Abstract
Merozoite surface protein 1 (MSP1) is a target for malaria vaccine development. Antibodies to the 19-kDa carboxy-terminal region referred to as MSP119 inhibit erythrocyte invasion and parasite growth, with some MSP1-specific antibodies shown to inhibit the proteolytic processing of MSP1 that occurs at invasion. We investigated a series of antibodies purified from rabbits immunized with MSP119 and AMA1 recombinant proteins for their ability to inhibit parasite growth, initially looking at MSP1 processing. Although significant inhibition of processing was mediated by several of the antibody samples, there was no clear relationship with overall growth inhibition by the same antibodies. However, no antibody samples inhibited processing but not invasion, suggesting that inhibition of MSP1 processing contributes to but is not the only mechanism of antibody-mediated inhibition of invasion and growth. Examining other mechanisms by which MSP1-specific antibodies inhibit parasite growth, we show that MSP119-specific antibodies are taken up into invaded erythrocytes, where they persist for significant periods and result in delayed intracellular parasite development. This delay may result from antibody interference with coalescence of MSP119-containing vesicles with the food vacuole. Antibodies raised against a modified recombinant MSP119 sequence were more efficient at delaying intracellular growth than those to the wild-type protein. We propose that antibodies specific for MSP119 can mediate inhibition of parasite growth by at least three mechanisms: inhibition of MSP1 processing, direct inhibition of invasion, and inhibition of parasite development following invasion. The balance between mechanisms may be modulated by modifying the immunogen used to induce the antibodies.
INTRODUCTION
Malaria remains a major disease in Africa, Asia, and Latin America with an estimated 300 to 500 million cases and up to a million deaths per year (41). To date, no registered vaccine has been developed, and artemisinin combination therapies (ACTs) are currently the main treatment for clinical disease due to infections with Plasmodium falciparum, the principal human pathogen. There is an ever-present risk of parasites developing drug resistance and thus a clear public health need to develop an effective vaccine. RTS,S, a vaccine based on the circumsporozoite surface protein, is currently undergoing phase III trials, but there is still a major interest to develop a vaccine that includes a component(s) directed at the asexual blood stage (22). Two P. falciparum proteins that have been researched extensively as vaccine targets are merozoite surface protein 1 (MSP1) and apical merozoite antigen 1 (AMA1), but there is still uncertainty about how a protective immune response against these targets may act. Recent vaccine trials with these antigens have produced disappointing results (31, 37), suggesting that we need to understand better the mechanisms of immunity and potentially engineer the antigens to improve the responses (21).
MSP1 is the most characterized merozoite surface protein (21) and is essential during the invasive blood stage (11). It is synthesized in schizonts as an ∼190-kDa protein, which is cleaved by P. falciparum subtilisin 1 (PfSUB1) at the end of schizogony into four polypeptides of characteristic length: p83, p42, p38, and p30 (9). These fragments remain associated together on the parasite's surface via noncovalent bonds, along with several other surface proteins (33, 38), and anchored to the plasma membrane via the C-terminal glycosylphosphatidylinositol (GPI) moiety located on the 42-kDa fragment (MSP142) (17). MSP1 may play a role in the initial binding of the merozoite to an erythrocyte (34). During the final stages of erythrocyte invasion, MSP142 undergoes a second cleavage event called secondary processing and mediated by another parasite subtilisin (PfSUB2 [20]), generating MSP133 and MSP119. The result of this cleavage is the shedding of the majority of the MSP1 and its associated protein complex, a process that has been linked with loss of the merozoite coat during erythrocyte invasion (6). However, MSP119 remains attached to the merozoite due to the GPI anchor and is taken into the erythrocyte (5, 7, 13). The role MSP119 plays in subsequent intracellular parasite development is unclear, although it is the first known marker for the developing food vacuole where it persists until the end of the intracellular cycle and is discarded in the residual body together with products of digestion such as hemozoin (13). There is abundant evidence that antibodies to MSP119 can interfere with parasite growth, and a range of mechanisms have been proposed, ranging from steric inhibition of parasite binding to erythrocytes and inhibition of SUB2-mediated secondary processing to the recruitment of cellular functions through Fc-mediated mechanisms (2, 7, 18, 19, 27, 30).
The structure of P. falciparum MSP119 has been elucidated and used to define the epitopes recognized by monoclonal antibodies (MAbs) that when bound inhibit secondary processing (28, 29, 35). In addition, the modification of MSP119 by substitution of different amino acid residues has been helpful not only to identify the epitopes of these classes of antibody, but also to define a third class of antibody (called blocking antibodies) that competes with inhibitory antibodies and therefore allows secondary processing to proceed even in the presence of the inhibitory antibodies (19, 39). Some of these modified MSP119 antigens have been proposed to offer advantages over the wild-type antigen in that they might induce fewer blocking antibodies when used as an immunogen (21, 23, 39).
AMA1 reaches the merozoite surface from the microneme organelles. Although its role in invasion is different from that of MSP1, it is also shed from the merozoite surface by the action of SUB2 (24). Antibodies to AMA1 have been shown to be highly effective at reducing parasite invasion in vitro and also to interfere with AMA1 processing (14, 15).
In a search for the antigens to include in an asexual blood-stage vaccine, a number of MSP1 constructs have been compared (1, 36). This comparison has included proteins produced via different expression systems, modified forms of MSP1 to include amino acid substitutions, and chimeric proteins consisting of fused AMA1 and MSP1 sequences (1, 16). In this work, we used reagents previously developed to some of these antigens (1) to further clarify the importance and mechanism by which MSP119 antibodies can inhibit parasite growth and development. We show that although MSP1 processing by SUB2 is inhibited by some of the IgGs tested, this activity alone cannot account for the observed inhibition of growth. However, in no instance was a high inhibition of MSP1 processing associated with a low inhibition of growth, suggesting that SUB2 processing of MSP1 is a key prerequisite for erythrocyte invasion. We provide evidence that MSP119-specific IgG is taken up along with the invading merozoite into an erythrocyte and that these antibodies can delay the intracellular development of the parasite, possibly by interfering with the formation of the food vacuole. Based on these observations, we propose a model in which MSP119-specific IgGs can inhibit parasite growth by at least three mechanisms: inhibition of MSP1 secondary processing, direct inhibition of invasion, and inhibition of an MSP1 function that is important postinvasion, for example at its location in the food vacuole. These results suggest that a modified protein is more effective than the wild-type recombinant protein in inducing antibodies that delay intracellular development.
MATERIALS AND METHODS
IgG production.
The antibodies raised against a variety of recombinant MSP119 and AMA1 proteins used in this study were purified from serum produced as part of the EUROMALVAC II Integrated Project (1). Groups of individual rabbits were immunized with one of six antigen preparations: AMA1, a mixture of AMA1 and wild-type MSP119 (mix), an AMA1-MSP119 fusion protein (fusion) (16), wild-type MSP119 expressed in Pichia pastoris (MSP1wt), wild-type MSP119 expressed from recombinant baculovirus in insect cells (MSP1bac; kindly provided by Shirley Longacre, Institut Pasteur), and mutant MSP119 expressed in Pichia pastoris (MSP1mut; Cys12 and Cys28 replaced by Ile and Trp, respectively); further details of antigens have been described previously (1). All IgGs used in these studies were purified from individual serum samples by affinity chromatography on protein G columns. Total IgG concentration was measured by spectroscopy.
MSP1 and AMA1 processing assay.
Each of the IgG fractions produced was tested for its ability to block secondary processing of MSP1, using a modified processing assay and merozoites (4). In brief, merozoites (3D7 line) were harvested in a medium containing EGTA to chelate Ca2+ and prevent SUB2 activation and then stored at −80°C. Prior to use, merozoites were thawed on ice, washed, and then resuspended in 10 mM Tris-HCl buffer (pH 8.0). To aliquots of merozoites, IgG samples were added to a final concentration of 5 mg ml−1 and SUB2 was activated by the addition of CaCl2 to 5 mM (final). Samples were incubated for 15 min at 37°C before centrifugation and supernatant collection. Processing was determined by SDS-PAGE and Western blotting to detect shed products: MSP133 using the specific antibody X509 (8), and the 44-kDa fragment of AMA1 using the 4G2 antibody (10). Following chemiluminescence and fluorography, signals were analyzed using ImageJ software to determine relative band intensities and calculate the percentage of processing in the presence of each IgG, compared to controls using the following equation: percentage processing = 100 × [(IgG Int − negative control Int)/(positive control Int − negative control Int)], where Int is band intensity. The positive control sample contained neither IgG nor EGTA, and the negative control contained an excess of EGTA to suppress further processing (6).
Growth and manipulation of P. falciparum cultures.
P. falciparum 3D7 parasites were routinely cultured at 2% hematocrit and 37°C in RPMI 1640 medium containing Albumax (Gibco 041-91762A) and glutamine. Merozoites were collected as described previously (4). For the inhibition of growth assays, parasites were first partially synchronized using magnetic collection of schizonts. Two hours postinvasion, the cells were pelleted by centrifugation and then suspended in a final concentration of 5% sorbitol at 37°C for 10 min to remove any remaining schizonts. Following sorbitol treatment, the cells were pelleted again and washed twice in fresh medium prior to resuspension in medium at 1% parasitemia and 2% hematocrit for further culture. Synchronized cultures were grown for approximately 40 h or until the next schizogony.
Growth assay by FACS analysis of hydroethidine (HE)-stained parasites and by microscopy of Giemsa-stained smears.
Assays to examine the effect of antibodies on parasite growth were carried out in a 96-well plate format, with a final volume of 100 μl for each culture (75 μl of stock culture plus 25 μl of a 20 mg ml−1 IgG stock). Each assay was performed in duplicate. Following setup, plates were incubated in a humid chamber containing 90% nitrogen, 5% carbon dioxide, and 5% oxygen at 37°C. After one cycle of invasion to the next schizont stage (typically 44 to 48 h), the samples were analyzed by fluorescence-activated cell sorter (FACS) (3). In brief, 50 μl of sample was added to 500 μl of freshly diluted hydroethidine fluorescence stain (HE; 17084; Polysciences Inc.; 1:200 dilution of 10 mg ml−1 stock in 100% dimethyl sulfoxide [DMSO]) and incubated for 20 min at 37°C. Samples were then diluted with 1 ml of phosphate-buffered saline (PBS) to enable appropriate FACS counting. Staining was stopped by storing the samples on ice in the dark. For negative controls, noninfected red blood cells were HE stained and processed in the same way.
Parasitemia was calculated using the FACSCalibur flow cytometer (Becton Dickson). In brief, cells to be counted were initially screened using forward and side scatter parameters and gated for erythrocytes. From this gated population, the HE staining was determined using the FL2 detector (585/42 nm) with 50,000 cells counted. When expressed as a histogram of HE intensity versus cell number, a distinct population representing trophozoite/schizont-infected erythrocytes is evident and can be used to calculate the parasitemia as follows: growth inhibition = 100 − (100 × average parasitemia in the presence of IgG/control parasitemia).
To confirm the stage of parasites within the asexual cycle, thin smears were prepared, methanol fixed, stained with Giemsa's reagent, and examined by microscopy. The parasites were divided into three classes: ring stage (early trophozoite), late trophozoite (hemozoin clearly evident, single nucleus), and schizont (multiple nuclei with or without segmentation).
IgG uptake assay.
Parasite cultures were set up as described above for FACS analysis, and IgG was added to each well at a final concentration of 0.5 mg ml−1 (a non-invasion-inhibitory concentration). Parasites were allowed to invade and then following invasion (determined by the presence of early-ring-stage parasites in control cultures), plates were briefly centrifuged to pellet cells. The cells were then washed with fresh medium (3 × 100 μl), resuspended to 100 μl, and incubated for various periods in the gassed humid chamber. To examine for the presence of IgG that had been taken up at various time points, smears were prepared, dried, and stored (−20°C) for subsequent immunolabeling. The smears were fixed in 4% formaldehyde-PBS for 15 min, extracted with 1% Triton X-100 (vol/vol) in PBS for 5 min, and then blocked in 3% bovine serum albumin (BSA) (wt/vol) in PBS for 60 min. Antibodies were detected by the addition of Alexa Fluor488-tagged goat anti-rabbit IgG (A11034; Molecular Probes) and mounted in ProLong Gold containing DAPI (4′,6-diamidino-2-phenylindole; P771644; Invitrogen). Slides were examined using a Zeiss Axiovert 200 microscope using a 100× objective, and images were acquired using Axiovision 4.0 software (Zeiss), with additional processing using Adobe Photoshop.
RESULTS
MSP142 processing assay.
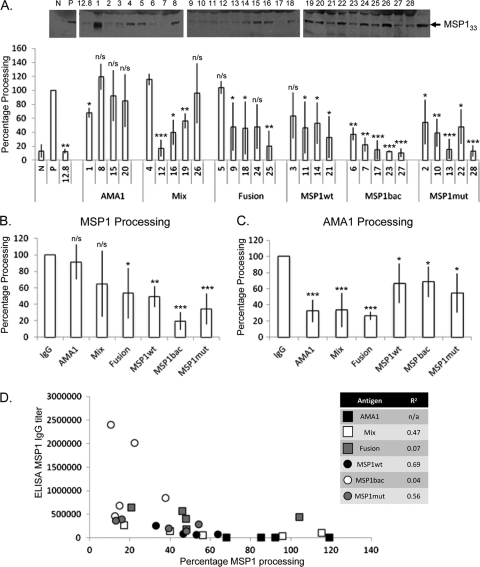
The initial aim of this study was to determine whether antibodies raised by immunization with a variety of recombinant MSP119 proteins could inhibit the secondary processing of MSP142, and if so to what extent this activity contributed to their overall ability to inhibit parasite growth in vitro. A previous study had shown that these sera contained antibodies that inhibited parasite growth (1). Secondary processing was determined by the detection of MSP133 using Western blotting and ImageJ analysis. By comparing the amount of MSP133 produced in the presence of antibody to that in the positive (absence of IgG) and negative (in the presence of EGTA to inhibit SUB2 processing) controls, we were able to calculate the percentage of MSP1 processing in the presence of each IgG (Fig. 1A). The assay showed a general trend for decreased MSP1 processing when the IgG samples were grouped according to the antigen they had been raised against, from high to low: AMA1 alone, AMA1-MSP1 mix/fusion, MSP1wt, MSP1mut, and MSP1bac (Fig. 1B). Although the assay was rigorously controlled at all steps through sample preparation, minimal handling, and the inclusion of controls on each gel, a significant variability in MSP1 processing was noted, which is reflected in the error bars. IgGs that were raised against antigen containing an AMA1 component also inhibited the processing of AMA1 (Fig. 1C). These assays were carried out at 2.5 mg ml−1 to avoid background problems. Importantly, when we grouped IgG samples according to their antigen specificity, there was a distinct difference in their inhibition of either AMA1 or MSP1 processing.
Fig 1.
Analysis of MSP1 processing in the presence of specific antibody. (A) Images representative of MSP1 blots used for analysis of MSP142 processing as estimated by ImageJ analysis of Western blot data. The intensity of the signal due to X509 binding to MSP133 was quantified by densitometry for each IgG sample expressed as a percentage relative to positive controls. Samples are displayed according to the antigen: AMA1, mixed AMA1 and MSP119wt (mix), AMA1-MSP119 fusion (fusion), MSP119wt (MSP1wt), baculovirus-expressed MSP119wt (MSP1bac), and mutant MSP119 (MSP1mut) with the numbers identifying particular IgG samples. The positive (P) and negative (N) controls, as well as a known processing-inhibitory monoclonal antibody (MAb 12.8), are included. Purified IgG (5.0 mg ml−1) was used, with standard deviation indicated. Student's t test was used to determine statistical significance in relation to the positive control (P values: *, 0.01 to 0.05; **, 0.01 to 0.001; and ***, <0.001). (B) Inhibition of MSP1 processing by specific IgG antigen group. Student's t test was used to determine statistical significance compared to the control. (C) Inhibition of AMA1 processing estimated by ImageJ analysis of Western blot data. Pooled IgG from the groups defined in A. IgG was added at 2.5 mg ml−1, and the percentage of processing relative to positive controls was calculated. Student's t test was used to determine statistical significance in relation to the positive control. (D) Graph depicting MSP1 processing in the presence of the various IgG samples compared with their MSP1 titer by ELISA.
Although for the MSP1 assay all IgGs were used at the same concentration, to clarify whether the observed inhibition of processing was due to MSP1-specific IgG, we compared the MSP1 processing inhibition to its MSP1-specific titer, using enzyme-linked immunosorbent assay (ELISA) data from reference 1 (Fig. 1D). From this analysis there was no clear relationship between ELISA titer and percentage processing in most cases; MSP1wt and MSP1mut gave the highest correlation (R2 = 0.69 and 0.56, respectively). Within the MSP1bac group, all IgGs gave significant inhibition of MSP1 processing (>60%), but there was a range of IgG titers (Fig. 1D).
Relationship between inhibition of MSP1 processing and inhibition of parasite growth.
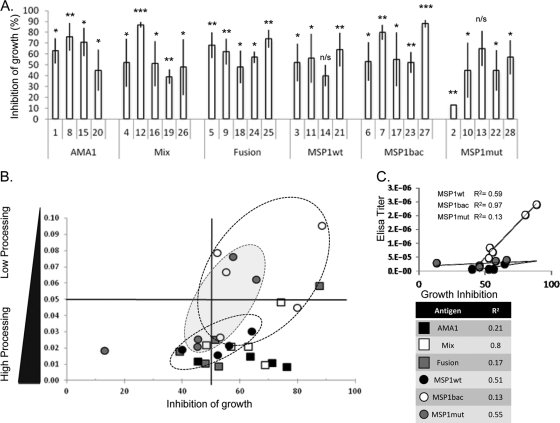
The primary role of MSP1 during invasion is believed to be in host cell recognition and attachment, with the SUB2-mediated cleavage acting to remove the majority of the complex, presumably after this role is completed. Accordingly, it has been proposed that a primary mechanism by which MSP1-specific antibodies may inhibit parasite growth is via the inhibition of secondary processing (7, 19). We therefore looked for any correlation between the extent of antibody-mediated inhibition of parasite growth and MSP1 secondary processing. Since our processing assays were performed with merozoites from the 3D7 line, we used the same parasite to carry out growth inhibition assays using the vital stain hydroethidine (HE) and a FACS procedure (Fig. 2A).
Fig 2.
Correlation between parasite growth inhibition, inhibition of MSP1 processing, and antibody titer. (A) Analysis of the inhibition of parasite growth in the presence of specific antibody, as determined by hydroethidine staining and FACS counting. The percentage of growth inhibition was calculated relative to controls. Antibodies from individual sera, identified and grouped as defined for Fig. 1, were used. The data are from three representative independent experiments; in each case, a final concentration of 5.0 mg ml−1 purified IgG was used. The standard deviation is indicated; Student's t test was used to determine statistical significance in relation to the positive control (P values: *, 0.01 to 0.05; **, 0.01 to 0.001; and ***, <0.001). (B) Comparison of the inhibition of parasite growth and MSP142 processing by individual antibody samples from rabbits immunized with AMA1 alone (AMA1, black squares), a mixture of AMA1 and wild-type MSP119 (mix, white squares), an AMA1-MSP119 fusion protein (fusion, gray squares), wild-type MSP119 expressed in Pichia pastoris (MSP1wt, black circles), wild-type MSP119 expressed from recombinant baculovirus in insect cells (MSP1bac, white circles), and mutant MSP119 expressed in Pichia pastoris (MSP1mut, gray circles). The antibodies from groups of animals immunized with MSP119 alone are ringed. The calculated R2 values for each group of antibody samples are listed in the table (bottom right). (C) Correlation of anti-MSP1 antibody titer as determined by ELISA (data from reference 1) with antibody-mediated inhibition of growth as determined by FACS. The antibody samples were from animals immunized with wild-type MSP119 expressed in Pichia pastoris (MSP1wt, black circles), wild-type MSP119 expressed from recombinant baculovirus in insect cells (MSP1bac, white circles), and mutant MSP119 expressed in Pichia pastoris (MSP1mut, gray circles). The calculated correlation for each slope is indicated.
If inhibition of secondary processing is a principal factor in the inhibition of parasite invasion and growth, then we would predict a direct relationship between the inhibition of growth and the reciprocal of the MSP1 processing (1/P) in the presence of antibody, i.e., the less MSP1 secondary processing the greater the inhibition of parasite growth. However, for none of the IgG groups tested was a tight correlation observed (Fig. 2B, R2 values), although we did note a general grouping of results based on the antigen used to raise the antibodies. Nevertheless, in no instance was there inhibition of MSP1 processing in the absence of growth inhibition (Fig. 2B, top left quadrant), indicating that when the antibodies did inhibit MSP1 processing it was contributing in some degree to the overall inhibition of parasite growth. IgG samples produced by immunization with AMA1-based antigens all fall in the lower half of the graph (Fig. 2B). This was expected as each IgG contains an AMA1 antibody component capable of contributing to parasite growth inhibition but which will have no effect on the measured MSP1 processing. Of the three groups of antibody samples raised to recombinant MSP1, those produced by immunization with the wild-type MSP1 produced in Pichia gave the most consistent but lowest inhibition of processing, while the two remaining groups (MSP1bac and MSP1mut) had several IgG samples giving significant inhibition of MSP1 processing, though as groups these also had the widest range. Based on the titer of MSP1-specific IgG in the samples, there was a strong direct relationship between the level of specific antibody and inhibition of parasite growth (Fig. 2C), which was not present when the level of specific antibody was compared to inhibition of processing (Fig. 1D).
IgG uptake at merozoite invasion delays intracellular parasite development.
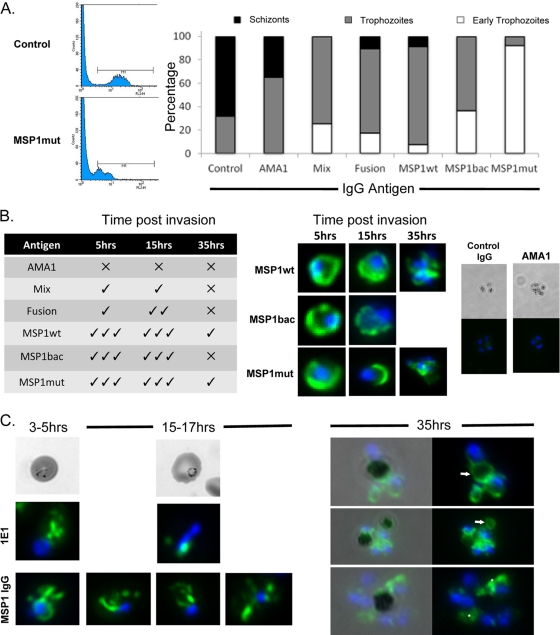
The FACS-based growth inhibition assay uses HE as a vital stain that requires the parasite to be metabolically active to convert the HE to ethidium, which then binds to DNA. The amount of DNA in the cell reflects the parasite's stage of development: ring (early trophozoite) and trophozoite stages have a single copy of the genome; schizogony requires extensive DNA synthesis, replication, and nuclear division. Therefore, as the parasite progresses through its intracellular development more DNA is present, and this higher DNA content results in greater incorporation of ethidium and higher fluorescence intensity. This is indicated by the right shift in the histogram generated by the FACS.
Although the overall parasitemia was measured and used to calculate growth inhibition mediated by the presence of specific IgG, it was noted that for some groups there was a left shift in the histograms, indicating the presence of earlier-stage or delayed parasites (Fig. 3A). To confirm this observation, the growth inhibition assays were repeated with a selection of IgGs and the parasite morphology analyzed by microscopy of smears stained with Giemsa's reagent. Interestingly, when the parasites were treated with antibodies raised to a recombinant protein including MSP1, there was a noted delay in parasite development in the parasites present compared to controls (Fig. 3A). This was particularly evident in the presence of antibodies raised to MSP1mut. For such developmental delay and morphological changes to occur, it was predicted that some of the antibody must be taken into the erythrocyte with the invading merozoite, a feature previously observed for the monoclonal antibodies 111.4 and 1E1, which accumulate together with MSP119 in the food vacuole (13). To determine if any IgG was taken into the erythrocyte, we repeated the assays using a low concentration of IgG (0.5 mg ml−1). Thin smears were prepared, and the parasites were examined at 5, 15, and 35 h postinvasion using immunofluorescence following permeabilization of the cells and the addition of a labeled second antibody. We identified antibody within infected erythrocytes, indicating that some of the IgG was taken in along with the invading merozoite and persisted until at least 35 h postinvasion (Fig. 3B). There was no detectable uptake of AMA1-specific antibodies or IgG controls. These results are consistent with the uptake of antibody bound directly to MSP1 and agree with our FACS and parasite staging data (Fig. 3A). Previous studies have reported the specific uptake of MSP119-specific antibodies (4, 13). Although labeling was present in the parasites incubated with all the MSP1-specific IgGs, the intensity and duration of label were greatest for those samples produced by immunization with MSP1wt and MSP1mut. In late-stage parasites, the IgG was detected in the food vacuole as identified by the hemozoin crystal (Fig. 3C, 35 h) but also elsewhere throughout the cytoplasm, presumably in vesicles that had failed to coalesce with the food vacuole; such vesicles had been identified previously in MSP1 uptake studies (13). In contrast, the MAb 1E1 labeling coalesced to a single location by 15 to 17 h postinvasion, as described previously (13). These results indicate that the MSP119-specific antibodies may delay or obstruct food vacuole formation (Fig. 3C).
Fig 3.
The effect of antibodies on intracellular parasite development. (A) Analysis of parasites ∼40 h postinvasion by FACS following hydroethidine staining and by microscopy following Giemsa staining. In the presence of specific antibodies, parasite development was delayed as shown by DNA fluorescence (ethidium labeling moved to left; MSP1mut antibody-treated parasites shown) and morphology compared to control parasites; the proportions of schizonts (black), trophozoites (gray), and early trophozoites (white) in each population are indicated in the graph. (B) MSP119-specific antibody is taken up into parasites at invasion and persists during development. The table on the left indicates the presence of detectable antibody at 5, 15, and 35 h postinvasion for parasites incubated with antibodies raised against the various proteins, and on the right are images of parasites from the different time points stained for the presence of rabbit IgG (green) and with DAPI (blue) to locate the nuclei. Representative images are shown for the groups that gave the most pronounced IgG labeling, along with the AMA1 and irrelevant IgG controls. (C) Comparison of MSP1 IgG labeling compared to control cultures containing MSP1-specific MAb 1E1 at early stages of parasite development, 3 to 5 and 15 to 17 h postinvasion. At later time points (35 h), MSP1 IgG labeling was evident and associated with hemozoin (arrows) and cytosolic structures (asterisks).
DISCUSSION
The initial aim of this work was to evaluate the contribution made by antibodies that inhibit secondary processing of MSP1 to the antibody-mediated inhibition of parasite growth in vitro. We used preparations of rabbit antibodies that had been raised by immunization with a range of recombinant proteins comprised of MSP119 sequences, either alone or in combination with AMA1-derived sequences (29). By applying tight protocols, the pooling of merozoite sample preparations and the inclusion of reference samples on each SDS-PAGE gel, we have been able to detect the contribution processing inhibition makes to the mechanisms of overall growth inhibition by these antibodies. The specificity of the assay was confirmed by including AMA1-specific antibodies, which as a group showed no MSP1 processing inhibition (Fig. 1A and B) (average 95% of control). Furthermore, the IgG inhibition of MSP1 processing by SUB2 does not affect the SUB2 processing of AMA1 or vice versa.
Although antibodies from these serum samples have been examined previously for their ability to inhibit parasite growth in vitro, these studies used the Wellcome and FCR3 lines of P. falciparum in a lactate dehydrogenase-based assay (1), and there is evidence for varying responses between parasite lines (2, 3). As part of this work, we retested each IgG sample in HE and FACS-based growth inhibition assay (3). This system incorporates a measure of parasite viability and gives an indication of the developmental stage of the parasite population by DNA content as measured by the amount of ethidium bound. From this, we show that for the MSP1-specific IgGs there was limited correlation between IgG-mediated inhibition of processing and growth. This correlation was reduced even further when antibodies to AMA1 were present (mix and fusion antigens) and was negligible for AMA1-specific antibodies. However, none of the IgG samples gave a high inhibition of MSP1 processing but a low level of growth inhibition, indicating that when present, inhibition of MSP1 processing contributes to growth inhibition.
Although there is evidence for a correlation between the MSP1-specific antibody titer and inhibition of growth, there was little if any correlation between MSP1 antibody titer and inhibition of MSP1 processing. It is of note that in two of the antigen groups (MSP1bac and MSP1mut), there were IgG samples with low MSP1 titers but which gave significant MSP1 processing inhibition (>80%), a level that was comparable with that of other IgG samples tested with significantly higher MSP1 titers by ELISA. Thus, MSP1 processing inhibition is not merely dependant on IgG concentration.
Together, these results indicate that antibodies that inhibit MSP1 secondary processing are not a major component of the IgG fraction and that IgGs act via other mechanisms to contribute to the overall inhibition of parasite growth. However, the results do not rule out the potential of engineering the antigen, for example enhancing the processing inhibition activity by manipulating the sequence of the antigen. Reducing the induction of blocking antibodies that prevent the binding of processing inhibitory antibodies is one strategy to achieve this (39).
Several aspects of our data show that when MSP1-specific IgG was present, the parasites that developed were significantly delayed compared to controls. This is in agreement with a previous study using the same sera that noted a change in parasite morphology (1). Using non-growth-inhibiting concentrations of IgG, we were able to detect MSP119-specific IgG within infected erythrocytes up to at least 35 h postinvasion, indicating that IgG was taken into the parasitophorous vacuole bound to MSP119 of the invading merozoite. The presence of MSP119-specific IgGs within erythrocytes is in agreement with a previous study using MAbs (13) and morphological observations made using polyclonal antibodies (1, 2, 40). It is known that MSP119 is carried into the infected cell and persists postinvasion (5, 26), accumulating in the food vacuole and finally shed within the residual body (13). The fact that IgGs are taken in with the merozoite and remain bound over a significant period of the parasite's life cycle places them in an appropriate location to inhibit intracellular development, thus contributing to inhibition of parasite growth. We observed IgG labeling around the hemozoin, indicating association with the food vacuole as well as IgG within subcytoplasmic compartments, possibly the early vesicles that coalesce to form the food vacuole (13). The extensive cytoplasmic labeling was not evident in the MAb 1E1 control, consistent with a possible disruption or delay in food vacuole formation.
The mechanisms by which an antibody response to MSP119 contributes to protective immunity in vivo are still not fully resolved (30). However, antibody fine specificity (12, 32) and Fc-mediated mechanisms (25, 27) are important. Data acquired in this study suggest that polyclonal antibodies to MSP119 can inhibit parasite invasion of erythrocytes by inhibiting MSP1 processing and by a second, potentially more important mechanism, perhaps steric hindrance of parasite binding. The fact that another factor other than MSP1 processing is involved means that the processing assay alone is an inadequate method to assay antibody-mediated inhibition of parasite growth. However, since some MAbs show good activity in both processing and invasion inhibition (7, 19, 25), it remains a viable tool to dissect the mode of action of MSP1-specific antibodies. The contribution inhibition of intracellular growth makes toward the protective effect of anti-MSP1 antibodies needs further investigation, but it is interesting that an engineered variant of MSP119 appears able to induce antibodies that are more active in this assay than those induced by the wild-type protein, suggesting that the fine specificity of the antibodies may contribute to this mechanism. These results are consistent with the notion that engineering or modifying the MSP1 antigen may convey properties that improve its potential for vaccine development.
ACKNOWLEDGMENTS
The study was funded in part by the UK Medical Research Council (file reference number U117532067) and the European Union (through the European Malaria Vaccine Development Association, EMVDA Integrated Project LSH-2005-037506; EviMalaR Network of Excellence Health-2009-2.3.2-1-242095; and European malaria vaccine development consortium EUROMALVAC II QLK2-CT-2002-01197).
We thank Shirley Longacre (Institut Pasteur, France) for the baculovirus-expressed MSP119 protein and Muni Grainger for merozoites.
Footnotes
Published ahead of print 27 December 2011
REFERENCES
- 1. Arnot DE, et al. 2008. Comparative testing of six antigen-based malaria vaccine candidates directed toward merozoite-stage Plasmodium falciparum. Clin. Vaccine Immunol. 15:1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergmann-Leitner ES, Duncan EH, Angov E. 2009. MSP-1p42-specific antibodies affect growth and development of intra-erythrocytic parasites of Plasmodium falciparum. Malar. J. 8:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergmann-Leitner ES, et al. 2006. Critical evaluation of different methods for measuring the functional activity of antibodies against malaria blood stage antigens. Am. J. Trop. Med. Hyg. 75:437–442 [PubMed] [Google Scholar]
- 4. Blackman MJ. 1994. Purification of Plasmodium falciparum merozoites for analysis of the processing of merozoite surface protein-1. Methods Cell Biol. 45:213–220 [DOI] [PubMed] [Google Scholar]
- 5. Blackman MJ, Heidrich HG, Donachie S, McBride JS, Holder AA. 1990. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J. Exp. Med. 172:379–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blackman MJ, Holder AA. 1992. Secondary processing of the Plasmodium falciparum merozoite surface protein-1 (MSP1) by a calcium-dependent membrane-bound serine protease: shedding of MSP133 as a noncovalently associated complex with other fragments of the MSP1. Mol. Biochem. Parasitol. 50:307–315 [DOI] [PubMed] [Google Scholar]
- 7. Blackman MJ, Scott-Finnigan TJ, Shai S, Holder AA. 1994. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J. Exp. Med. 180:389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blackman MJ, Whittle H, Holder AA. 1991. Processing of the Plasmodium falciparum major merozoite surface protein-1: identification of a 33-kilodalton secondary processing product which is shed prior to erythrocyte invasion. Mol. Biochem. Parasitol. 49:35–44 [DOI] [PubMed] [Google Scholar]
- 9. Child MA, Epp C, Bujard H, Blackman MJ. 2010. Regulated maturation of malaria merozoite surface protein-1 is essential for parasite growth. Mol. Microbiol. 78:187–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collins CR, et al. 2007. Fine mapping of an epitope recognized by an invasion-inhibitory monoclonal antibody on the malaria vaccine candidate apical membrane antigen 1. J. Biol. Chem. 282:7431–7441 [DOI] [PubMed] [Google Scholar]
- 11. Combe A, et al. 2009. Clonal conditional mutagenesis in malaria parasites. Cell Host Microbe 5:386–396 [DOI] [PubMed] [Google Scholar]
- 12. Corran PH, et al. 2004. The fine specificity, but not the invasion inhibitory activity, of 19-kilodalton merozoite surface protein 1-specific antibodies is associated with resistance to malarial parasitemia in a cross-sectional survey in The Gambia. Infect. Immun. 72:6185–6189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dluzewski AR, et al. 2008. Formation of the food vacuole in Plasmodium falciparum: a potential role for the 19 kDa fragment of merozoite surface protein 1 (MSP1(19)). PLoS One 3:e3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dutta S, et al. 2005. Mode of action of invasion-inhibitory antibodies directed against apical membrane antigen 1 of Plasmodium falciparum. Infect. Immun. 73:2116–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dutta S, Haynes JD, Moch JK, Barbosa A, Lanar DE. 2003. Invasion-inhibitory antibodies inhibit proteolytic processing of apical membrane antigen 1 of Plasmodium falciparum merozoites. Proc. Natl. Acad. Sci. U. S. A. 100:12295–12300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Faber BW, et al. 2007. Malaria vaccine-related benefits of a single protein comprising Plasmodium falciparum apical membrane antigen 1 domains I and II fused to a modified form of the 19-kilodalton C-terminal fragment of merozoite surface protein 1. Infect. Immun. 75:5947–5955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gerold P, Schofield L, Blackman MJ, Holder AA, Schwarz RT. 1996. Structural analysis of the glycosyl-phosphatidylinositol membrane anchor of the merozoite surface proteins-1 and -2 of Plasmodium falciparum. Mol. Biochem. Parasitol. 75:131–143 [DOI] [PubMed] [Google Scholar]
- 18. Gilson PR, et al. 2008. MSP1(19) miniproteins can serve as targets for invasion inhibitory antibodies in Plasmodium falciparum provided they contain the correct domains for cell surface trafficking. Mol. Microbiol. 68:124–138 [DOI] [PubMed] [Google Scholar]
- 19. Guevara Patino JA, Holder AA, McBride JS, Blackman MJ. 1997. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J. Exp. Med. 186:1689–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harris PK, et al. 2005. Molecular identification of a malaria merozoite surface sheddase. PLoS Pathog. 1:241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holder AA. 2009. The carboxy-terminus of merozoite surface protein 1: structure, specific antibodies and immunity to malaria. Parasitology 136:1445–1456 [DOI] [PubMed] [Google Scholar]
- 22. Holder AA. 2009. Malaria vaccines: where next? PLoS Pathog. 5:e1000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holder AA, et al. 1999. Merozoite surface protein 1, immune evasion, and vaccines against asexual blood stage malaria. Parassitologia 41:409–414 [PubMed] [Google Scholar]
- 24. Howell SA, et al. 2003. A single malaria merozoite serine protease mediates shedding of multiple surface proteins by juxtamembrane cleavage. J. Biol. Chem. 278:23890–23898 [DOI] [PubMed] [Google Scholar]
- 25. Lazarou M, et al. 2009. Inhibition of erythrocyte invasion and Plasmodium falciparum merozoite surface protein 1 processing by human immunoglobulin G1 (IgG1) and IgG3 antibodies. Infect. Immun. 77:5659–5667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McBride JS, Heidrich HG. 1987. Fragments of the polymorphic Mr 185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol. Biochem. Parasitol. 23:71–84 [DOI] [PubMed] [Google Scholar]
- 27. McIntosh RS, et al. 2007. The importance of human FcgammaRI in mediating protection to malaria. PLoS Pathog. 3:e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morgan WD, et al. 1999. Solution structure of an EGF module pair from the Plasmodium falciparum merozoite surface protein 1. J. Mol. Biol. 289:113–122 [DOI] [PubMed] [Google Scholar]
- 29. Morgan WD, Frenkiel TA, Lock MJ, Grainger M, Holder AA. 2005. Precise epitope mapping of malaria parasite inhibitory antibodies by TROSY NMR cross-saturation. Biochemistry 44:518–523 [DOI] [PubMed] [Google Scholar]
- 30. Murhandarwati EE, et al. 2010. Growth-inhibitory antibodies are not necessary for protective immunity to malaria infection. Infect. Immun. 78:680–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ogutu BR, et al. 2009. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PLoS One 4:e4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okech BA, et al. 2004. Fine specificity of serum antibodies to Plasmodium falciparum merozoite surface protein, PfMSP-1(19), predicts protection from malaria infection and high-density parasitemia. Infect. Immun. 72:1557–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pachebat JA, et al. 2007. Extensive proteolytic processing of the malaria parasite merozoite surface protein 7 during biosynthesis and parasite release from erythrocytes. Mol. Biochem. Parasitol. 151:59–69 [DOI] [PubMed] [Google Scholar]
- 34. Perkins ME, Rocco LJ. 1988. Sialic acid-dependent binding of Plasmodium falciparum merozoite surface antigen, Pf200, to human erythrocytes. J. Immunol. 141:3190–3196 [PubMed] [Google Scholar]
- 35. Pizarro JC, et al. 2003. Crystal structure of a Fab complex formed with PfMSP1-19, the C-terminal fragment of merozoite surface protein 1 from Plasmodium falciparum: a malaria vaccine candidate. J. Mol. Biol. 328:1091–1103 [DOI] [PubMed] [Google Scholar]
- 36. Reed ZH, et al. 2009. Comparison of immunogenicity of five MSP1-based malaria vaccine candidate antigens in rabbits. Vaccine 27:1651–1660 [DOI] [PubMed] [Google Scholar]
- 37. Sagara I, et al. 2009. A randomized controlled phase 2 trial of the blood stage AMA1-C1/Alhydrogel malaria vaccine in children in Mali. Vaccine 27:3090–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Trucco C, et al. 2001. The merozoite surface protein 6 gene codes for a 36 kDa protein associated with the Plasmodium falciparum merozoite surface protein-1 complex. Mol. Biochem. Parasitol. 112:91–101 [DOI] [PubMed] [Google Scholar]
- 39. Uthaipibull C, et al. 2001. Inhibitory and blocking monoclonal antibody epitopes on merozoite surface protein 1 of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 307:1381–1394 [DOI] [PubMed] [Google Scholar]
- 40. Woehlbier U, Epp C, Hackett F, Blackman MJ, Bujard H. 2010. Antibodies against multiple merozoite surface antigens of the human malaria parasite Plasmodium falciparum inhibit parasite maturation and red blood cell invasion. Malar. J. 9:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. World Health Organization 2010. World Malaria Report 2010. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/world_malaria_report_2010/en/ [Google Scholar]