Abstract
The gastrointestinal mucosa has a remarkable ability to repair damage with the support of epidermal growth factor (EGF), which stimulates epithelial migration and proliferative reepithelialization. For the treatment of mucosal injuries, it is important to develop efficient methods for the localized delivery of mucoactive biotherapeutics. The basic idea in the present study came from the assumption that an intestinal probiotic vehicle can carry and deliver key recombinant medicinal proteins to the injured epithelial target in patients with intestinal ulcerative diseases, including inflammatory bowel disease. The study was focused on the use of the safe probiotic E. coli Nissle 1917, which was constructed to secrete human EGF in conjunction with the lipase ABC transporter recognition domain (LARD). Using the in vitro physically wounded monolayer model, ABC transporter-mediated EGF secretion by probiotic E. coli Nissle 1917 was demonstrated to enhance the wound-healing migration of human enterocytes. Moreover, the epithelial wound closure was dependent on EGF receptor-linked activation, which exclusively involved the subsequent signaling pathway of the mitogen-activated protein kinase kinase (MEK) extracellular-related kinases 1 and 2 (ERK1/2). In particular, the migrating frontier of the wounded edge displayed the strongest EGF receptor-linked signaling activation in the presence of the recombinant probiotic. The present study provides a basis for the clinical application of human recombinant biotherapeutics via an efficient, safe probiotic vehicle.
INTRODUCTION
Epidermal growth factor (EGF) was originally isolated from mouse salivary gland extract as a factor accelerating corneal wound healing (10). EGF now is recognized as a general growth factor that exerts various actions, including cell migration and proliferation, on a wide variety of cells (7, 43, 44). EGF stimulates the proliferation of keratinocytes in culture, and the topical administration of EGF accelerates the epidermal regeneration of partial-thickness burns or split-thickness incisions in vivo. EGF acts by binding with high affinity to epidermal growth factor receptor (EGFR) on the cell surface and stimulating the intrinsic protein-tyrosine kinase activity of the receptor. Although EGFR activation by its ligands, including transforming growth factor-α (TGF-α), heparin-bound EGF, betacellulin, and amphiregulin, can regulate many cellular processes (12), the main physiological roles of EGF are associated with epithelial wound healing.
Upon exposure to ulcerogenic and/or necrotizing agents, such as aspirin, indomethacin, bile acids, alcohol, and ischemia, the gastrointestinal mucosa develops characteristic morphological, ultrastructural, and functional changes reflecting the disruption of the mucosal barrier. The healing of deep mucosal erosions requires the reconstruction (reepithelialization) of the surface glandular epithelial structures and the lamina propria, including the mucosal microvascular network, nerves, and connective tissue cells. The gastrointestinal mucosa has a remarkable ability to repair damage with the support of EGF, which stimulates cell migration and increases blood flow (3, 5, 27, 36, 45). During reepithelialization, the repair of deeper injuries (erosions) requires epithelial cells to compensate for the mucosal defect. EGF, which is mitogenic for progenitor cell populations, increases the release of gastric mucin, attenuates gastric acid secretion, and stimulates cell migration (9).
Only a small fraction of orally administrated mucoactive biotherapeutics, including recombinant protein drugs, reaches the intended target site because of strong digestive degradation in the gastrointestinal tract. As a consequence of inefficient drug delivery, it is important to develop new methods for the localized delivery of mucoactive biotherapeutics. The basic idea in the present study was derived from the assumption that intestinal commensal bacteria or probiotics can carry and deliver key medicinal components to the injured epithelial target in patients with intestinal ulcerative diseases, including inflammatory bowel disease. Genetically modified probiotics might be a good alternative in mucosal biotherapy as a live safe carrier. Escherichia coli strain Nissle 1917 (O6:K5:H1) is a nonpathogenic fecal isolate that has been used as a probiotic agent in human and animal medicine since the early 1920s to treat chronic inflammatory and infectious diseases of the human and animal intestine (15, 16, 29). Probiotics are defined as viable microorganisms with physiologically beneficial or therapeutic activities. Various in vitro and animal studies with probiotics, including E. coli Nissle 1917, have demonstrated the capacity of probiotics to reduce intestinal inflammation (16, 20), strengthen the integrity of the intestinal epithelial barrier (42, 47), lessen host hypersensitivity (4), or suppress epithelial adherence and invasion of pathogenic bacteria (31, 33). Moreover, limited clinical investigations using E. coli Nissle 1917 and other microorganisms have demonstrated that probiotic-based therapeutic application can be efficacious in patients with chronic ulcerative colitis (13, 23, 32) and irritable bowel syndrome (38). E. coli Nissle 1917 is relatively safe for therapeutic applications, although its administration to immunocompromised hosts with defective intestinal microbiota after antibiotic therapy may lead to severe adverse effects (21). Regardless of the high numbers of E. coli Nissle 1917 cells that can colonize the intestinal tract, the bacterium does not cause colitis even in gnotobiotic animals monoinoculated with the strain (21). Molecular genetics as well as functional analyses have revealed that E. coli Nissle 1917 does not produce any virulence factors or carry any genes for pathogenicity traits, and it does not form enterotoxins, cytotoxins, or hemolysins (6, 39). The collective observations support the general recognition of biotherapeutic applications using E. coli Nissle 1917 as a safe organism for human use.
Type I secretion occurs in a continuous process across both the inner and the outer membrane of Gram-negative bacteria. One representative type I transporter is the ATP binding cassette (ABC) protein, which recognizes the C-terminal signal sequence of the target protein and hydrolyzes ATP for protein translocation (11, 28). In this study, we focused on the potential of E. coli Nissle 1917 as the vehicle for the targeted delivery of therapeutic proteins to ulcerated intestinal epithelia. E. coli Nissle 1917 secreting human EGF in conjunction with the lipase ABC transporter recognition domain (LARD) by the ABC transporter system (8) was established. In advance of the clinical application of the recombinant probiotic, the secretory and delivery systems were biologically assessed in human intestinal epithelial monolayer cell cultures. In particular, the recombinant bacterially mediated wound-healing process in the ulcerated epithelial layer was quantified and was shown to be mechanistically associated with EGF-linked receptor signals. The present study provides a basis for the clinical application of human biotherapeutics with efficiency and enhanced safety via the probiotic E. coli Nissle 1917.
MATERIALS AND METHODS
Cell cultures.
HCT-8 intestinal epithelial cells and nontransformed intestinal epithelial cell 18 (IEC-18) cells were purchased from the American Type Culture Collection (Rockville, MD). Cells were maintained in RPMI 1640 medium containing 10% fetal bovine serum (FBS; Wellgene, Daegu, South Korea) and 1% penicillin-streptomycin (Wellgene). For the culture of IEC-18 cells, 25 mM HEPES was added. Cells were maintained at 37°C in humidified 5% CO2 incubators. Cell numbers and viability were assessed by the exclusion of trypan blue dye (Sigma-Aldrich, St. Louis, MO) using a hemocytometer.
Bacterial strains used in this study.
Bacterial strains used in this study include Escherichia coli XL1-Blue and Escherichia coli Nissle 1917. Escherichia coli Nissle 1917 was kindly provided by Ardeypharm GmbH (Herdecke, Germany). Transformed E. coli strains were routinely grown at 37°C in Luria-Bertani (LB) broth or agar supplemented with appropriate antibiotics, such as 100 μg/ml chloramphenicol and 100 μg/ml ampicillin, after transformation. All bacteria were grown in LB medium to an optical density at 600 nm (OD600) of approximately 1, washed by centrifugation, and resuspended in serum-free RPMI medium. To determine the effects of bacterially released EGF, bacteria were grow in RPMI at 37°C to an OD600 of 0.6 to ∼0.7 and adjusted by dilution with RPMI to provide a multiplicity of infection (MOI) of 50:1 (bacterial to host cells). Bacteria in RPMI containing 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) were incubated for 8 to 12 h. The IPTG-induced supernatants were sterile filtered (pore size, 0.45 μm) and used for the incubation of human epithelial monolayer cells.
Plasmid construction.
Plasmids used in this study are depicted in Table 1. In this study, the export of recombinant human EGF attached to the C-terminal fragment (residues 302 to 476) of thermostable lipase (TliA) of Pseudomonas fluorescens SIK W1 is reported. These fragments, defined in previous work as a lipase ABC transporter recognition domain (LARD) (8), are secretion signals derived from TliA. Among the different LARDs, LARD3 (residues 372 to 476) was attached to the C terminus of human EGF. LARD3 was PCR amplified using pTOTAL (1) as a template, and the PCR product was linked to EGF gene primers containing different enzyme sites (EGF forward primer, 5′-GGGGAATTC ATGAATAGTGACTCTGAATGTCC-3′; reverse primer, 5′-GGGTCTAGA GCGCAGTTCCCACCACTTC-3′). The EGF gene and LARD3 coding sequences from pTOTAL were PCR amplified using primers with EcoRI/XbaI and XbaI/HindIII sites, respectively. A factor Xa protease cleavage site (IEGR) was added between EGF and LARD3 as a linker by attaching the corresponding oligonucleotide. The EGF and LARD3 sequences were inserted into the EcoRI-XbaI and XbaI-HindIII sites, respectively, of pKK223-3 downstream of the tac promoter to construct pEGF-LARD3. The PrtDEF gene was inserted into the SacI-NdeI site of pACYC-184 to construct pEcPrtDEF. These plasmids were introduced simultaneously into E. coli via heat shock transformation.
Table 1.
Characterization of expression plasmids
| Constructed plasmid | Insert(s) | Vector |
|---|---|---|
| pEGF-LARD3 | EGF and LARD3 | pKK223-3 |
| pEcPrtDEF | PrtDEF transporter gene (from E. chrysanthemi) | pACYC-184 |
Western blot analysis.
The activations of EGFR and EGF were determined by Western blot analysis. Protein in the same amount of cell lysates, collected at the indicated times, was resolved by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the resolved proteins were transferred to polyvinylidene difluoride membranes (Amersham Pharmacia Biotech, Piscataway, NJ). Each membrane was blocked with 5% nonfat skim milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) for 2 h and then incubated with specific antibody overnight at 4°C. The antibodies used were anti-total EGFR, anti-phospho-EGFR, anti-phospho-ERK, anti-actin (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-phospho-AKT (Cell Signaling Technologies, Cambridge, MA). After primary antibody incubation, each membrane was washed with TBST and then incubated with horseradish peroxidase-conjugated secondary antibodies for 2 h. The proteins were detected by enhanced chemiluminescence substrates (ELPIS Biotech, Taejon, South Korea). For some samples, the cell lysate was centrifuged at 13,000 × g at 4°C for 40 min. Target proteins in the supernatants were immunoprecipitated using antibody and protein G-agarose (Santa Cruz Biotechnology) and then detected using Western blot analysis.
EGF purification from culture supernatant of Nissle-AC (E. coli Nissle 1917 with the EGF expression plasmid and PrtDEF transporter).
Released recombinant EGF protein was purified by coimmunoprecipitation using anti-LARD3 antibody and protein G agarose. Bound resin was extensively washed with 20 mM sodium phosphate, pH 7.0, three times. The recombinant protein was eluted with 100 mM glycine buffer, pH 2.5. Eluted proteins were immediately adjusted to pH 7.4 using 1 M Tris-HCl (pH 9.0) to neutralize eluted solution. Eluted samples were tested for the biological activity of activating EGFR and subjected to a wound-healing assay.
Wound-healing assay.
Cells were seeded in culture plates and were cultured in RPMI 1640 medium containing 10% FBS until growth was confluent. Before wound manipulation, epithelial cells were starved overnight in serum-free conditions. All wound-healing processes were observed in serum-free conditions after wound injury to minimize the basal wound-healing effects by growth factors in the serum. A wound was made in the center of each confluent monolayer using a plastic blade. The wounded monolayers then were cultured in serum-free RPMI 1640 in the presence or absence of recombinant E. coli. The changes in cell-free area were monitored for up to 48 h using a digital image processor connected to a microscope (Nikon, Tokyo, Japan), and the areas were measured three times with an image-analyzing program (Leica, Cambridge, United Kingdom). The wound-healing migration from the wound area was determined by measuring the length of the epithelial migration from the wounded edges. Each relative migration was compared to the migration length of control monolayer cells with no bacterial supernatant. The unit of measurement was the fold change in migration length compared to that of the control after 24 h. For each treatment, three dishes were replicated. Ten fields were collected from one dish. For each field, there were more than 10 measurements.
Confocal microscopy.
Cells were incubated in a glass-bottomed culture dish. After treatment with EGF-LARD3 or vehicle (dimethyl sulfoxide [DMSO]), cells were fixed with 4% paraformaldehyde diluted in phosphate-buffered saline (PBS). Fixed cells were permeabilized with 0.2% Triton X-100 in PBS for 10 min. After blocking for 2 h with 3% bovine serum albumin (BSA) in PBS, cells were incubated with a buffer (3% BSA in PBS) at a 1:200 dilution of mouse polyclonal anti-phospho-EGFR (Epitomics, Burlingame, CA) and phospho-ERK (p-ERK) antibody (Santa Cruz Biotechnology) at room temperature for 1.5 h and repeatedly washed using PBS. Incubation with Alexa Fluor 546 goat anti-mouse IgG (H+L) and Alexa Fluor 488 goat anti-rabbit IgG (H+L) was done for 1.5 h at room temperature, followed by repeated washes using PBS. After subsequent staining with 100 ng/ml 4′-6-diamidino-2-phenylindole (DAPI) in PBS for 30 min, which has measurable absorbance at 405 nm, confocal images were obtained using a model FV1000 confocal microscope (Olympus, Tokyo, Japan) using single-line excitation (546 nm) or multitrack sequential excitation (546 and 633 nm). Images were acquired and processed with FV10-ASW software (Olympus). The intensity of signals from the four selected fields was measured using Multi Gauge software (Fuji Film, Tokyo, Japan). The edge of the wound was partitioned into four zones (1 to 4) from the end, and each zone was 25 pixels in width.
Statistical analyses.
Data were analyzed using SigmaStat for Windows (Jandel Scientific, San Rafael, CA). For the comparative analysis of two groups of data, Student's t test was performed. For the comparative analysis of multiple groups, data were subjected to analysis of variance (ANOVA), and pairwise post hoc ANOVA comparisons were made by the Student-Newman-Keuls (SNK) method. Data not meeting the normality assumption were subjected to Kruskal-Wallace ANOVA on ranks, and then pairwise comparisons were made by the SNK method.
RESULTS
Expression of human EGF in E. coli Nissle 1917.
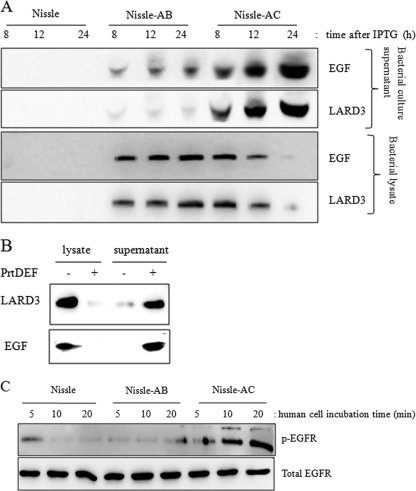
The EGF expression plasmid used in this study encodes an in-frame fusion of human mature EGF polypeptide and one fragment of LARD3, which harbors a set of secretion signals derived from the thermostable lipase (TliA) (Table 1). Together with this plasmid, the ABC transporter gene, including PrtDEF, was introduced for the secretion of LARD3-linked recombinant EGF protein. The PrtDEF transporter of Erwinia chrysanthemi functions at 37°C, the optimum growth temperature for E. coli, while the Pseudomonas fluorescens TliDEF transporter must be expressed at 25 to 27°C for its proper function (1, 8). E. coli Nissle 1917 or E. coli XL1-Blue was transformed with plasmids of EGF and the ABC transporter (PrtDEF) and were designated Nissle-AC and XL1-AC, respectively (Table 2). Bacteria with the EGF expression plasmid and empty vector (pACYC-184) were named Nissle-AB and XL1-AB. Secreted EGF was detected by immunoprecipitation using anti-EGF or anti-LARD antibody. The bands detected using each antibody were shown to be secreted by LARD3-linked EGF protein with an estimated molecular mass of 21 kDa (Fig. 1A). Nissle-AC secreted EGF protein, which increased in a time-dependent manner, whereas EGF was trapped inside the Nissle-AB cells and only a slight amount of EGF was detected (Fig. 1A). A similar pattern was observed in E. coli XL1-Blue expressing LARD3-linked EGF (Fig. 1B). The introduction of the PrtDEF transporter gene led to the extracellular secretion of the recombinant EGF protein. Otherwise, most EGF proteins were trapped in the bacterial cells. Because of the absence of the PrtDEF transporter in Nissle-AB, the extracellular transporting of LARD3-linked EGF was restricted compared to that in Nissle-AC.
Table 2.
Designation of recombinant bacteria
| Recombinant E. coli strain | Properties |
|---|---|
| XL1-AB | E. coli XL1-Blue with pEGF-LARD3 and pACYC-184 |
| XL1-AC | E. coli XL1-Blue with pEGF-LARD3 and pEcPrtDEF |
| Nissle-AB | E. coli Nissle 1917 with pEGF-LARD3 and pACYC-184 |
| Nissle-AC | E. coli Nissle 1917 with pEGF-LARD3 and pEcPrtDEF |
Fig 1.
Expression of human EGF in E. coli Nissle 1917. (A) The recombinant EGF-LARD3 gene was expressed in E. coli Nissle 1917 after 1 mM IPTG treatment in LB broth; the released product was detected using immunoprecipitation with anti-EGF or anti-LARD3 antibody. Each recombinant bacterium was designated Nissle (the wild type of E. coli Nissle 1917), Nissle-AB (E. coli Nissle 1917 with the EGF expression plasmid and pACYC-184), or Nissle-AC (E. coli Nissle 1917 with the EGF expression plasmid and PrtDEF transporter). (B) The PrtDEF transporter gene was introduced into E. coli XL1-Blue expressing LARD3-linked EGF. After 1 mM IPTG treatment, the bacterial lysate and supernatant were assessed by protein analysis. (C) The bacterial culture supernatants were administered to human intestinal epithelial cells (HCT-8) for up to 20 min, and the cellular lysate was subjected to immunoprecipitation with either anti-EGFR or anti-phospho-EGFR antibody for the monitoring of biological activity.
Secreted EGF protein binds to the EGF receptor with intrinsic protein-tyrosine kinase activity, subsequently leading to the autophosphorylation of several tyrosine (Y) residues in the C-terminal domain of EGFR (17). EGF-producing E. coli Nissle 1917 supernatant was applied to the human intestinal epithelial cell culture system to monitor the biological activity of the recombinant probiotic in the in vitro human gut epithelial wound-healing model. HCT-8 human intestinal epithelial cells were treated with the bacterial cell culture medium, and the activation of the human EGF receptor was analyzed. HCT-8 cells are a frequently used human epithelial cell culture model for microbial infection, ulceration, and inflammatory diseases (2, 40, 41) and were used in the assessment of intestinal wound healing. Following the addition of each bacterial culture supernatant, the tyrosine phosphorylation of EGFR was observed with a peak at 10 to 20 min after exposure. The activation of EGFR was more prominent in the presence of Nissle-AC than in the presence of Nissle-AB or wild-type E. coli Nissle 1917 (Fig. 1C).
EGF-LARD3 protein promotes human intestinal epithelial repair in vitro.
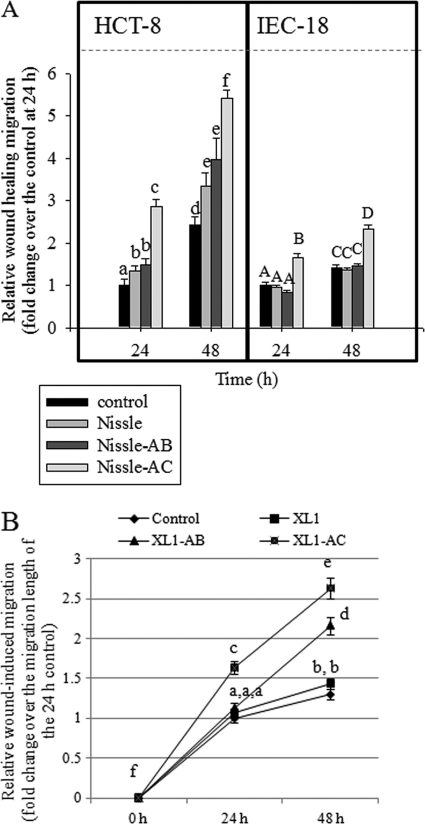
As an EGF-linked biological function in human epithelia, the wound-healing process was the focus of the present study. The efficacy of E. coli Nissle 1917-delivering EGF protein in epithelial healing in vitro was assessed in physically injured human enterocyte monolayers, particularly focusing on epithelial migration. Compared to that in the control E. coli Nissle 1917-treated group, EGF-producing Nissle-AC and Nissle-AB significantly enhanced epithelial healing (Fig. 2A). The wound-healing enhancement by the recombinant bacteria (using Nissle-AB and Nissle-AC supernatants) also was observed in the nontransformed intestinal cell line IEC-18 (Fig. 1A). Since human EGF is about 80% homologous to murine EGF and is known to function effectively in murine models (46), we tested the effect of released human EGF on available nontransformed IEC-18, although it is a murine cell line. In addition to the assessment in E. coli Nissle 1917-mediated EGF delivery, similar effects were observed in the E. coli XL1-Blue-mediated system (Fig. 2B). The EGF-LARD3 fusion protein that was secreted by E. coli XL1-Blue also promoted epithelial wound closure. Moreover, although Nissle-AB does not have the PrtDEF ABC transport system, its induced wound-healing effect was still higher than that on the control E. coli XL1-Blue-treated HCT-8 epithelial monolayers at 48 h, indicating that even a small amount of nonspecific release of EGF was effective for epithelial reconstitution.
Fig 2.
Effect of recombinant EGF-secreting bacteria on epithelial wound healing. The y axis indicates relative migratory activity as described in Materials and Methods. The HCT-8 or IEC-18 cellular monolayer was starved overnight and physically wounded. (A) Control supernatant (RPMI 1640 medium without FBS) and each bacterial (Nissle, Nissle-AB, or Nissle-AC) culture supernatant were administered to wounded epithelial cells, and the relative length of epithelial migration was measured at each time point. Values on the y axis indicate fold change above the migration length of the control after 24 h. Values are means ± standard errors of the means (SEM) (n = 10 to 14). Bars with different letters are significantly different from each other (P < 0.05). Pairwise comparison was performed using post hoc ANOVA SNK methods in each experiment with different epithelial cell lines. (B) The experimental procedure for HCT-8 cells was performed using another general nonpathogenic E. coli strain, designated XL1 (the wild type of E. coli XL1-Blue), XL1-AB (E. coli XL1-Blue with the EGF expression plasmid and pACYC-184), or XL1-AC (E. coli XL1-Blue with the EGF expression plasmid and PrtDEF transporter). Values are means ± SEM (n = 10 to 14). Bars with different letters are significantly different from each other (P < 0.05). Pairwise comparison was performed using post hoc ANOVA SNK methods.
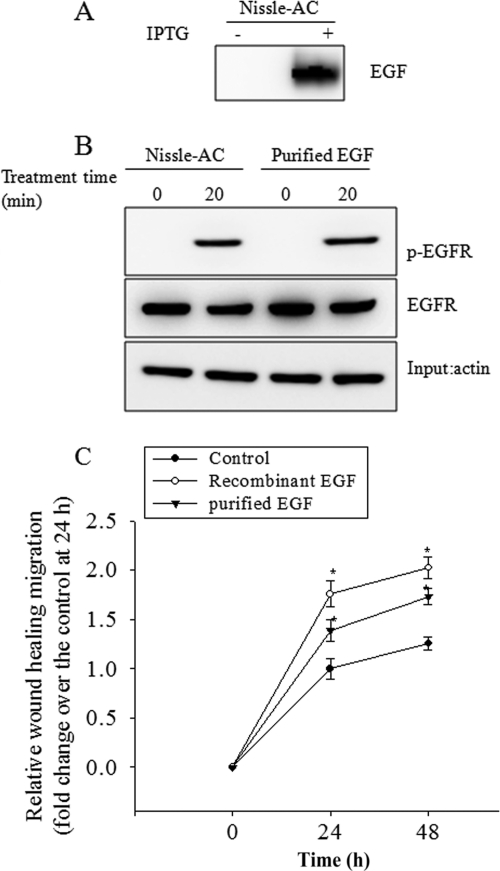
Furthermore, to address the direct action of EGF, we purified released EGF from the Nissle-AC supernatant via the immunoaffinity method (Fig. 3A). The isolated EGF also was biologically effective by activating EGFR as EGF-producing Nissle-AC supernatant (Fig. 3B). The purified EGF had enhancive effects on the wound-healing migration activity in HCT-8 cells (Fig. 3C). As a positive control, the commercially available recombinant EGF was analyzed for its effects on wound healing. Taken together, the data indicate that ABC transporter-mediated EGF secretion by probiotic E. coli Nissle 1917 and E. coli XL1-Blue enhanced the epithelial-wound healing process in vitro in enterocyte monolayers.
Fig 3.
Effect of purified EGF on epithelial wound healing. (A) EGF was purified using the immunoaffinity method and detected as described in Materials and Methods. (B) The bacterial culture supernatants or purified EGF were administered to wounded monolayers of human intestinal epithelial cells (HCT-8) for 20 min, and the cellular lysate was subjected to immunoprecipitation with either anti-EGFR or anti-phospho-EGFR antibody for the monitoring of biological activity. (C) The y axis indicates relative migratory activity as depicted in Materials and Methods. The HCT-8 cellular monolayer was starved overnight and physically wounded. Control (RPMI 1640 medium without FBS), recombinant EGF, and purified EGF from Nissle-AC was administered to wounded epithelial cells, and the relative length of epithelial migration was measured at each time point. Values are means ± SEM (n = 10 to 14). An asterisk represents a significantly different result (P < 0.05) compared to that of each control group at each time point. For comparative analyses of two groups of data, Student's t test was performed.
E. coli Nissle 1917-delivered EGF protein induces wound closure by the activation of the ERK signaling pathway.
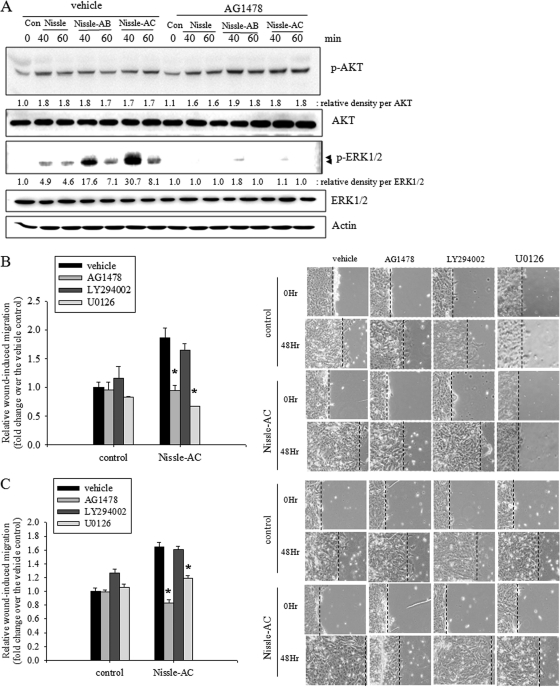
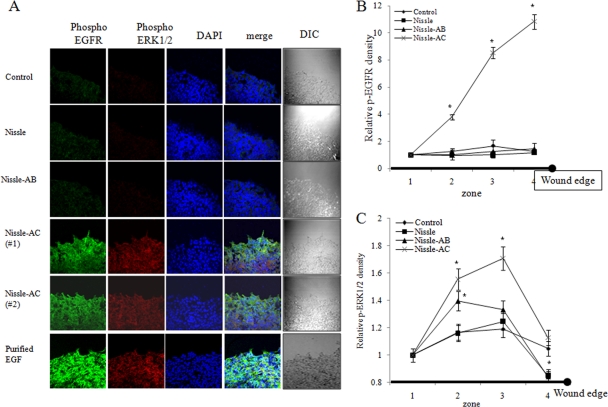
EGFR is the prototype of the ErbB family, which is expressed in nearly all epithelial tissues. The activation of EGFR triggers several main signaling effectors, including the mitogen-activated protein kinase kinase (MEK) extracellular-related kinase (ERK) pathway or the phosphatidylinositol 3-kinase-AKT pathway, which both are responsible for multiple epithelial biophysical processes of proliferation, migration, and other wound-repairing cellular activities. Therefore, we investigated the possibility that EGFR activation by E. coli Nissle 1917-delivered EGF plays a role in mediating AKT and ERK activation and subsequent migratory wound healing in human enterocyte monolayers. To study the signaling pathway induced by EGF-producing recombinant probiotic bacteria, we assessed the effects of EGF signaling inhibition on the phosphorylation of AKT and ERK in HCT-8 cells. Treatment with both wild-type and recombinant E. coli Nissle 1917 enhanced the phosphorylation of ERK1/2 and AKT proteins. In particular, the Nissle-AC group potently activated ERK1/2 and AKT signals (Fig. 4A). In particular, strong transient ERK1/2 activation was observed after around 40 min and decreased soon, while slightly activated AKT was sustained for 60 min. Moreover, the EGFR family tyrosine kinase inhibitor AG1478 completely attenuated EGF-LARD3-induced ERK activation, but the reducing effects on AKT signals were marginal (Fig. 4A), indicating that the secreted recombinant EGF-LARD3 was exclusively involved in ERK activation via the EGF receptor. Moreover, we determined whether the ERK signal was critical for the EGF-linked wound-healing process in Nissle-AC-exposed epithelial monolayers. ERK1/2 signaling inhibition using U0126 MEK inhibitor or the blocking of EGFR-linked signal using AG1478 reduced Nissle-AC-triggered wound-healing migration, whereas AKT inhibition using the LY294002 phosphatidylinositol 3-kinase inhibitor was not significantly effective in HCT-8 and IEC-18 monolayers (Fig. 4B and C). Additionally, the secreted EGF-induced EGFR activation and subsequent phosphorylation of ERK1/2 were confirmed in physically wounded monolayers of human enterocytes using confocal microscopy. In particular, the migrating frontier of the wounded edge displayed the strongest signals in EGFR phosphorylation in the presence of Nissle-AC (Fig. 5A). The edge of the wound was partitioned into four zones (1 to 4). Color signals in each zone were quantified and compared (Fig. 5B and C). Whereas the signal of phospho-EGFR was the highest in the marginal end (zone 4), phospho-ERK1/2 reached maximal levels in the next zone (zone 3), implicating the phosphorylation sequences from EGFR and ERK1/2 in the migrating epithelial edge.
Fig 4.
Involvement of EGF receptor-linked signals in wound healing. (A) Serum-starved intestinal epithelial cells were pretreated with vehicle (DMSO) or 10 μM AG1478 and then stimulated with control supernatant (Con; RPMI 1640 medium without FBS) and each bacterial (Nissle, Nissle-AB, or Nissle-AC) supernatant. The total epithelial cellular lysate was subjected to Western blot analysis. (B and C) Serum-starved wounded intestinal epithelial monolayer cells (HCT-8) (B) or IEC-18 (C) were treated with control and Nissle-AC supernatants in the presence of vehicle (DMSO) or each inhibitor (10 μM AG1478, 5 μM LY294002, and 2 μM U0126). Relative wound closure at 48 h was measured, and the representative pictures (×100 magnification) are shown on the right. Values are means ± SEM (n = 10 to 14). An asterisk indicates significant differences from each vehicle group (P < 0.05). For the comparative analysis of two groups of data, Student's t test was performed.
Fig 5.
Visualization of epithelial signaling activation by recombinant bacteria. (A) Control supernatant (RPMI 1640 medium without FBS), each bacterial (Nissle, Nissle-AB, or Nissle-AC) supernatant, or commercially available purified EGF was administered to wounded epithelial cells for 40 min. Phosphorylated ERK1/2 (red) and phosphorylated EGF receptor (green) was determined by immunofluorescence confocal microscopy. DIC, differential interference contrast. (B and C) The phosphorylation signals were quantified as described in Materials and Methods. Values are means ± SEM (n = 10 to 14). An asterisk indicates significant differences between each control group at each zone (P < 0.05). For comparative analyses of two groups of data, Student's t test was performed.
DISCUSSION
In the present study, we used the PrtDEF ABC transporter system, which works optimally at 37°C. Therefore, Nissle-AC showed more secretion of EGF and subsequently higher biological activity for epithelial wound healing than Nissle-AB and Nissle. However, Nissle-AB also partially enhanced wound-healing migration, although it does not have the PrtDEF transporter. Although most EGF proteins were trapped in the bacterial cells, a small amount of released EGF can be detected in Nissle-AB culture media (Fig. 1A). Regardless of the absence of the PrtDEF transporter, a small amount of intracellular EGF can be exported via other endogenous transporters or autolysis. Even the small amount of EGF thus can account for the partial enhancement of wound-healing migration in the present study. In response to mucosal injury, EGF plays an important role in intestinal cell restitution, proliferation, and maturation. Although the salivary levels of EGF are low in patients with an active gastrointestinal ulcer, these levels normalize during periods of ulcer healing (34). Clinical intravenous treatment with EGF in gastric ulcer patients is effective in triggering ulcer healing (24). Moreover, an oral trial with high doses of recombinant human EGF also enhanced the process of wound repair in the gastrointestinal tract in the patient (22, 37). Generally, less than 1% of protein drugs administered orally get to the target because of their instability and degradation in the digestive tract. To overcome the application problem of the oral recombinant therapeutics for intestinal ulcerative disease, a protected and enhanced delivery system is required. In the present study, probiotic E. coli strain Nissle 1917 was designed as a protein factory and capsule vector against digestive fluid. The intent is that stably established recombinant probiotic bacteria in the mucosal barrier would secrete proteins to the neighboring wounded region, hastening the epithelial healing process with a reduced chance of exposure to proteolytic degradation by digestive enzymes. Moreover, probiotic-delivered EGF protein would be favorable for stimulating the surface EGF receptor via a type I secretion system ABC transporter instead of a type III secretion system, which directly injects proteins into the epithelial cytoplasm. For these goals to be realized, further study is necessary to confirm the in vivo wound-healing action by the recombinant bacteria and its secreted protein EGF in experimental animals and patients with ulcerative colitis.
Probiotic bacteria, including E. coli Nissle 1917, have been evaluated in the last few years as an alternative, safe therapeutic modality for treating gastrointestinal ulcerative diseases (23, 26, 32). In the present study, E coli Nissle 1917 alone was not a good enhancer of in vitro wound healing, although it marginally enhanced wound healing and the production of proliferation-linked kinases such as AKT and ERK1/2 (Fig. 2 and 4). Instead of a direct effect of the bacteria on the migratory activity of intestinal epithelial cells, it is assumed that the probiotic bacteria can act favorably by enhancing mucosal barrier integrity, particularly through the upregulation of tight junction-associated genes in the epithelial layer (42). Epithelial integrity via tight junctions is critical for maintaining active fluid absorption. Otherwise, leaky gut syndrome can occur, which leads to noninfective diarrhea as well as the translocation of the commensal bacteria and subsequent pathological hypersensitive immune activation of underlying lymphocytes. Although clinical investigations have been promising, evidence for the efficacy of the probiotic bacteria in Crohn's disease is weak, and many questions still remain unanswered. In the present study, the functional supplementation of E. coli Nissle 1917 with EGF protein provided host cells with an additional guarantee for the wound-healing process. Enhanced cell migration via the EGF receptor-linked signaling pathway positively contributed to the wound-healing process, which also can cooperate with the bacterial action of the enhanced barrier integrity via tight junctions against ulcerative colitis.
In terms of the signaling cascade in the wound-healing process, EGF-loaded E. coli was shown to stimulate EGF receptor phosphorylation and the subsequent kinase cascade ERK1/2, which importantly influenced epithelial migration into the wounded region of the cell monolayer. Although EGF receptor activation could trigger AKT signals, activation can be EGF receptor independent. The EGF family of receptor-linked signals can be differentially regulated in different downstream pathways involved in cell proliferation and differentiation, including the Raf-MEK-ERK and AKT pathways (14, 30). Despite evidence that AKT plays critical roles in cancer cell motility (19), the EGF receptor-ERK1/2 signaling cascade can be important in the regulation of multiple key biophysical processes underlying cell migration both in pathogenic and physiological states (18, 25, 35). In the present study, the blockade of the EGF receptor could not suppress the activated AKT signals, which implicates the EGF receptor-independent phosphorylation of AKT by the recombinant bacteria and their products. Moreover, EGF receptor-mediated epithelial migration can be driven by sustained ERK signals from autocrine stimulation. The extended activation of the EGF receptor signal is not always desirable, because uncontrolled EGF receptor activation can be associated with epithelial malignancy. Therefore, the intestinal residency half-life of recombinant bacteria should be controlled to limit the persistent overstimulation of EGF receptor-linked signals in the intestinal epithelial barrier, which is another unresolved issue for the safe use of recombinant probiotics.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education, Science, and Technology (no. 2009-0087028 and 2009-0065479).
Footnotes
Published ahead of print 19 December 2011
REFERENCES
- 1. Ahn JH, Pan JG, Rhee JS. 1999. Identification of the tliDEF ABC transporter specific for lipase in Pseudomonas fluorescens SIK W1. J. Bacteriol. 181:1847–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alcantara Warren C, et al. 2008. Detection of epithelial-cell injury, and quantification of infection, in the HCT-8 organoid model of cryptosporidiosis. J. Infect. Dis. 198:143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barrandon Y, Green H. 1987. Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor-alpha and epidermal growth factor. Cell 50:1131–1137 [DOI] [PubMed] [Google Scholar]
- 4. Bickert T, et al. 2009. Probiotic Escherichia coli Nissle 1917 suppresses allergen-induced Th2 responses in the airways. Int. Arch. Allergy Immunol. 149:219–230 [DOI] [PubMed] [Google Scholar]
- 5. Blay J, Brown KD. 1985. Epidermal growth factor promotes the chemotactic migration of cultured rat intestinal epithelial cells. J. Cell. Physiol. 124:107–112 [DOI] [PubMed] [Google Scholar]
- 6. Blum G, Marre R, Hacker J. 1995. Properties of Escherichia coli strains of serotype O6. Infection 23:234–236 [DOI] [PubMed] [Google Scholar]
- 7. Carpenter G, Cohen S. 1990. Epidermal growth factor. J. Biol. Chem. 265:7709–7712 [PubMed] [Google Scholar]
- 8. Chung CW, et al. 2009. Export of recombinant proteins in Escherichia coli using ABC transporter with an attached lipase ABC transporter recognition domain (LARD). Microb. Cell Fact. 8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coffey RJ, Romano M, Goldenring J. 1995. Roles for transforming growth factor-alpha in the stomach. J. Clin. Gastroenterol. 21(Suppl. 1):S36–S39 [PubMed] [Google Scholar]
- 10. Cohen S, Elliott GA. 1963. The stimulation of epidermal keratinization by a protein isolated from the submaxillary gland of the mouse. J. Investig. Dermatol. 40:1–5 [DOI] [PubMed] [Google Scholar]
- 11. Delepelaire P. 1994. PrtD, the integral membrane ATP-binding cassette component of the Erwinia chrysanthemi metalloprotease secretion system, exhibits a secretion signal-regulated ATPase activity. J. Biol. Chem. 269:27952–27957 [PubMed] [Google Scholar]
- 12. Derynck R, Roberts AB, Winkler ME, Chen EY, Goeddel DV. 1984. Human transforming growth factor-alpha: precursor structure and expression in E. coli. Cell 38:287–297 [DOI] [PubMed] [Google Scholar]
- 13. Do VT, Baird BG, Kockler DR. 2010. Probiotics for maintaining remission of ulcerative colitis in adults. Ann. Pharmacother. 44:565–571 [DOI] [PubMed] [Google Scholar]
- 14. Downward J. 2003. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 3:11–22 [DOI] [PubMed] [Google Scholar]
- 15. D'Souza AL, Rajkumar C, Cooke J, Bulpitt CJ. 2002. Probiotics in prevention of antibiotic associated diarrhoea: meta-analysis. BMJ 324:1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duncker SC, Lorentz A, Schroeder B, Breves G, Bischoff SC. 2006. Effect of orally administered probiotic E. coli strain Nissle 1917 on intestinal mucosal immune cells of healthy young pigs. Vet. Immunol. Immunopathol. 111:239–250 [DOI] [PubMed] [Google Scholar]
- 17. Firth LC, Baker NE. 2003. EGF receptor signaling: a prickly proposition. Curr. Biol. 13:R773–R774 [DOI] [PubMed] [Google Scholar]
- 18. Forsyth CB, et al. 2007. Regulation of oxidant-induced intestinal permeability by metalloprotease-dependent epidermal growth factor receptor signaling. J. Pharmacol. Exp. Ther. 321:84–97 [DOI] [PubMed] [Google Scholar]
- 19. Gan Y, et al. 2010. Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene 29:4947–4958 [DOI] [PubMed] [Google Scholar]
- 20. Grabig A, et al. 2006. Escherichia coli strain Nissle 1917 ameliorates experimental colitis via toll-like receptor 2- and toll-like receptor 4-dependent pathways. Infect. Immun. 74:4075–4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gronbach K, et al. 2010. Safety of probiotic Escherichia coli strain Nissle 1917 depends on intestinal microbiota and adaptive immunity of the host. Infect. Immun. 78:3036–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haedo W, et al. 1996. Oral human recombinant epidermal growth factor in the treatment of patients with duodenal ulcer. Rev. Esp. Enferm. Dig. 88:409–418 [PubMed] [Google Scholar]
- 23. Henker J, Muller S, Laass MW, Schreiner A, Schulze J. 2008. Probiotic Escherichia coli Nissle 1917 (EcN) for successful remission maintenance of ulcerative colitis in children and adolescents: an open-label pilot study. Z. Gastroenterol. 46:874–875 [DOI] [PubMed] [Google Scholar]
- 24. Itoh M, Matsuo Y. 1994. Gastric ulcer treatment with intravenous human epidermal growth factor: a double-blind controlled clinical study. J. Gastroenterol. Hepatol. 9(Suppl. 1):S78–S83 [DOI] [PubMed] [Google Scholar]
- 25. Joslin EJ, Opresko LK, Wells A, Wiley HS, Lauffenburger DA. 2007. EGF-receptor-mediated mammary epithelial cell migration is driven by sustained ERK signaling from autocrine stimulation. J. Cell Sci. 120:3688–3699 [DOI] [PubMed] [Google Scholar]
- 26. Konturek PC, et al. 2009. Probiotic bacteria Escherichia coli strain Nissle 1917 attenuates acute gastric lesions induced by stress. J. Physiol. Pharmacol. 60(Suppl. 6):41–48 [PubMed] [Google Scholar]
- 27. Konturek SJ, et al. 1992. Transforming growth factor alpha and epidermal growth factor in protection and healing of gastric mucosal injury. Scand. J. Gastroenterol. 27:649–655 [DOI] [PubMed] [Google Scholar]
- 28. Koronakis E, Hughes C, Milisav I, Koronakis V. 1995. Protein exporter function and in vitro ATPase activity are correlated in ABC-domain mutants of HlyB. Mol. Microbiol. 16:87–96 [DOI] [PubMed] [Google Scholar]
- 29. Kruis W, et al. 1997. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 11:853–858 [DOI] [PubMed] [Google Scholar]
- 30. Kyriakis JM, et al. 1992. Raf-1 activates MAP kinase-kinase. Nature 358:417–421 [DOI] [PubMed] [Google Scholar]
- 31. Leatham MP, et al. 2009. Precolonized human commensal Escherichia coli strains serve as a barrier to E. coli O157:H7 growth in the streptomycin-treated mouse intestine. Infect. Immun. 77:2876–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matthes H, Krummenerl T, Giensch M, Wolff C, Schulze J. 2010. Clinical trial: probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli Nissle (EcN). BMC Complement. Altern. Med. 10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mondel M, et al. 2009. Probiotic E. coli treatment mediates antimicrobial human beta-defensin synthesis and fecal excretion in humans. Mucosal Immunol. 2:166–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ohmura E, et al. 1987. Salivary immunoreactive human epidermal growth factor (IR-hEGF) in patients with peptic ulcer disease. Hepatogastroenterology 34:160–163 [PubMed] [Google Scholar]
- 35. Osaki LH, Figueiredo PM, Alvares EP, Gama P. 2011. EGFR is involved in control of gastric cell proliferation through activation of MAPK and Src signalling pathways in early-weaned rats. Cell Prolif. 44:174–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pai R, Tarnawski A. 1998. Signal transduction cascades triggered by EGF receptor activation: relevance to gastric injury repair and ulcer healing. Dig. Dis. Sci. 43:14S–22S [PubMed] [Google Scholar]
- 37. Palomino A, et al. 2000. A multicenter, randomized, double-blind clinical trial examining the effect of oral human recombinant epidermal growth factor on the healing of duodenal ulcers. Scand. J. Gastroenterol. 35:1016–1022 [DOI] [PubMed] [Google Scholar]
- 38. Plassmann D, Schulte-Witte H. 2007. Treatment of irritable bowel syndrome with Escherichia coli strain Nissle 1917 (EcN): a retrospective survey. Med. Klin. (Munich) 102:888–892 [DOI] [PubMed] [Google Scholar]
- 39. Schulze J, Sonnenborn U. 1995. Re.: Oral administration of a certain strain of live Escherichia coli for intestinal disorders? (Infection 23 [1995] 51–54). Infection 23:184–188 [DOI] [PubMed] [Google Scholar]
- 40. Sifuentes LY, Di Giovanni GD. 2007. Aged HCT-8 cell monolayers support Cryptosporidium parvum infection. Appl. Environ. Microbiol. 73:7548–7551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thebault S, et al. 2006. Proteomic analysis of glutamine-treated human intestinal epithelial HCT-8 cells under basal and inflammatory conditions. Proteomics 6:3926–3937 [DOI] [PubMed] [Google Scholar]
- 42. Ukena SN, et al. 2007. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One 2:e1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wells A. 1999. EGF receptor. Int. J. Biochem. Cell Biol. 31:637–643 [DOI] [PubMed] [Google Scholar]
- 44. Wells A, Ware MF, Allen FD, Lauffenburger DA. 1999. Shaping up for shipping out: PLCgamma signaling of morphology changes in EGF-stimulated fibroblast migration. Cell Motil. Cytoskeleton 44:227–233 [DOI] [PubMed] [Google Scholar]
- 45. Wu HM, Yuan Y, McCarthy M, Granger HJ. 1996. Acidic and basic FGFs dilate arterioles of skeletal muscle through a NO-dependent mechanism. Am. J. Physiol. 271:H1087–H1093 [DOI] [PubMed] [Google Scholar]
- 46. Wu W, et al. 2007. Expression of epidermal growth factor (EGF)/transforming growth factor-alpha by human lung cancer cells determines their response to EGF receptor tyrosine kinase inhibition in the lungs of mice. Mol. Cancer Ther. 6:2652–2663 [DOI] [PubMed] [Google Scholar]
- 47. Zyrek AA, et al. 2007. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 9:804–816 [DOI] [PubMed] [Google Scholar]