Abstract
NGO0579 is annotated copA in the Neisseria gonorrhoeae chromosome, suggesting that it encodes a cation-transporting ATPase specific for copper ions. Compared to wild-type cells, a copA mutant was more sensitive to killing by copper ions but not to other transition metals. The mutant also accumulated a greater amount of copper, consistent with the predicted role of CopA as a copper efflux pump. The copA mutant showed a reduced ability to invade and survive within human cervical epithelial cells, although its ability to form a biofilm on the surface of these cells was not significantly different from that of the wild type. In the presence of copper, the copA mutant exhibited increased sensitivity to killing by nitrite or nitric oxide. Therefore, we concluded that copper ion efflux catalyzed by CopA is linked to the nitrosative stress defense system of Neisseria gonorrhoeae. These observations suggest that copper may exert its effects as an antibacterial agent in the innate immune system via an interaction with reactive nitrogen species.
INTRODUCTION
Neisseria gonorrhoeae is a human-adapted bacterial pathogen that causes the sexually transmitted infection gonorrhoea. Gonococcal infection is associated primarily with an inflamed mucosa of the urethra in males. However, in females, it often results in asymptomatic infection of the cervix (4). During colonization of the human host, the gonococcus interacts with epithelial cells and phagocytic cells of the innate immune system. These cells employ a variety of strategies to defend against gonococcal infection, including production of reactive oxygen and reactive nitrogen species (ROS and RNS, respectively) (24). Defense against RNS may be of particular importance because cervical epithelial cells produce nitric oxide (NO) in response to gonococcal infection (3). The gonococcus itself generates NO, as it possesses a nitrite reductase (AniA) which converts nitrite (NO2−) to NO under microaerobic conditions (13). An NO reductase (NorB) further catalyzes the conversion of NO to nitrous oxide (N2O) via an energy-conserving respiratory process (10), and notably, formation of a gonococcal biofilm on cervical epithelial cells requires both AniA and NorB (5).
Recently, we described the properties of NmlR, the only transcription factor from the MerR family of regulators encoded within the N. gonorrhoeae genome (12). NmlR controls the expression of a small regulon comprised of nmlR itself (NGO0602), adhC-estD (NGO0601-NGO0600, encoding an alcohol dehydrogenase pseudogene and a thioesterase gene, respectively), trxB (NGO0580, encoding thioredoxin reductase), and copA (NGO0579, encoding a putative cation-transporting ATPase). In subsequent studies, we showed that a trxB mutant was sensitive to killing by NO, whereas an estD mutant was sensitive to killing by S-nitrosoglutathione (GSNO) and was unable to grow in liquid cultures in the presence of nitrite (20, 21). Both mutants showed reduced abilities to form a biofilm on transformed human cervical epithelial cells (THCECs) and also to invade and survive within primary cervical epithelial (pex) cells.
The presence of a gene annotated copA within the NmlR regulon is intriguing. In many Gram-negative bacteria, copA protects against copper toxicity, but its expression is under the control of a copper-sensing MerR family regulator, CueR (18). CueR is not present in N. gonorrhoeae, and the gonococcus also lacks additional proteins involved in copper tolerance, such as the cuprous oxidase CueO. In Escherichia coli, there is an additional system (the cus system) that confers tolerance to excess copper under microaerobic conditions (18). However, this set of genes is also absent from the chromosome of N. gonorrhoeae. Although copA (NGO0579) is inferred to encode a copper-transporting ATPase, there has been no analysis to define its function in the gonococcus. We report herein the identification of CopA as a copper efflux pump and, furthermore, the characterization of a copA mutant of N. gonorrhoeae.
The antimicrobial effects of copper have been known for centuries (2), and mechanisms of copper toxicity at the biochemical level are understood reasonably well. Yet the recognition that copper might have a role in the innate immune system is a more recent development (32), although it has been realized for some time that the copper deficiency observed in Menkes syndrome patients makes these individuals more susceptible to bacterial infection (1). This leads to the question of how copper might be used by the host to exert an antimicrobial effect. We now provide evidence that the antimicrobial effects of copper involve its interaction with reactive nitrogen species. These are the first data to describe a role for copper in mediating the nitrosative stress response to infection and, furthermore, provide a mechanistic explanation for the bactericidal effect exerted by copper.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
N. gonorrhoeae strain 1291 was cultured routinely on GC base (Oxoid) supplemented with 1% (vol/vol) IsoVitalex (Becton Dickinson) and 5% (vol/vol) sodium bicarbonate. Growth on solid medium was performed at 37°C in the presence of 5% CO2. Where required, kanamycin or spectinomycin was used at a concentration of 100 or 50 μg/ml, respectively. Unless otherwise specified, liquid cultures were grown aerobically at 37°C by shaking 150-ml cultures in 250-ml Erlenmeyer flasks at 100 rpm.
Construction of mutant strains.
The copA gene was amplified by PCR using the primers copA-F (5′-CCATATCGCCTTTGAGTGC-3′) and copA-R (5′-ATTGGTTGGGATTTTTCAGC-3′), cloned into pGEM-T Easy, and disrupted through insertion of a kanamycin resistance cassette into the unique CspCI restriction site. The resulting plasmid, pGEM-T::copA::kan, was linearized with EaeI and used to transform N. gonorrhoeae 1291. Kanamycin-resistant colonies were evident after 2 days. Correct insertion into the chromosome was verified by PCR using primers external to the construct used for mutagenesis (copA-F-check [5′-CTTCCTCACTCGGCAAACC-3′] and copA-R-check [5′-GCAACACTTGAATGCTGTCG-3′]), as well as primers complementary to the kanamycin resistance cassette (Kan-F/R).
The copA mutant was complemented by inserting an active copy of the copA gene under the control of its own promoter into the proB gene in the chromosome (29). The wild-type copA gene, including 300 bp each of the upstream and downstream flanking regions, was amplified by PCR using the primers copA-cmpl-F (5′-CATCGTCCCGGGGAGAAACCGCGCCATCAATTC-3′) and copA-cmpl-R (5′-CATCGTCTTAAGGTTGCCGCCTTCGTCAAAAAAAC-3′). The PCR product was inserted into vector pCTS32 (29) between the XmaI and AfiII sites, and the resulting plasmid, pCTS32::copA, was linearized with NdeI and transformed into N. gonorrhoeae strain 1291 copA. Spectinomycin-resistant colonies, indicative of complementation, were evident after 2 days. Correct recombination into the chromosome was verified by PCR using primers copA-cmpl-F and proB-check-F (5′-AATTGCATGGTAGTCCTTG-3′).
Bacterial killing assays.
Disc diffusion susceptibility assays were performed by placing paper discs (Oxoid) containing 5 μl of each stress reagent onto a lawn of N. gonorrhoeae (approximately 106 cells) on solid medium. Plates were incubated overnight, and the zones of clearing around the discs were measured. Where required, copper nitrate (1 to 10 μM) was included during preparation of the solid medium. For liquid copper sensitivity assays, cultures were grown to the early exponential phase (optical density at 600 nm [OD600], ∼0.3), and 1 ml of the appropriate concentration of copper sulfate (0 to 600 μM) was added. Growth rates were monitored for a further 3 to 4 h, and cell viability was determined by serial dilution and plating on solid medium. Stocks of copper and sodium nitrite were prepared immediately before use, using deionized water and phosphate-buffered saline (PBS), respectively. Bacterial sensitivity to nitrosative stress was assayed by plating out 5-μl serial dilutions of cells (approximately 107 bacteria/ml) onto solid medium containing various combinations of copper nitrate (1 to 10 μM) plus sodium nitrite (0 to 5 mM) or GSNO (0 to 2 mM). All reagents were purchased from commercial suppliers, except for GSNO, which was prepared following the methods outlined by Hart (9).
Measurement of intracellular copper content.
Bacteria were grown in liquid medium to an OD600 of 0.3 and challenged with 0.2 mM copper sulfate for 30 min. Bacteria were harvested by centrifugation, washed twice with cold PBS, and then washed once with PBS containing 1 mM EDTA to remove any adventitiously bound metal. Cell pellets were dried at 50°C overnight and digested with concentrated nitric acid at 80°C for 2 h. Copper levels were measured using inductively coupled plasma mass spectroscopy (ICP MS) at the School of Earth Sciences, The University of Queensland. The results are presented as the weight ratio of copper to total protein.
Cervical cell invasion/survival and biofilm assays.
Primary cervical epithelial (pex) cells were procured and maintained as described previously (3). Cervical biopsy specimens used to grow primary cell cultures were obtained through the Cooperative Human Tissue Network at The Research Institute at Nationwide Children's Hospital and The Ohio State University (Columbus, OH). The ability of N. gonorrhoeae strains to associate with, invade, or survive within pex cells was determined using a modified gentamicin survival assay (24). N. gonorrhoeae biofilm growth in continuous-flow chambers, either over glass or on THCECs, was determined as described previously (24).
RESULTS
NGO0579 encodes a copper efflux pump and protects N. gonorrhoeae from copper toxicity.
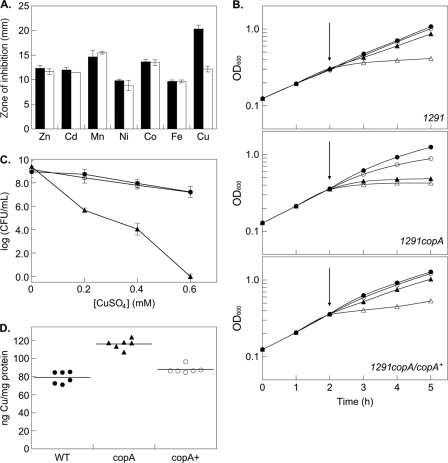
Analysis of the product of NGO0579 showed that it contains a heavy metal-associated (HMA) domain (PF00403) near its N terminus, and an E1-E2 ATPase domain (PF00122). We also identified conserved copper-binding CXXC and CPC motifs in the predicted N-terminal cytoplasmic domain and transmembrane domain, respectively. This in silico analysis suggested that NGO0579 encodes a cation-transporting ATPase that most probably functions as a copper efflux pump. To test this prediction, NGO0579 was disrupted by the insertion of a kanamycin resistance cassette (Fig. 1), and the sensitivity of this mutant to a range of divalent cations was measured using a disc diffusion assay. Figure 2A shows that the zones of clearance for wild-type N. gonorrhoeae 1291 and an NGO0579 mutant were identical for all metal ions tested except for copper, for which a greater zone of clearance was observed for the mutant. This result suggested that NGO0579 was associated with resistance to copper and not to other metal ions. To confirm this observation, cells were grown in liquid culture and challenged with copper sulfate. Figure 2B shows that the addition of increasing concentrations (0 to 0.6 mM) of copper ions attenuated the growth of N. gonorrhoeae 1291, but this effect was more pronounced for the NGO0579 mutant. Viability assays further confirmed that the NGO0579 mutant was more sensitive to killing by copper ions than were wild-type gonococci (Fig. 2C). Complementation of the NGO0579 mutant by inserting a single wild-type copy of NGO0579 into the gonococcal chromosome restored copper resistance to the level seen for wild-type cells (Fig. 2B and C). Given its proposed role in encoding a copper efflux pump, it was also expected that inactivation of NGO0579 would lead to an increase in the intracellular copper concentration. To test this hypothesis, liquid cultures were challenged with a sublethal amount of copper ions, and the concentration of intracellular copper was subsequently measured using ICP MS. Figure 2D shows that the NGO0579 mutant accumulated 40 to 50% more copper ions than did wild-type cells (P < 0.0001) and that the copper level in the complemented mutant returned to the wild-type level (P < 0.0001). Taken together, the above data are consistent with the view that NGO0579 encodes a copper efflux pump, and it is referred to as copA from this point forward.
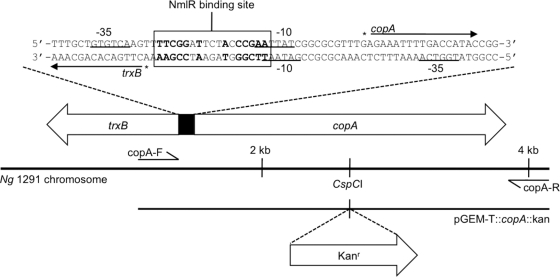
Fig 1.
Arrangement of copA (NGO0579) on the N. gonorrhoeae chromosome. The inset shows the nucleic acid sequence of the promoter region of copA and that of the divergent trxB gene. The −10 and −35 RNA polymerase binding sites are underlined, the NmlR binding site is boxed, the dyad symmetrical sequence is shown in bold, and the transcription start sites are indicated with asterisks (arrows represent the direction of transcription). The method for construction of the copA mutant is also illustrated, with the primers (copA-F and copA-R) used to amplify copA and the unique restriction enzyme site (CspCI) used to insert the kanamycin resistance cassette indicated.
Fig 2.
Copper tolerance in N. gonorrhoeae. (A) Sensitivity of N. gonorrhoeae 1291 wild type (black bars) and 1291 copA (white bars) to zinc sulfate (Zn; 1 M), cadmium chloride (Cd; 10 mM), manganese sulfate (Mn; 100 mM), nickel sulfate (Ni; 50 mM), cobalt chloride (Co; 50 mM), iron nitrate (Fe; 1 M), and copper sulfate (Cu; 40 mM) (P = 0.005), as determined by disc diffusion assays. Error bars indicate ±1 standard deviation from the mean. (B) Effect of copper on the growth rate of N. gonorrhoeae 1291 (wild type), 1291 copA, and complemented 1291 copA. Wild-type and mutant gonococci were allowed to grow in liquid culture for 2 h (OD600, ∼0.3), after which copper sulfate (denoted by arrows) was added to a final concentration of 0 mM (filled circles), 0.2 mM (empty circles), 0.4 mM (filled triangles), or 0.6 mM (empty triangles). These experiments were performed on at least three independent occasions, and representative growth curves are shown. (C) Survival of N. gonorrhoeae 1291 (wild type) (filled circles), 1291 copA (filled triangles), and complemented 1291 copA (empty circles) after 3 h of incubation with various defined concentrations of copper. CFU were determined by recovery of liquid cultures that had been challenged with copper sulfate as described in Materials and Methods. Experiments were performed on at least three independent occasions, and representative growth curves are shown. Error bars indicate ±1 standard deviation from the mean. (D) Intracellular copper content of N. gonorrhoeae 1291 (wild type) (filled circles), 1291 copA (filled triangles), and complemented 1291 copA (empty circles), as determined by ICP MS. Each horizontal line indicates the mean. P values were <0.0001 for all strains.
A copA mutant is defective in growth and survival in cervical epithelial cells but is unaffected in biofilm formation on glass and on transformed cervical cells.
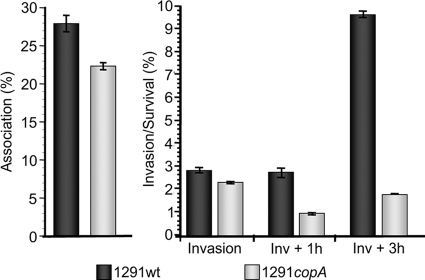
In women, gonococci are typically associated with the cervical epithelium and are able to invade, survive, and replicate within these cells (4). We previously reported that inactivation of estD and trxB, two genes in the NmlR regulon, results in reduced survival of N. gonorrhoeae during challenge of cervical epithelial cells (20, 21). To determine if copA is also important during cervical infection, we tested the ability of N. gonorrhoeae 1291 and 1291 copA to associate with, invade, and survive within pex cells. There was a small but significant difference in the ability of the copA mutant to associate with and invade pex cells in comparison to wild-type gonococci (Fig. 3). In further contrast to wild-type gonococci, survival of the mutant continued to decrease during the first hour following invasion, whereas there was no significant change in invasion/survival in parallel assays performed using wild-type gonococci. Over the next 2 h of infection, the copA mutant partially recovered to survive and replicate within pex cells. However, survival and replication of this mutant were severely attenuated compared to those of the wild-type bacteria, among which the number of viable intracellular bacteria had increased >3-fold over the same challenge period.
Fig 3.
Gonococcal association with and intracellular survival within primary human cervical epithelial (pex) cells. Bars depicted in each graph represent the mean percent total association (left) and invasion or survival (Inv + 1h or Inv + 3h) (right), calculated from three experiments performed in triplicate. Error bars denote the variance of the mean. Percentages were determined as a function of the original inoculum and the number of CFU evident upon plating the cervical cell lysates. A Kruskal-Wallis nonparametric analysis of variance was used to determine the statistical significance of the data obtained for the copA mutant upon comparison to assays performed using wild-type gonococci. The P value was <0.02 for the copA mutant under each condition assayed.
In earlier publications, we also showed that mutation of estD and trxB resulted in a greatly diminished ability of N. gonorrhoeae to form biofilms both on glass and over THCECs (20, 21). Therefore, we evaluated biofilm formation by wild-type 1291 and 1291 copA after 2 days of growth under continuous flow (Table 1; see Fig. S1 in the supplemental material). COMSTAT analyses showed that unlike the trxB and estD mutants, which were deficient in biofilm formation compared to the wild type, the copA mutant showed no significant difference in the ability to form a biofilm on glass or over THCECs (Table 1).
Table 1.
Summary of COMSTAT analysis of biomass, average thickness, and maximum thickness of wild-type strain 1291 and 1291 copA mutant biofilms grown over glass or on THCECsa
| Biofilm substrate | COMSTAT parameter | Value of parameter |
P value | |
|---|---|---|---|---|
| 1291 | 1291 copA | |||
| Glass | Biomass (μm3/μm2) | 22.0 ± 11.2 | 25.0 ± 10.9 | 0.5577 |
| Avg thickness (μm) | 82.7 ± 27.5 | 75.8 ± 23.1 | 0.5663 | |
| Maximum thickness (μm) | 102.8 ± 26.4 | 94.7 ± 26.4 | 0.4592 | |
| THCECs | Biomass (μm3/μm2) | 12.3 ± 7.3 | 11.6 ± 8.0 | 0.8224 |
| Avg thickness (μm) | 47.7 ± 22.7 | 40.5 ± 30.4 | 0.4624 | |
| Maximum thickness (μm) | 103.3 ± 27.3 | 99.3 ± 39.6 | 0.7394 | |
These experiments were performed on at least three different occasions. No statistical difference was observed, as determined using Student's t test.
Role for CopA in protection of N. gonorrhoeae against nitrosative stress.
Although our results clearly showed that copA was associated with copper tolerance, analysis of the N. gonorrhoeae chromosome revealed the absence of other genes that are typically involved in copper tolerance in Gram-negative bacteria. For example, CueR, the copper-responsive MerR family regulator, is absent from the gonococcus. Consistent with this observation, measurement of the transcription levels of copA by quantitative reverse transcription-PCR (qRT-PCR) showed that its expression was independent of the presence of copper ions (data not shown). However, the presence of an NmlR binding site within the trxB-copA intergenic region (Fig. 1) hinted that copA expression and copper homeostasis might be associated with defense against RNS in N. gonorrhoeae.
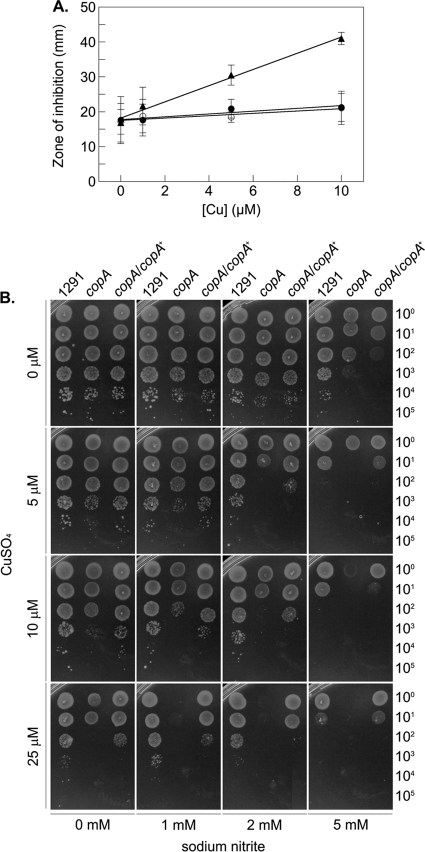
There are a variety of ways in which copper ions can become toxic toward living cells, and this toxicity is largely, but not exclusively, based on the redox chemistry of copper ions (11, 31). It is established that Cu(II) can cause oxidation of NO, thereby leading to formation of the nitrosonium cation, a strong electrophile that reacts with thiols to form S-nitrosothiols (28), whereas the reduction of S-nitrosothiols by Cu(I) can lead to the production of NO (26, 33). Thus, copper ions have the potential to drive RNS cycling between NO and S-nitrosothiols. To test the effect of copper ions on nitrosative stress, we developed a disc diffusion assay in which copper ions were included in the solid medium, and sodium nitrite, functioning as a generator of nitrosative stress, was added to a disc placed upon the agar surface. Figure 4A shows that in the absence of copper ions, the sensitivities to nitrite were similar for wild-type N. gonorrhoeae and the copA mutant, as indicated by comparable zones of clearance. However, as the amount of copper ions in the agar was increased to the sublethal concentration of 10 μM, the zone of clearance for strain 1291 copA was increased compared to that for wild-type or copA-complemented gonococci. This trend was also observed when serial dilutions of cells were grown on solid medium containing combinations of copper and nitrite. As shown in Fig. 4B, the sensitivity of gonococci to nitrite was enhanced in the presence of copper, as evidenced by the increase in the number of bacteria required to obtain visible growth. However, this effect was more pronounced for the 1291 copA mutant.
Fig 4.
Gonococcal sensitivity to nitrite in the presence of increasing concentrations of copper. (A) Disc diffusion assay using 5 M sodium nitrite. Each average zone of clearance obtained for wild-type strain 1291 (filled circles), 1291 copA (empty circles), and complemented 1291 copA (filled triangles) was plotted as a function of the concentration of added copper ions in the medium. Experiments were performed on at least three independent occasions. Error bars indicate ±1 standard deviation from the mean. (B) Growth of serial dilutions (noted on the right axis) of gonococcal strains on solid medium containing 0 to 25 μM copper sulfate and 0 to 5 mM sodium nitrite. These experiments were performed on at least three independent occasions, and representative results are shown.
It seemed likely that the toxicity of nitrite represented an increase in sensitivity to the nitrosonium cation, rather than deriving from nitrite itself, following the conversion of nitrite to NO by nitrite reductase. To test this hypothesis, we replaced nitrite in the above assays with GSNO, a generator of NO. Using the disc diffusion assay, GSNO had no apparent toxic effect on the different strains (data not shown). However, a clear, GSNO-sensitive phenotype was observed for the copA mutant in the serial dilution assay in the presence of sublethal concentrations of copper, whereas no change in survival was observed in the absence of copper (Fig. 5). These data support our hypothesis that copper ions increase the sensitivity of gonococci to NO.
Fig 5.
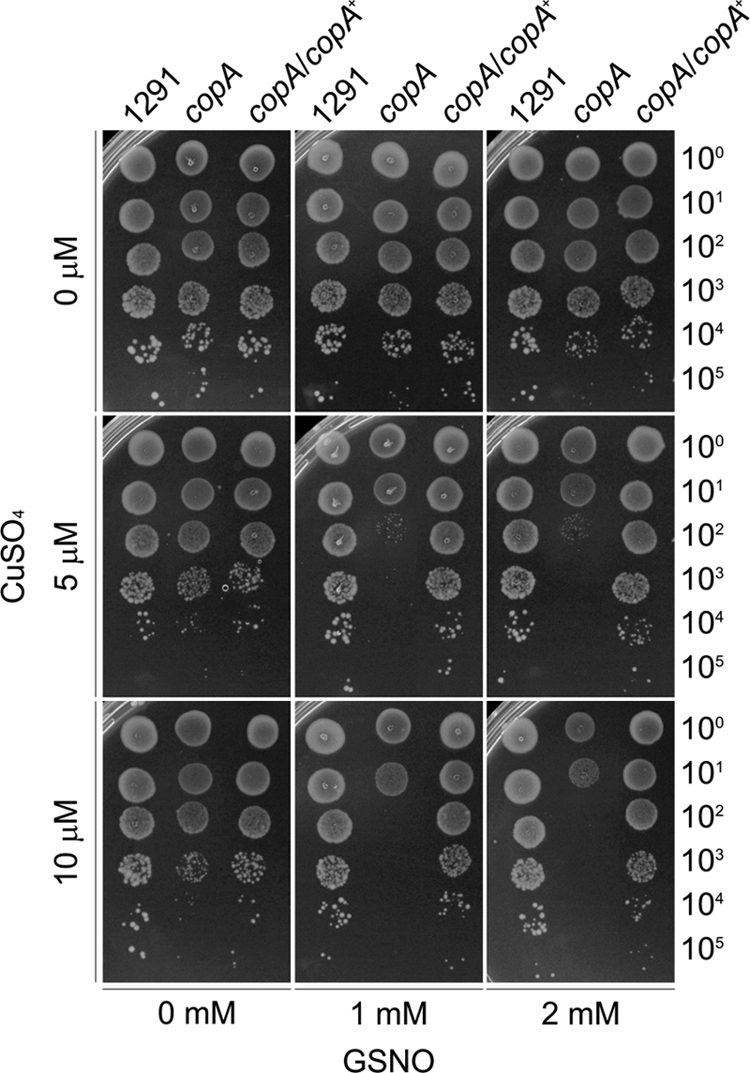
Gonococcal sensitivity to GSNO in the presence of increasing concentrations of copper. Growth is shown for serial dilutions (noted on the right axis) of gonococcal strains on solid medium containing 0 to 10 μM added copper ions and 0 to 2 mM GSNO. These experiments were performed on at least three independent occasions, and representative results are shown.
DISCUSSION
Genes that confer tolerance to copper are widely distributed among bacteria (23), and a common feature of copper tolerance systems is the presence of the cation-transporting ATPase CopA (7). In Gram-negative bacteria, as exemplified by E. coli, copA is part of the cue regulon, and activation of gene expression is dependent upon the transcription factor CueR, a MerR-like transcriptional factor that directly senses the presence of copper in the cytoplasm (18). In Gram-positive bacteria, a copper-sensing repressor, CopY, controls the expression of a set of cop genes involved in copper homeostasis (15). More recently, CsoR, a ubiquitous copper sensor in prokaryotes that controls the cso operon, was also identified (14). However, N. gonorrhoeae appears to possess only the copA gene; none of the other genes associated with the Cue, Cop, and Cso systems of copper tolerance are present in this bacterium. The absence of CueR or CopY in N. gonorrhoeae is also consistent with our observation that copA in N. gonorrhoeae is not inducible by copper ions. In contrast, copA expression in N. gonorrhoeae is controlled by NmlR, a transcription factor from the MerR family that is quite distinct from those involved in sensing cations (16). The mechanism of action of NmlR has not been defined, but the signal for its activation is almost certainly linked to oxidative and/or nitrosative stress (12). Although regulation of the copA gene in N. gonorrhoeae appears to be different from that reported for other bacteria, our observation that mutation of the copA gene led to increased intracellular copper as well as increased sensitivity to copper ions but not to any of the other transition metal ions confirms that this transporter is involved in removal of copper ions from the bacterial cytoplasm.
Apart from regulating its own expression and that of copA, gonococcal NmlR activates the expression of two functional genes, estD and trxB, encoding a thioesterase and a thioredoxin reductase, respectively (12). We have observed that an estD mutant is sensitive to killing in vitro by nitrite and by GSNO (20), whereas a trxB mutant is sensitive to killing by an NO generator (21). These results led us to conclude that components of the NmlR regulon are involved in protection of N. gonorrhoeae against nitrosative stress, although their precise biochemical roles are still under investigation (16). It is established that Cu(I) ions can interact with S-nitroso compounds, leading to the release of nitric oxide (8, 33). Therefore, our observation that a copA mutant was more sensitive to killing by nitrite and an NO generator in the presence of copper is consistent with the view that CopA is also part of a defense system against nitrosative stress.
Recently, we showed that gonococcal biofilm formation was actually enhanced by the addition of an NO generator, suggesting that this molecule was being used as an electron acceptor (6). Under these conditions, a number of genes, including norB, estD, and trxB, are required for biofilm formation. This is consistent with the role of these proteins in protecting the gonococcus against nitrosative stress (20, 21). In contrast, biofilm formation in the copA mutant was essentially the same as that of wild-type gonococci, suggesting that copper ions do not enhance nitrosative stress under these conditions.
It is recognized that during its interaction with cervical epithelial cells, the gonococcus must defend against NO that is produced endogenously, from the reduction of nitrite by AniA (6), as well as exogenously, by the host cell (3). N. gonorrhoeae has well-defined extracytoplasmic defenses against NO: cytochrome c′ is located in the outer membrane (30), and NorB is located in the cytoplasmic membrane. Expression of norB is controlled by NsrR, an NO-sensing repressor that also controls the expression of aniA and dnrN (encoding an iron-sulfur cluster repair enzyme) (19), thereby providing the gonococcus with a dedicated NO defense system. NO is known to inactivate iron-sulfur clusters by forming dinitrosyl-iron complexes (22). This could inhibit key enzymes associated with respiration (e.g., NADH dehydrogenase) and/or glucose metabolism (e.g., 6-phosphogluconate dehydratase). Our observation that a copA mutant is unable to survive in cervical epithelial cells suggests that during cervical infection, control of copper within the bacterial cell is important for an intracellular existence. The presence of copper in the bacterial cytoplasm could potentiate nitrosative stress by causing the uncontrolled release of NO from S-nitrosothiols, which may become toxic to the bacterial cell in the presence or absence of additional host-derived NO. While NorB and cytochrome c′ are able to defend against externally generated NO, they would be much less effective in defending against NO generated within the bacterial cytoplasm. Additionally, given that NO is a freely diffusible molecule, it is not clear if these external defense mechanisms could adequately protect intracellular gonococci from host-derived NO early during the course of infection, as the expression of norB requires NO-dependent derepression of NsrR. Therefore, copper homeostasis may also exist as a second line of defense against host-derived NO.
Copper has long been recognized as an antimicrobial agent in medicine (2). Petris and coworkers recently demonstrated that as part of the inflammatory response, reorganization of copper homeostasis occurs to mobilize copper into the macrophage phagosome (32). Thus, an increasing body of evidence indicates that copper efflux pumps are emerging as potentially important participants in the host-pathogen dynamic. Operons conferring copper resistance are found in Gram-negative pathogens, for example, Salmonella enterica serovar Typhimurium. A copA-golT mutant that lacks the two ATPases able to export copper ions from Salmonella showed significantly reduced survival in macrophages after 24 h compared to that of wild-type cells (17). It is tempting to suggest that this effect of copper may involve an interaction with reactive nitrogen species generated by the macrophage. The presence of a cop operon in a variety of Gram-positive pathogens, including Streptococcus pneumoniae, has also been noted (27). Recently, it was observed that a copA mutant of S. pneumoniae showed significantly reduced virulence in a mouse model of pneumococcal pneumonia (25). The pneumococcus copA mutant was observed to be sensitive to copper when this ion was present at a concentration exceeding 0.1 mM. This is similar to the concentration required to kill gonococci but higher than would be expected if copper were to have a direct antimicrobial role in vivo. However, our observation of RNS toxicity occurring in the presence of only micromolar concentrations of copper supports an important potentiating role for copper in the NOx-dependent killing of pathogens by the innate immune system. To our knowledge, these are the first data to describe a role for copper in contributing to the nitrosative stress response and, furthermore, provide one explanation for the mechanism by which copper exerts a bactericidal effect. Our data suggest that the control of copper efflux is likely essential for N. gonorrhoeae survival in vivo, where the presence of NO may enhance the antimicrobial properties of copper ions.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by program grants 284214 and 565526 from the National Health and Medical Research Council of Australia to M.P.J. and A.G.M. and by Australian Research Council Discovery grant DP0986578 to A.G.M. Additional support for this work came from National Institutes of Health grants AI045728 (to M.A.A.) and AI076398 (to J.L.E.), as well as from a Young Investigator Award from The Research Institute at Nationwide Children's Hospital to J.L.E.
We gratefully acknowledge the Cooperative Human Tissue Network (Columbus, OH) for providing cervical tissue specimens.
Footnotes
Published ahead of print 19 December 2011
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Danks DM, Campbell PE, Stevens BJ, Mayne V, Cartwright E. 1972. Menkes's kinky hair syndrome. An inherited defect in copper absorption with widespread effects. Pediatrics 50:188–201 [PubMed] [Google Scholar]
- 2. Dollwet HHA, Sorenson JRJ. 1985. Historic uses of copper-compounds in medicine. Trace Elem. Med. 2:80–87 [Google Scholar]
- 3. Edwards JL. 2010. Neisseria gonorrhoeae survival during primary human cervical epithelial cell infection requires nitric oxide and is augmented by progesterone. Infect. Immun. 78:1202–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edwards JL, Apicella MA. 2004. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin. Microbiol. Rev. 17:965–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Falsetta ML, et al. 2009. Transcriptional profiling identifies the metabolic phenotype of gonococcal biofilms. Infect. Immun. 77:3522–3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Falsetta ML, McEwan AG, Jennings MP, Apicella MA. 2010. Anaerobic metabolism occurs in the substratum of gonococcal biofilms and may be sustained in part by nitric oxide. Infect. Immun. 78:2320–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fan B, Rosen BP. 2002. Biochemical characterization of CopA, the Escherichia coli Cu(I)-translocating P-type ATPase. J. Biol. Chem. 277:46987–46992 [DOI] [PubMed] [Google Scholar]
- 8. Gaston B. 1999. Nitric oxide and thiol groups. Biochim. Biophys. Acta 1411:323–333 [DOI] [PubMed] [Google Scholar]
- 9. Hart TW. 1985. Some observations concerning the S-nitroso and S-phenylsulfonyl derivatives of L-cysteine and glutathione. Tetrahedron Lett. 26:2013–2016 [Google Scholar]
- 10. Householder TC, Fozo EM, Cardinale JA, Clark VL. 2000. Gonococcal nitric oxide reductase is encoded by a single gene, norB, which is required for anaerobic growth and is induced by nitric oxide. Infect. Immun. 68:5241–5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Imlay JA, Macomber L. 2009. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. U. S. A. 106:8344–8349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kidd SP, Potter AJ, Apicella MA, Jennings MP, McEwan AG. 2005. NmlR of Neisseria gonorrhoeae: a novel redox responsive transcription factor from the MerR family. Mol. Microbiol. 57:1676–1689 [DOI] [PubMed] [Google Scholar]
- 13. Knapp JS, Clark VL. 1984. Anaerobic growth of Neisseria gonorrhoeae coupled to nitrite reduction. Infect. Immun. 46:176–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu T, et al. 2007. CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator. Nat. Chem. Biol. 3:60–68 [DOI] [PubMed] [Google Scholar]
- 15. Lu ZH, Dameron CT, Solioz M. 2003. The Enterococcus hirae paradigm of copper homeostasis: copper chaperone turnover, interactions, and transactions. Biometals 16:137–143 [DOI] [PubMed] [Google Scholar]
- 16. McEwan AG, et al. 2011. Novel bacterial MerR-like regulators their role in the response to carbonyl and nitrosative stress. Adv. Microb. Physiol. 58:1–22 [DOI] [PubMed] [Google Scholar]
- 17. Osman D, et al. 2010. Copper homeostasis in Salmonella is atypical and copper-CueP is a major periplasmic metal complex. J. Biol. Chem. 285:25259–25268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Outten FW, Huffman DL, Hale JA, O'Halloran TV. 2001. The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J. Biol. Chem. 276:30670–30677 [DOI] [PubMed] [Google Scholar]
- 19. Overton TW, et al. 2006. Coordinated regulation of the Neisseria gonorrhoeae-truncated denitrification pathway by the nitric oxide-sensitive repressor, NsrR, and nitrite-insensitive NarQ-NarP. J. Biol. Chem. 281:33115–33126 [DOI] [PubMed] [Google Scholar]
- 20. Potter AJ, et al. 2009. Esterase D is essential for protection of Neisseria gonorrhoeae against nitrosative stress and for bacterial growth during interaction with cervical epithelial cells. J. Infect. Dis. 200:273–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Potter AJ, et al. 2009. Thioredoxin reductase is essential for protection of Neisseria gonorrhoeae against killing by nitric oxide and for bacterial growth during interaction with cervical epithelial cells. J. Infect. Dis. 199:227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ren B, Zhang N, Yang J, Ding H. 2008. Nitric oxide-induced bacteriostasis and modification of iron-sulphur proteins in Escherichia coli. Mol. Microbiol. 70:953–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rensing C, Grass G. 2003. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 27:197–213 [DOI] [PubMed] [Google Scholar]
- 24. Seib KL, et al. 2007. Characterization of the OxyR regulon of Neisseria gonorrhoeae. Mol. Microbiol. 63:54–68 [DOI] [PubMed] [Google Scholar]
- 25. Shafeeq S, et al. 2011. The cop operon is required for copper homeostasis and contributes to virulence in Streptococcus pneumoniae. Mol. Microbiol. 81:1255–1270 [DOI] [PubMed] [Google Scholar]
- 26. Singh RJ, Hogg N, Joseph J, Kalyanaraman B. 1996. Mechanism of nitric oxide release from S-nitrosothiols. J. Biol. Chem. 271:18596–18603 [DOI] [PubMed] [Google Scholar]
- 27. Solioz M, Abicht HK, Mermod M, Mancini S. 2010. Response of Gram-positive bacteria to copper stress. J. Biol. Inorg. Chem. 15:3–14 [DOI] [PubMed] [Google Scholar]
- 28. Stamler JS, Singel DJ, Loscalzo J. 1992. Biochemistry of nitric-oxide and its redox-activated forms. Science 258:1898–1902 [DOI] [PubMed] [Google Scholar]
- 29. Steichen CT, Shao JQ, Ketterer MR, Apicella MA. 2008. Gonococcal cervicitis: a role for biofilm in pathogenesis. J. Infect. Dis. 198:1856–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Turner SM, et al. 2005. Mutational and biochemical analysis of cytochrome c′, a nitric oxide-binding lipoprotein important for adaptation of Neisseria gonorrhoeae to oxygen-limited growth. Biochem. J. 388:545–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Valko M, Morris H, Cronin MTD. 2005. Metals, toxicity and oxidative stress. Curr. Med. Chem. 12:1161–1208 [DOI] [PubMed] [Google Scholar]
- 32. White C, Lee J, Kambe T, Fritsche K, Petris MJ. 2009. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J. Biol. Chem. 284:33949–33956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Williams DLH. 1999. The chemistry of S-nitrosothiols. Acc. Chem. Res. 32:869–876 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.