Abstract
Suitably controlled serosurveillance surveys are essential for evaluating human papillomavirus (HPV) immunization programs. A panel of plasma samples from 18-year-old females was assembled, the majority of the samples being from recipients of the bivalent HPV vaccine. Antibody specificities were evaluated by three independent laboratories, and 3 pools that displayed no antibodies to any HPV type tested or intermediate or high levels of antibody to HPV16, HPV18, HPV31, and HPV45 were created. These pools will be useful as control reagents for HPV serology.
TEXT
The link between persistent infection with high-risk human papillomavirus (HPV) and the development of cervical cancer, the second most common cancer in women worldwide, is well established. The development of highly efficacious vaccines against the two most prevalent genotypes, HPV16 and HPV18 (13), represents one of the most significant advances in human vaccination for many years (12, 15, 19). Emerging data from efficacy trials also suggest some degree of cross-protection against nonvaccine types, including HPV31 and HPV45 (1, 15).
Serologic assays for the evaluation of HPV vaccine responses are currently limited to an enzyme-linked immunosorbent assay (ELISA) (9), three multiplex assay systems (4, 6, 14), and a pseudovirus neutralization assay (2), and emerging data suggest that each system has some utility for characterizing HPV vaccine antibody specificity (3, 18). Protection against vaccine types is thought to be mediated by neutralizing antibodies (17), and while the mechanism of vaccine-induced cross-protection is uncertain, the measurement of antibodies against nonvaccine types (5, 11) may be useful as a potential correlate or surrogate of cross-protection (16). The only internationally available serologic standard is a WHO International Standard (IS) for HPV16 antibodies, derived from subjects with natural HPV16 infection (7), although a candidate IS for HPV18 antibodies, derived from subjects with natural HPV18 infection, is currently being characterized.
The aim of this study was to create serologic reference reagents for use as quality controls in postimmunization serosurveillance surveys able to control for responses against vaccine (HPV16 and HPV18) and nonvaccine (HPV31 and HPV45) types. While ISs are essential for assigning an international unitage of antibody levels, the daily quality control of serological tests needs to have access to secondary standards that are available in larger amounts than the IS itself. Such secondary standards should preferably be characterized by analysis of antibody level in parallel with the IS, to assign a traceable international unitage to them (21). The reference reagents described in this paper have the high antibody levels that are typical of vaccinated subjects, and this makes them easier to use as reference standards for laboratories that perform serology mostly on vaccinated subjects, who have antibody levels substantially higher than found in the IS.
Twenty-seven citrated plasma packs not required for transfusion were obtained from NHS Blood and Transplant and tested negative for anti-HIV antibodies, anti-hepatitis C virus (HCV) antibodies, and HBsAg. The plasma packs were selected from females 18 years old in September 2009, of which a high proportion would have been vaccinated with the bivalent vaccine as part of the United Kingdom National HPV Immunization Programme “catch up” campaign (20). Serum is thought to be the ideal sample for HPV neutralization assays, due to the potential for heparin to interfere with the assay (2); however, as these plasma samples were collected as citrated plasma packs, this is not expected to be an issue.
A plasma panel containing one aliquot of each coded sample was formally distributed to (i) laboratory A (Centre for Infections, Health Protection Agency, United Kingdom) for testing in a neutralization assay containing Optiprep-purified pseudoviruses representing HPV16, HPV18, HPV31, HPV45, and the control bovine papillomavirus (BPV) made by transfection of 293TT cells with the appropriate bicistronic psheLL L1-L2 plasmid and the secreted alkaline phosphatase (SEAP) reporter vector (http://home.ccr.cancer.gov/lco/plasmids.asp) (2) with transduction of susceptible target cells resolved using the chemiluminescent SEAP reporter gene assay (Roche) and Glomax multidetection system (Promega), (ii) laboratory B (Global WHO HPV Reference Laboratory, Centers for Disease Control and Prevention, Atlanta, GA) for testing in the pseudovirus neutralization assay containing HPV16, HPV18, and the control BPV and detected using the SEAP reporter gene assay (BD Biosciences) and a Victor 2 luminometer (Perkin Elmer), and (iii) laboratory C (Global WHO HPV Reference Laboratory, Malmö University Hospital, Sweden) for testing in a multiplex serology assay with the following non-reporter-containing HPV L1-L2 pseudoviruses: α1 (HPV32), α2 (HPV3), α7 (HPV18, HPV45, and HPV68), α9 (HPV16, HPV31, HPV33, HPV52, and HPV58), α10 (HPV6 and HPV11), β1 (HPV5), β2 (HPV15 and HPV38), and β3 (HPV76) according to published methodology (6).
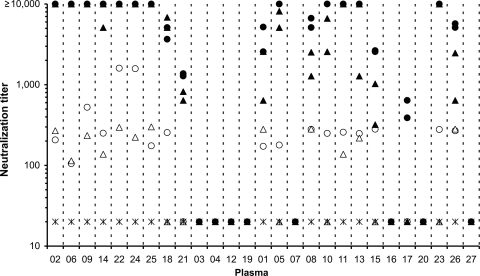
Eight plasma samples (29.6%) demonstrated no neutralization against any of the four HPV types tested and 18 (66.7%) neutralized both HPV16 and HPV18 (12 of these also neutralized both HPV31 and HPV45), while 1 sample (3.7%) was positive for HPV16 alone, suggesting a natural HPV16 infection (Fig. 1). No neutralization of the control BPV pseudovirus was seen (all titers < 40). Based on sample positivity alone, there was 100% concordance (interrater agreement, κ = 1.000; [Stata 10.1; StataCorp, TX]) between the neutralization data sets from laboratories A and B. In addition, there was also very good agreement between the magnitudes of neutralizing antibody titers obtained by both laboratories for HPV16 (96% concordance, κ = 0.945) and HPV18 (85% concordance, κ = 0.797) when stratified by discrete titer intervals (<40, 40 to 160, 160 to 640, 640 to 2,560, 2,560 to 10,240, and >10,240).
Fig 1.
Neutralization of HPV16 (filled circles), HPV18 (filled triangles), HPV31 (open circles), HPV45 (open triangles), and control BPV (asterisks) pseudoviruses by using individual plasmas. HPV16, HPV18, and BPV neutralization titers were assembled using data generated by both laboratories A and B, while HPV31 and HPV45 titers were determined by laboratory A only. Negative values (<40) were assigned a titer of 20, and a maximum level was assigned when titers were ≥10,000. Plasma samples are arranged according to whether they were selected for inclusion in the high HPV16/18 (P02, P06, P09, P14, P22, P24, and P25), intermediate HPV16/18 (P18 and P21), and HPV-negative (P03, P04, P12, and P19) antibody pools.
A comparison of neutralization (laboratory A) and multiplex serological (laboratory C) assay data sets showed 100% concordance (κ = 1.000) for HPV16 and HPV18, while for HPV31 and HPV45, concordance was lower, at 96.3% (κ = 0.922; McNemar test for discrepancies, P = 1.000 [Stata 10.1]) and 85.2% (κ = 0.705; P = 0.617), respectively, although these discrepancies were not significant. Of the eight plasma samples for which no neutralization activity was detected, four of these had serological reactivity against one or more α or β HPV types not included in the neutralization panel (P07 [HPV6 and HPV32], P16 [HPV6], P20 [HPV68 and HPV76], and P27 [HPV38]).
These data were used to assemble HPV-negative, intermediate HPV16/18 antibody, and high HPV16/18 antibody plasma pools whose specificity was confirmed by laboratory A in the pseudovirus neutralization assay (Table 1). The WHO International Standard for HPV16 antibodies (IS16; code 05/134, 10 international units [IU]/ml) demonstrated type-specific neutralization of HPV16 at levels consistent with natural infection (Table 1) (7).
Table 1.
Neutralization of vaccine and nonvaccine HPV pseudoviruses by plasma pools and IS16
| HPV antibody reagent | Avg neutralization titer (%CV) against indicated HPV pseudovirusa |
||||
|---|---|---|---|---|---|
| HPV16 | HPV18 | HPV31 | HPV45 | BPV | |
| HPV negative | <40 (0) | <40 (0) | <40 (0) | <40 (0) | <40 (0) |
| Intermediate HPV16/18 | 3,538 (2.1) | 5,655 (1.7) | 40 (13.9) | <40 (0) | <40 (0) |
| High HPV16/18 | 161,838 (2.4) | 163,866 (4.8) | 1,271 (6.7) | 148 (3.2) | <40 (0) |
| IS16b | 295 (12.9) | <40 (0) | <40 (0) | <40 (0) | <40 (0) |
Average neutralization titers derived from three experiments (with percent coefficient of variation [%CV] of the log10 titers in parentheses).
IS16, International Standard for HPV16 antibodies.
The HPV-negative plasma pool (Table 1) comprised four plasma samples (P03, P04, P12, and P19) that were negative for neutralizing antibodies against HPV16, HPV18, HPV31, and HPV45 (Fig. 1) and negative for binding antibodies against all of the α and β HPV types by multiplex serology.
The intermediate HPV16/18 antibody plasma pool (Table 1) comprised two plasma samples (P18 and P21) that displayed intermediate levels of neutralizing antibody against HPV16 and HPV18; plasma sample P18 also displayed some neutralizing antibody activity against HPV31 (Fig. 1). Both plasma samples were positive for binding antibodies against HPV16 and HPV18, and apart from P18 having some reactivity against HPV32 (α1), these samples were negative for binding antibodies against other α and β HPV types tested by multiplex serology. The median HPV16 neutralizing antibody titer for this plasma pool when expressed as IU per milliliter was 187 IU/ml (interquartile range [IQR], 126 to 211 IU/ml; n = 3), consistent with a low-level to intermediate vaccine response (7).
The high HPV16/18 antibody plasma pool (Table 1) comprised seven plasma samples (P02, P06, P09, P14, P22, P24, and P25) that displayed high levels of neutralizing antibody against HPV16 and HPV18 and intermediate levels against HPV31 and HPV45 (Fig. 1). The individual plasmas were positive for multiple α and β HPV types tested by multiplex serology, including α1 (HPV32), α2 (HPV3), α7 (HPV18, HPV45, and HPV68), α9 (HPV16, HPV31, HPV33, HPV52, and HPV58), α10 (HPV6), β1 (HPV5), β2 (HPV15 and HPV38), and β3 (HPV76). The median HPV16 neutralizing antibody level was 6,668 IU/ml (IQR, 5,356 to 8,023 IU/ml; n = 3), consistent with a high-level vaccine antibody response (7).
Overall, the results obtained from testing the plasma pools were similar to those expected from the averages of the responses for the individual plasma samples.
Antibody titers derived from natural infection are significantly lower than from vaccinees (8), and HPV16, HPV18, HPV31, and HPV45 coinfections, particularly in this age group, are rare (10; R. Howell-Jones, N. de Silva, M. Akpan, P. Oakeshott, C. Carder, L. Coupland, M. Sillis, H. Mallinson, V. Ellis, D. Frodsham, T. I. Robinson, O. N. Gill, S. Beddonis, and K. Soldan, submitted for publication). Together, these data suggest that the plasma samples with high-titer antibodies against both HPV16 and HPV18 (including those with reactivity against HPV31 and HPV45) are almost certainly a result of vaccination. Our panel is not perfectly representative of most vaccinees, due to the age of vaccination in the catch-up cohort being rather older than the target age for routine immunization. However, the neutralization responses measured using these plasma pools are indistinguishable from the responses seen with sera taken from 13- to 14-year-old vaccinees (5). Low levels of antibodies generated by vaccination and/or natural infection toward other α and β HPV types, as suggested from multiplex serology, cannot be ruled out.
Surveillance studies are important for estimating the prevalence of an infectious agent in, or for monitoring the impact of a vaccine on, a population or demographic group. With the recent introduction of the HPV vaccines, many countries are conducting postvaccine surveillance, including seroepidemiology surveys. These plasma pools will be useful as reference reagents. They are currently available as 250-μl aliquots of liquid plasma archived at −80°C and can be obtained from the National Institute for Biological Standards and Control.
ACKNOWLEDGMENTS
This work was supported by the United Kingdom Medical Research Council (grant number G0701217). The work performed in Sweden and the United States was supported by the WHO.
The findings and conclusions in this report are ours and do not necessarily represent the views of the funding agency.
We are indebted to John T. Schiller and Christopher B. Buck (National Cancer Institute, Bethesda, MD) for access to the pseudovirus clones used in this study.
We declare no conflict of interest.
Footnotes
Published ahead of print 25 January 2012
REFERENCES
- 1. Brown DR, et al. 2009. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J. Infect. Dis. 199:926–935 [DOI] [PubMed] [Google Scholar]
- 2. Buck CB, Pastrana DV, Lowy DR, Schiller JT. 2005. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol. Med. 119:445–462 [DOI] [PubMed] [Google Scholar]
- 3. Dessy FJ, et al. 2008. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum. Vaccin. 4:425–434 [DOI] [PubMed] [Google Scholar]
- 4. Dias D, et al. 2005. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin. Diagn. Lab Immunol. 12:959–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Draper E, et al. 2011. Neutralization of non-vaccine human papillomavirus pseudoviruses from the A7 and A9 species groups by bivalent HPV vaccine sera. Vaccine 29:8585–8590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Faust H, Knekt P, Forslund O, Dillner J. 2010. Validation of multiplexed human papillomavirus serology using pseudovirions bound to heparin-coated beads. J. Gen. Virol. 91:1840–1848 [DOI] [PubMed] [Google Scholar]
- 7. Ferguson M, Wilkinson DE, Heath A, Matejtschuk P. 2011. The first international standard for antibodies to HPV 16. Vaccine 29:6520–6526 [DOI] [PubMed] [Google Scholar]
- 8. Frazer IH. 2010. Measuring serum antibody to human papillomavirus following infection or vaccination. Gynecol. Oncol. 118:S8–S11 [DOI] [PubMed] [Google Scholar]
- 9. Harper DM, et al. 2004. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 364:1757–1765 [DOI] [PubMed] [Google Scholar]
- 10. Jit M, et al. 2007. Prevalence of human papillomavirus antibodies in young females in England. Br. J. Cancer 97:989–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kemp TJ, et al. 2011. HPV16/18 L1 VLP vaccine induces cross-neutralizing antibodies that may mediate cross-protection. Vaccine 29:2011–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kjaer SK, et al. 2009. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev. Res. (Phila.) 2:868–878 [DOI] [PubMed] [Google Scholar]
- 13. Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. 2011. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int. J. Cancer 128:927–935 [DOI] [PubMed] [Google Scholar]
- 14. Michael KM, et al. 2008. Seroprevalence of 34 human papillomavirus types in the German general population. PLoS Pathog. 4:e1000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paavonen J, et al. 2009. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 374:301–314 [DOI] [PubMed] [Google Scholar]
- 16. Plotkin SA. 2008. Vaccines: correlates of vaccine-induced immunity. Clin. Infect. Dis. 47:401–409 [DOI] [PubMed] [Google Scholar]
- 17. Schiller JT, Day PM, Kines RC. 2010. Current understanding of the mechanism of HPV infection. Gynecol. Oncol. 118:S12–S17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schiller JT, Lowy DR. 2009. Immunogenicity testing in human papillomavirus virus-like-particle vaccine trials. J. Infect. Dis. 200:166–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schiller JT, Lowy DR. 2010. Vaccines to prevent infections by oncoviruses. Annu. Rev. Microbiol. 64:23–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. United Kingdom Department of Health 2011. Human papillomavirus (HPV). In Salisbury D, Ramsay M, Noakes K. (ed), Immunisation against infectious disease—“the green book.” HMSO, London, United Kingdom [Google Scholar]
- 21. World Health Organization 2010. Human papillomavirus laboratory manual, 1st ed, 2009. http://whqlibdoc.who.int/hq/2010/WHO_IVB_10.12_eng.pdf World Health Organization, Geneva, Switzerland [Google Scholar]