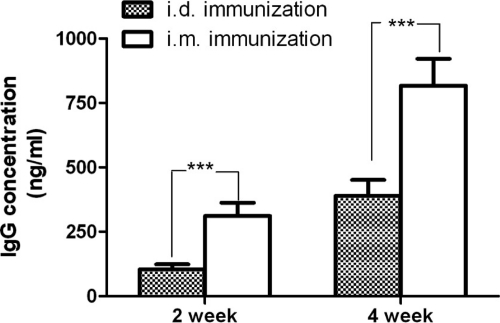
Increasing challenge doses of F. tularensis LVS decreased the survival rate in Ad/opt-Tul4 (Table 2). The route of immunization resulted in different protection rates. An i.d. vaccination with 3 doses of Ad/opt-Tul4 provided only 12.5% protection against challenge, which is significantly different (P < 0.05) than i.m. vaccination with Ad/opt-Tul4 (Fig. 1). Additionally, antibody titers were lower for groups of mice vaccinated with Ad/opt-Tul4 via the i.d. route than via the i.m. route (Fig. 2).
Table 2.
Comparison of challenge doses of F. tularensis LVS and survival rates of mice in different vaccination groups
| Dose of LVS (CFU) used for challenge (i.p.) | % survival (no. of mice that survived/total no. of mice) |
||
|---|---|---|---|
| Ad/opt-Tul4 (i.m.) | Ad/Null (i.m.) | LVS (i.d.) | |
| 210 | 60 (6/10) | 0 | NAa |
| 300 | 40 (2/5) | 0 | 100 (5/5) |
| 400 | 20 (1/5) | 0 | 100 (5/5) |
NA, not applicable; the challenge of 210 CFU LVS was not performed with mice vaccinated i.d. with F. tularensis LVS.
Fig 2.
Comparison of serum Anti-Tul4 IgG antibody level of Ad/opt-Tul4 immunization given intradermally (i.d.) versus intramuscularly (i.m.) after 2 and 4 weeks of primary immunization in mice (n = 8). A significant difference (P < 0.001) was found for IgG titers between i.d. versus i.m. immunization groups in weeks 2 and 4. The values shown in the column are means ± standard errors of the mean (n = 8).
Recombinant Tul4 protein vaccine elicited a high level of Tul4-specific IgG antibody but did not protect against challenge.
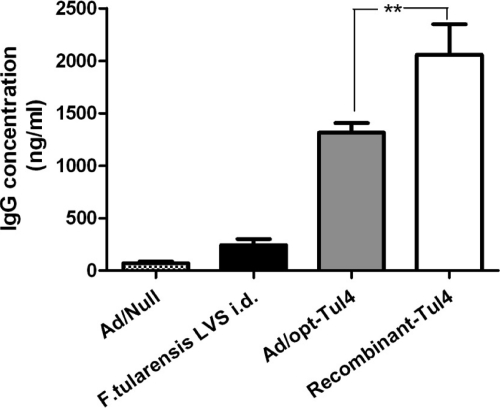
The total serum IgG antibody levels (anti-Tul4) were determined for the groups Ad/Null, F. tularensis LVS, Ad/opt-Tul4, and recombinant Tul4. Mice vaccinated with recombinant Tul4 protein showed a significantly higher antibody response (2,000 ng/ml) than mice vaccinated with Ad/opt-Tul4 (Fig. 3). However, the higher antibody response did not correlate with protection against challenge.
Fig 3.
Anti-Tul4 IgG antibodies in sera of different groups of mice after 6 weeks of initial immunization with Ad/opt-Tul4 (10 mice), Ad/Null (5 mice), recombinant-Tul4 (10 mice), and F. tularensis LVS (5 mice). There was a significant difference (P < 0.05) between Ad/opt-Tul4- and recombinant-Tul4-vaccinated mice.
Tul4-specific IgG antibody titers at different time points in the vaccination schedule of Ad/opt-Tul4 were assayed, and groups with booster immunizations and without were compared. After the second week, antibody titers showed significant differences (P < 0.01) from week to week (Fig. 4A). Regardless of whether the mice received booster doses of Ad/opt-Tul4 or not, there were no significant differences in antibody titer. However, the protection rate was higher in mice vaccinated with booster shots of Ad/opt-Tul4 (Fig. 4B).
Fig 4.
Effect of booster immunization. (A) Anti-Tul4 IgG antibody response after 0, 2, 4, and 6 weeks of immunization with Ad/opt-Tul4 without booster and with booster. The difference between the titers after 2 to 6 weeks and 4 to 6 weeks of primary immunization was very significant, with a P value < 0.001 (***) and a P value < 0.01 (**), respectively. (B) Survival curve of Ad/opt-Tul4 mice with and without booster. As a control, the survival rate of Ad/Null group is also shown.
Intramuscular vaccination with Ad/opt-Tul4 elicited a Th1-biased antibody response.
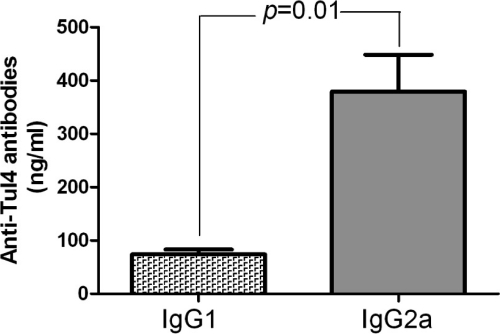
The proportion of Tul4-specific serum IgG1 and IgG2a antibody subclasses in mice i.m. vaccinated with Ad/opt-Tul4 was also assessed by ELISA. IgG2a antibody levels specific to Tul4 protein were three times higher than IgG1 antibody levels (Fig. 5).
Fig 5.
Anti-Tul4 IgG subtype IgG1/IgG2a comparison. Anti-Tul4 response in vaccinated mice with Ad/opt-Tul4 immunization (i.m.). IgG1 and IgG2a concentrations were measured by ELISA in the serum samples collected after 6 weeks of vaccination. The values shown in the column are means ± standard errors of the mean (n = 8).
DISCUSSION
A new generation of adenovirus-vectored tularemia vaccine encoding a Tul4 lipoprotein sequence, Ad/opt-Tul4, has been assessed in this study. A murine model was used to test serum Tul4-specific antibody levels, and protection against challenge with F. tularensis LVS was observed for mice immunized with Ad/opt-Tul4. The sequence for Tul4 was human codon optimized, and the adenovirus vector containing the optimized insert elicited a more robust immune response than that with a native insert (R. Kaur and M. Zeng, unpublished data); this effect has also been observed in other studies (1). The ease of genetic manipulation provides some advantages for Ad/opt-Tul4 over a live attenuated strain of F. tularensis. Although F. tularensis LVS can protect against challenge with the same strain, the efficacy of LVS derived from F. tularensis subsp. holarctica may be compromised if a different subspecies strain were to be used in the event of a bioterrorist attack. An adenovirus-vectored vaccine can be easily adjusted to code for subspecies-specific, codon-optimized sequences. Thus, the vaccine's flexible design makes it an appealing candidate.
Ad/opt-Tul4 may also be an attractive candidate vaccine due to its ability to provide dose-dependent protection against F. tularensis LVS. When mice were challenged with 210 CFU F. tularensis LVS via the i.p. route, we observed a 60% protection rate in mice immunized three times with Ad/opt-Tul4 (i.m.). Without the booster shots protection dropped to 12.5%. Similarly, when mice were vaccinated with recombinant Tul4 protein (three times), protection hovered at 20%. Both vaccines elicited a robust antibody titer, but protection was observed only in mice immunized three times with Ad/opt-Tul4. This high vaccine dose activated humoral immunity and, potentially, cell-mediated immunity. However, more studies are needed to assess whether there is a high enough dose of Ad/opt-Tul4 to elicit the most potent immune response.
Comparing the Tul4-specific antibody titers between mice immunized with Ad/opt-Tul4 with and without booster shots, both groups were observed to have antibody titers at around 1,400 ng/ml at 6 weeks after immunization. A closer look at the booster group's IgG antibodies revealed that subclass IgG2a was predominantly produced at levels three times higher than IgG1. While the predominance of IgG2a to IgG1 has been observed previously in mice immunized i.d. with 1,000 CFU LVS (21), we are the first to report this observation using a Tul4-based vaccine. Thus, a strong and predominant IgG2a response may signal protection against a lethal tularemia challenge in mice. The more significant IgG2a response also indicates a bias toward the Th1 immune response, and because high IgG antibody titers alone are not associated with protection against a lethal dose of LSV, this implies that cell-mediated immunity may be responsible for the additional clearance of tularemia pathogen and survival of mice. In fact, while passive transfer of high-titer anti-LSV IgG protects against i.p. challenge with 3,000 LD50 in normal BALB/c mice, transfer of anti-LSV IgG alone does not protect athymic nu/nu+ mice from challenge (21). However, once nu/nu+ mice are reconstituted with T cells from normal mice, passive transfer of anti-LSV IgG does protect against challenge with LSV. Furthermore, gamma interferon (IFN-γ) may provide additional protection, as anti-LSV IgG passive transfer to normal and nu/nu+ mice reconstituted with T cells did not survive LSV challenge in the presence of anti-IFN-γ (21). Moreover, it has also been demonstrated that T-cell immunity is essential for long-term survival of mice challenged with tularemia (9) In humans, a recent study has demonstrated that cellular immunity persists for 30 years after vaccination with LVS (10) and highlights the importance of cell-mediated immunity to tularemia for long-term protection. Thus, humoral responses alone may not be enough to protect against challenge with tularemia. Therefore, future studies should also characterize cellular immunity rendered via immunization with Ad/opt-Tul4 vaccine with and without booster shots.
The role of humoral immunity to intracellular pathogens has been equivocal. Typically, antibodies are thought to work against extracellular pathogens, whereas cell-mediated immunity has a more important role in the clearance of intracellular pathogens such as F. tularensis. However, it has been shown that antibodies are more complex than we previously understood, enhancing innate immunity and regulating the inflammatory response, and are active in clearing intracellular pathogens such as Cryptococcus neoformans and Mycobacterium tuberculosis (3). Without an appropriate antibody response, the immune system may not be able to clear a tularemia infection. Strong early protection against colonization is highly dependent on B-cell activity (6), and F. tularensis has been shown to spread from the lungs via the hematogenous route to systemic organs (11). We observed a low antibody titer (about 250 ng/ml IgG) in mice immunized with F. tularensis LVS. Therefore, from this study and others (9, 21), it can be inferred that B-cell activity is required for full protection. Additionally, a highly virulent strain of F. tularensis may completely shut down the inflammatory responses (17); thus, circulating anti-Tul4 antibodies raised in response to the Ad/opt-Tul4 vaccine may be especially important in the early stages of preventing high colonization rates of F. tularensis. Our Ad/opt-Tul4 vaccine has been shown to elicit sufficient B-cell activity and provides a 60% protection rate against LVS i.p. challenge.
Mucosal antibody responses producing IgA antibodies have previously been observed following vaccination with F. tularensis LVS (18). Although not evaluated in this study, IgA antibodies in the mucosal lining of respiratory tracts can prohibit adhesion and motility of F. tularensis. Our Ad/opt-Tul4 vaccine is an attractive candidate because of the innate ability of adenovirus to elicit a mucosal response. Further experimentation involving intranasal (i.n.) immunization may provide exciting results. Theoretically, challenge with F. tularensis LVS via the i.n. route may show that our vaccine can provide better protection due to the strong humoral response compared with that for LVS. A bioterrorist attack would conceivably be directed toward the inhalation route, and the spread of the bacteria may be halted before vital organs are reached. In this study, Ad/opt-Tul4's efficacy has been assessed via the i.m. and i.d. routes. Although some studies have observed that adenovirus-based vaccines against melanoma are optimal when given through the i.d. route (14), we observed that the i.d. route is ineffective for an adenovirus-vectored vaccine against tularemia. This raises the question of the efficacy of additional routes; perhaps an i.n. or oral route could elicit a more varied and robust immunogenic response. Therefore, additional experimentation is also needed to assess these possibilities.
We reiterate the need for further experimentation to assess the T-cell activity that Ad/opt-Tul4 induces to provide a more complete profile for the vaccine. In an effort to develop a safe next-generation subunit vaccine, a multitude of membrane proteins have been identified in F. tularensis LVS that can stimulate T cells from LVS-primed individuals (22, 26, 27). Recombinant Tul4 protein has been shown to be recognized by T cells, and the responding T cells produced IL-2 and IFN-γ. Our Ad/opt-Tul4 vaccine utilizes Tul4, but mechanisms of protection against tularemia challenge must be further elucidated. Given the efficacy of the Ad/opt-Tul4 vaccine and its ability to elicit a strong humoral response, our next-generation vaccine candidate shows potential pending additional studies.
ACKNOWLEDGMENTS
This work was supported by the U.S. Public Service research grant AI059225 (M.Z.) from the National Institute of Allergy and Infectious Diseases and an internal research fund (M.Z.) from the Texas Tech University Health Sciences Center Paul L. Foster School of Medicine.
Footnotes
Published ahead of print 25 January 2012
REFERENCES
- 1. André S, et al. 1998. Increased immune response elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usage. J. Virol. 72: 1497–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boyer JL, Kobinger G, Wilson JM, Crystal RG. 2005. Adenovirus-based genetic vaccines for biodefense. Hum. Gene Ther. 16: 157–168 [DOI] [PubMed] [Google Scholar]
- 3. Casadevall A, Pirofski LA. 2006. A reappraisal of humoral immunity based on mechanisms of antibody-mediated protection against intracellular pathogens. Adv. Immunol. 91: 1–44 [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention 2000. Biological and chemical terrorism: strategic plan for preparedness and response. MMWR Recommend. Rep. 49(RR04): 1–14 [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention 2002. Tularemia, United States, 1990–2000. MMWR Morb. Mortal. Wkly. Rep. 51: 182–184 [Google Scholar]
- 6. Culkin SJ, Rhinehart-Jones T, Elkins KL. 1997. A novel role for B cells in early protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J. Immunol. 158: 3277–3284 [PubMed] [Google Scholar]
- 7. Dennis DT, et al. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 285: 2763–2773 [DOI] [PubMed] [Google Scholar]
- 8. Eigelsbach HT, Hornick RB, Tulis JJ. 1967. Recent studies on live tularemia vaccine. Med. Ann. Dist. Columbia 36: 282–286 [PubMed] [Google Scholar]
- 9. Elkins KL, Rhinehart-Jones T, Nacy CA, Winegar RK, Fortier AH. 1993. T-cell-independent resistance to infection and generation of immunity to Francisella tularensis. Infect. Immun. 61: 823–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eneslätt K, et al. 2011. Persistence of cell-mediated immunity three decades after vaccination with the live vaccine strain of Francisella tularensis. Eur. J. Immunol. 41: 974–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Forestal CA, et al. 2007. Francisella tularensis has a significant extracellular phase in infected mice. J. Infect. Dis. 196: 134–137 [DOI] [PubMed] [Google Scholar]
- 12. Fortier AH, Slayter MV, Ziemba R, Meltzer MS, Nacy CA. 1991. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect. Immun. 59: 2922–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Golovliov I, et al. 1995. Adjuvanticity of ISCOMs incorporating a T cell-reactive lipoprotein of the facultative intracellular pathogen Francisella tularensis. Vaccine 13: 261–267 [DOI] [PubMed] [Google Scholar]
- 14. Hangalapura BN, et al. 2011. Delivery route, MyD88 signaling and cross-priming events determine the anti-tumor efficacy of an adenovirus based melanoma vaccine. Vaccine 29: 2313–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He TC, et al. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. U. S. A. 95: 2509–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Isherwood KE, Titball RW, Davies DH, Felgner PL, Morrow WJ. 2005. Vaccination strategies for Francisella tularensis. Adv. Drug Deliv Rev. 57: 1403–1414 [DOI] [PubMed] [Google Scholar]
- 17. Kirimanjeswara GS, Olmos S, Bakshi CS, Metzger DW. 2008. Humoral and cell-mediated immunity to the intracellular pathogen Francisella tularensis. Immunol. Rev. 225: 244–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koskela P, Salminen A. 1985. Humoral immunity against Francisella tularensis after natural infection. J. Clin. Microbiol. 22: 973–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lubeck MD, et al. 1997. Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat. Med. 3: 651–658 [DOI] [PubMed] [Google Scholar]
- 20. National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 21. Rhinehart-Jones TR, Fortier AH, Elkins KL. 1994. Transfer of immunity against lethal murine Francisella infection by specific antibody depends on host gamma interferon and T cells. Infect. Immun. 62: 3129–3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sandström G, Tarnvik A, Wolf-Watz H. 1987. Immunospecific T-lymphocyte stimulation by membrane proteins from Francisella tularensis. J. Clin. Microbiol. 25: 641–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shen H, Chen W, Conlan JW. 2004. Susceptibility of various mouse strains to systemically- or aerosol-initiated tularemia by virulent type A Francisella tularensis before and after immunization with the attenuated live vaccine strain of the pathogen. Vaccine 22: 2116–2121 [DOI] [PubMed] [Google Scholar]
- 24. Shiver JW, et al. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415: 331–335 [DOI] [PubMed] [Google Scholar]
- 25. Sjöstedt A, Sandstrom G, Tarnvik A. 1992. Humoral and cell-mediated immunity in mice to a 17-kilodalton lipoprotein of Francisella tularensis expressed by Salmonella typhimurium. Infect. Immun. 60: 2855–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sjöstedt A, Sandstrom G, Tarnvik A, Jaurin B. 1990. Nucleotide sequence and T cell epitopes of a membrane protein of Francisella tularensis. J. Immunol. 145: 311–317 [PubMed] [Google Scholar]
- 27. Surcel HM. 1990. Diversity of Francisella tularensis antigens recognized by human T lymphocytes. Infect. Immun. 58: 2664–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tan Y, Hackett NR, Boyer JL, Crystal RG. 2003. Protective immunity evoked against anthrax lethal toxin after a single intramuscular administration of an adenovirus-based vaccine encoding humanized protective antigen. Hum. Gene Ther. 14: 1673–1682 [DOI] [PubMed] [Google Scholar]
- 29. Vorburger SA, Hunt KK. 2002. Adenoviral gene therapy. Oncologist 7: 46–59 [DOI] [PubMed] [Google Scholar]
- 30. Xu Q, Arevalo MT, Pichichero ME, Zeng M. 2006. A new complementing cell line for replication-incompetent E1-deleted adenovirus propagation. Cytotechnology 51: 133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zeng M, Cerniglia GJ, Eck SL, Stevens CW. 1997. High-efficiency stable gene transfer of adenovirus into mammalian cells using ionizing radiation. Hum. Gene Ther. 8: 1025–1032 [DOI] [PubMed] [Google Scholar]
- 32. Zeng M, et al. 2001. AdEasy system made easier by selecting the viral backbone plasmid preceding homologous recombination. Biotechniques 31: 260–262 [DOI] [PubMed] [Google Scholar]
- 33. Zeng M, et al. 2007. Protective immunity against botulism provided by a single dose vaccination with an adenovirus-vectored vaccine. Vaccine 25: 7540–7548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeng M, Xu Q, Hesek ED, Pichichero ME. 2006. N-fragment of edema factor as a candidate antigen for immunization against anthrax. Vaccine 24: 662–670 [DOI] [PubMed] [Google Scholar]