Abstract
Mycoplasma pneumoniae is a significant human respiratory pathogen that causes high morbidity worldwide. No vaccine to prevent M. pneumoniae infection currently exists, since the mechanisms of pathogenesis are poorly understood. To this end, we constructed a P30 cytadhesin mutant (P-130) with a drastically reduced capacity for binding to erythrocytes and an inability to glide on glass substrates. This mutant was determined to be avirulent and cannot survive in the lungs of BALB/c mice. We also ascertained that the previously identified P30 gliding motility mutant II-3R is avirulent and also cannot be recovered from the lungs of mice after infection. Mutant P130 was then assessed for its efficacy as a live attenuated vaccine candidate in mice after challenge with wild-type M. pneumoniae. After vaccination with the P-130 P30 mutant, mice showed evidence of exacerbated disease upon subsequent challenge with the wild-type strain PI1428, which appears to be driven by a Th17 response and corresponding eosinophilia. Our results are in accordance with other reports of vaccine-induced disease exacerbation in rodents and emphasize the need to better understand the basic mechanisms of M. pneumoniae pathogenesis.
INTRODUCTION
Mycoplasma pneumoniae is a chronic human pathogen and the etiological agent of many cases of bronchitis and community-acquired pneumonia. Infection with this bacterium can also cause and/or exacerbate other diseases including asthma, myocarditis, sickle cell disease, and encephalitis. Outbreaks are common at such institutions as military bases, schools, and hospitals, where individuals are in close contact for long periods of time, reflecting the community-acquired nature of infections. Diagnosis is difficult as most medical laboratories do not screen for this pathogen, since quick and inexpensive tests are not readily available. Common first-line β-lactam antibiotics are ineffective treatments since mycoplasmas lack a cell wall; consequently, this pathogen is often overlooked during diagnosis of affected individuals, and common treatments do not target the source of the disease. This results in considerable economic and societal hardships due to lost and ineffective work/school time, making this pathogen a burden to public health (1, 42).
Several virulence determinants of M. pneumoniae have been previously identified and characterized. This bacterium is thought primarily to exploit an extracellular niche and requires a complex tip structure to attach to its host's mucosal epithelium. The tip structure is also involved in gliding motility and cell division. Densely clustered on the surface of the tip structure are the major cytadhesins P1 and P30 and cytadherence-accessory proteins B/C, which are supported by a framework of interdependent cytoskeletal proteins, including HMW1, HMW2, HMW3, P200, P65, P41, and P24 (reviewed in reference 2). Cytadhesin protein P30 has been shown to be critical for both attachment (11, 32) and gliding motility, although its requirements for each are distinguishable (18). Thus, mutant II-3R, having an altered protein sequence at amino acids 135 to 151, cytadheres at a level approximately 60% of the wild-type level but exhibits a drastically reduced gliding velocity, approximately 5% of that of the wild type (18). The 17-residue substitution in mutant II-3R occurs in extracellular domain II, indicating that this region is particularly important for gliding motility, whereas the downstream C-terminal domain III was shown to be essential for cytadherence (4). Interestingly, intranasal inoculation in golden Syrian hamsters of the related P30 null mutant strain II-3 resulted in reduced lung lesions and mycoplasmal recovery relative to wild-type results at day 14 postinoculation, indicating that P30 may be important for the virulence of M. pneumoniae (24). While complementation with the wild-type allele by transposon delivery restores cytadherence and gliding motility (18, 32), we cannot rule out the possibility of secondary mutations impacting its virulence in vivo. Furthermore, the contributions of attachment and gliding motility in M. pneumoniae virulence cannot be distinguished with II-3, since both phenotypes are absent from this mutant.
After attaching to the host respiratory epithelium, M. pneumoniae initiates inflammatory responses characterized by the infiltration of lymphocytes, neutrophils, and sometimes eosinophils. M. pneumoniae engages the host's immune system by Toll-like receptor 1/2/6 (TLR1/2/6) ligation with bacterial lipoproteins, leading to the secretion of cytokines and chemokines (20, 34). Rather than mediating clearance, the inflammatory response instead contributes to immunopathology in both rodents and humans, thereby complicating vaccine design. For example, several vaccine trials conducted in military personnel during the 1960s using inactivated bacteria resulted in minimal efficacy (reviewed in reference 27), and in some instances vaccination appeared to exacerbate disease upon subsequent challenge with virulent M. pneumoniae (38, 39). Disease exacerbation has also been observed in rodent models of M. pneumoniae vaccination and challenge (8). However, few vaccine studies have been conducted in humans or rodents using a live attenuated strain or mucosal administration of a vaccine, making such approaches a possible means to achieve protective immunity.
In this work, we further characterized the role of M. pneumoniae protein P30 in disease pathogenesis using specific mutants and have assessed the relative contributions of hemadsorption and gliding motility in P30's virulence. We also sought to determine if a P30 mutant could potentially be used as a vaccine candidate to prevent acute pneumonia in a mouse model of infection. Interpretation of the data and potential immune mechanisms associated with enhanced disease after vaccination and challenge are discussed.
MATERIALS AND METHODS
Bacteria and culture conditions.
All M. pneumoniae strains, including PI1428 (passage 12) (5) and M129 (passage 13), were cultured at 37°C in fortified commercial (FC) or Hayflick's medium (10% horse serum, 5% yeast extract). The generation and characterization of P30 mutants II-3 and II-3R have been previously described (24, 32). Isogenic P30 mutant P-130 was generated from an M. pneumoniae transposon (Tn) mutant library (min-Tn pMT85, an aliquot of the same mouse challenge lot of strain PI1428, was used to generate the library) using the haystack mutagenesis approach, as previously described (40). Gentamicin sulfate (80 μg/ml) was added to the medium for the propagation of the transposon mutant. For mouse studies, frozen 50-μl aliquots of each strain were thawed, and 10 ml of FC medium was added to the tube. Cultures were incubated at 37°C with gentle shaking at 120 RPM on an orbital shaker. After 5 h of incubation, optical density at 620 nm (OD620) measurements were aseptically conducted on a spectrophotometer to estimate CFU counts per ml of culture, and color changing unit (CCU) measurements were conducted using 10-fold serial dilutions to ensure that spectrophotometric measurements represented live bacteria. Samples were then centrifuged at 2,000 × g for 10 min at 4°C, the supernatant was decanted, and the pellet was suspended in a volume of fresh medium that was calculated to give the desired concentration, given the total CFU measured in the tube (calculated as follows: CFU/ml × volume [in ml] before centrifugation = CFU [pellet]; CFU [pellet]/X volume = CFU [desired]/volume [desired], where “X” is the variable volume added to the pellet).
Phenotypic characterization of P-130.
Protein expression was analyzed by Western blotting as previously described (4) but using antibodies and concentrations described elsewhere (10). Transposon mutant P-130 was assessed qualitatively for hemadsorption as previously described (16). Satellite colony formation was examined after 60 h of growth in borosilicate glass chamber slides (Nunc Nalgene, Naperville, IL) as a qualitative assessment of gliding motility or at 37°C for 18 h for quantitative calculation of gliding motility for individual cells (>1,000 cells observed for gliding frequency and at least 38 cells per strain for mean and corrected gliding velocities), as previously described (18).
In vivo virulence assessment and vaccination/challenge study.
All animal experiments were conducted in accordance with our approved Institutional Animal Care and Use Committee protocol (protocol A09-012). Mycoplasma and murine virus-free BALB/c mice (2 months old, female) were acquired from Charles River Laboratories (Worcester, MA). Mice were allowed to acclimate in filter-top cages (5 mice per cage) for 1 week in a biosafety hood prior to use. Mice were sedated using vaporized isoflurane before being handled. Four animal experiments were conducted: one to assess the virulence of mutant P-130 (study 1), another to assess the virulence of mutant II-3R (study 2), a vaccination and challenge study to determine the efficacy of the P30 mutant P-130 as a vaccine candidate (study 3), and a vaccination and challenge study of the immune response after challenge (study 4). Groups of mice were inoculated by the intranasal route with 50 μl of 7 × 107 CFU (studies 1 and 2) or 1 × 107 CFU (studies 3 and 4) of the respective M. pneumoniae strain (n = 5 for each group except study 4, for which n = 3). For studies 1 and 2, negative-control mice were inoculated with sterile FC medium and positive-control mice were inoculated with wild-type PI1428 (study 1) or M129 (study 2). For studies 3 and 4, negative-control mice were given sterile FC medium on day 0 (vaccination) and day 21 (challenge), positive-control mice were sham vaccinated with sterile FC medium (day 0) and challenged with virulent strain PI1428 (day 21), and test groups were vaccinated with P30 mutant strain P-130 (day 0) and challenged with strain PI1428 (day 21). Mice were humanely sacrificed 4 days postchallenge via cervical dislocation after all experiments, and the lungs were immediately harvested for histopathology and mycoplasma culture (studies 1, 2, and 3) or subjected to bronchoalveolar lavage (study 4). For mycoplasma recovery, the lower right lung was removed and placed into 3 ml of FC medium, briefly vortexed, and incubated for 3 h at 37°C. The remaining tissue was inflated with 10% neutral buffered formalin and allowed to fix for histopathologic evaluation. After 3 h of incubation, mycoplasma recovery samples were passed through a 0.45-μm filter and transferred to new tubes. Acidic cultures (as indicated by a color shift from red to orange or yellow) were adjusted to an approximate pH of 7.4 by adding 10 N NaOH until a red color was restored. Quantification of recovery cultures was performed by assessing CCU in neat and 10-fold serial dilutions (50 μl into 450 μl FC medium, out to 10−5 dilution). Samples were incubated for 28 days or until a color shift was observed.
Histopathology.
After fixation for 48 h in 10% buffered formalin, tissues were routinely processed and stained as previously described (40). Perivascular and peribronchial lymphocytic infiltrates were graded in increasing severity with scores ranging from 0 (no visible lesions) to 4 (marked lesions), in increments of 1. Lesion score evaluations were performed in a blinded fashion by a board-certified veterinary pathologist who was unaware of the experimental groups.
Differential leukocyte count and quantitative measurement of cytokines in BALF.
Processing of bronchoalveolar lavage fluid (BALF) and subsequent differential leukocyte counts were conducted as described by Siddiqui et al. (36). Data are represented as the percentage of each leukocyte cell type per 500 total white blood cells. BALF supernatants were concentrated 10-fold using Amicon Ultra-4 3K centrifugal filter devices (Millipore Corp., Billerica, MA), per the manufacturer's instructions, before cytokine analysis. Cytokine concentrations in 10× BALF (interleukin 1β [IL-1β], IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-13, IL-17, gamma interferon [IFN-γ], and tumor necrosis factor alpha [TNF-α]) were measured using a custom-made Bioplex array system (Bio-Rad, Hercules, CA) on a Bio-Rad Bioplex-200 system, with recombinant murine cytokines as standards. The postassay sample concentration and limits of detection were reported, taking into consideration the 10× sample concentration prior to analysis. Luminex bead array software was used to analyze the data.
Statistical analyses.
Lesion scores for all experiments were analyzed between the test group and the positive- and negative-control groups by the nonparametric Kruskal-Wallis analysis of variance (ANOVA) on ranks test with a Student-Newman-Keuls (SNK) post hoc test for multiple pairwise comparisons between groups (α = 0.05). Quantitative gliding motility, differential leukocyte counts, and cytokine concentrations for all experiments were analyzed between the test group and the positive- and negative-control groups by the parametric one-way ANOVA test with a Student-Newman-Keuls post hoc test for multiple pairwise comparisons between groups (*, P < 0.05; **, P < 0.01; ***, P < 0.001). A surrogate value which fell below the limit of detection (lod) was used for statistical analysis of BALF cytokine concentrations in animals by subtracting 0.1 from the lod. All data were analyzed using the software program SigmaPlot for Windows, version 11.0 (Systat Software Inc., San Jose, CA).
RESULTS
Generation and characterization of P30 mutant P-130.
An isogenic M. pneumoniae P30 mutant was isolated from a library of more than 1,900 Tn mutants generated in strain PI1428 using the haystack mutagenesis approach. This mutant was designated P-130, as the 130th clone picked from the PI1428 mutant library. The Tn insertion mapped to nucleotide 156 (of 825) of the P30 gene, resulting in disruption of 81% of the coding sequence.
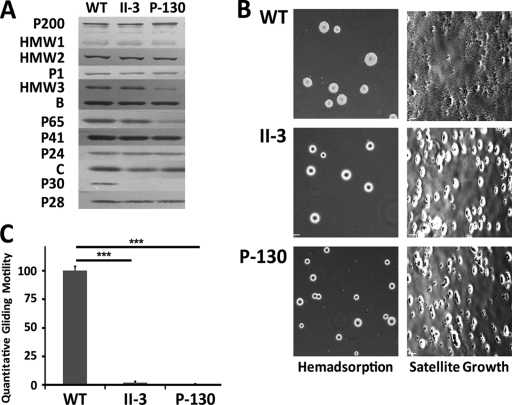
P-130 protein expression of cytadherence and cytoskeletal proteins (P200, HMW1, HMW2, HMW3, P1, B, C, P65, P41, P30, P28, and P24) was assessed by Western blotting and compared to the previously characterized P30 mutant II-3 and wild-type M129 (Fig. 1A). No P30 protein was detected in P-130 (as expected), and P65 was also noticeably reduced, as previously described for other P30 mutants (21). Of note, mutant P-130 also had reduced steady-state levels of the HMW3 protein, a phenotype that has not been previously described for P30 mutants. The gene for HMW3 is immediately downstream of the gene for P30, and they appear to be cotranscribed (43). Disruption of P30 in transposon mutant P-130 may have a polar effect on HMW3 transcription, whereas the frameshift in mutant II-3 may not, accounting for the difference in HMW3 expression in these mutants. To ensure that the observed reduction in protein levels for P30, P65, and HMW3 was not due to nucleotide (and hence amino acid/antigenic) differences between strains M129 (for which antibody binding has been verified for each protein) and PI1428 (the parental strain of P-130), we generated a draft sequence of the complete genome of strain PI1428 (data not shown) and compared these genes with the published M129 genome (12). No amino acid differences were observed in these predicted proteins, consistent with the conclusion that levels of proteins P30, P65, and HMW3 in mutant P-130 were reduced as a result of disruption of the P30 gene.
Fig 1.
Phenotypic characterization of P30 mutant P-130. (A) Western blot of cytoskeletal and cytadherence proteins in wild-type strain M129, mutant II-3, and mutant P-130. (B) Qualitative hemadsorption and satellite growth of wild-type and P30 mutant colonies. (C) Quantitative gliding motility of wild-type and P30 mutant strains on a glass substrate. Data represent the mean motility and one standard deviation from the mean. Significance was determined by one-way ANOVA with SNK post hoc multiple pairwise comparison (***, P < 0.001).
The previously characterized P30 mutant II-3 has been shown to have marked reductions in hemadsorption and gliding motility (18, 32). Erythrocyte binding, satellite growth, and individual cell gliding for mutant P-130 were also negligible compared to those for M129 (Fig. 1B and C). Thus, the P30 mutant P-130 exhibits deficient attachment and motility phenotypes, similar to those observed in previously characterized P30 mutants.
Virulence assessment of P30 mutants P-130 and II-3R in BALB/c mice.
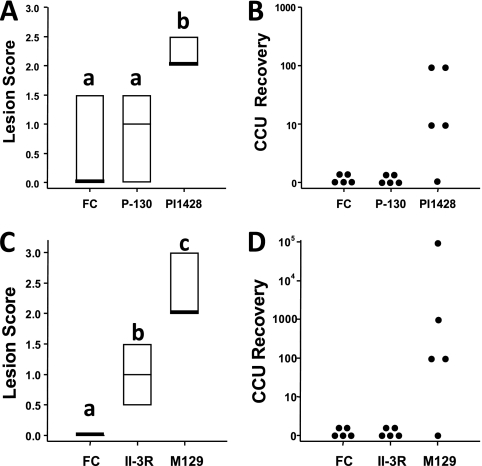
In previous work, mutant II-3 was inoculated into hamsters to assess its virulence in vivo and was shown to be avirulent 14 days postinoculation (24). Given that II-3 was a naturally arising hemadsorption-negative mutant, it is not known if other mutations that contribute to the attenuated phenotype potentially exist in this strain. To confirm that P30 is indeed essential for virulence, we intranasally inoculated BALB/c mice with a high dose of 7 × 107 CFU of isogenic mutant P-130, PI1428 (positive control), or sterile FC medium (negative control) and performed pathological assessments (Fig. 2A) and mycoplasma recovery (Fig. 2B) from the lungs 4 days postinoculation (the time point which corresponds with peak lesions in these mice [17]). Consistent with the attenuation of mutant II-3 in hamsters, P-130 was avirulent in the lungs of mice based on pathological assessments and the absence of mycoplasma recovery. These data indicate that cytadhesin P30 is essential for the virulence of M. pneumoniae in BALB/c mice.
Fig 2.
Lesion scores and mycoplasma recovery from the analysis of virulence of P30 mutants in BALB/c mice. Lesion scores for mutant P-130 (A) or II-3R (C). FC, growth medium (negative control). Box plot results represent the 25th percentile, median, and 75th percentile. Groupings for significance (denoted as a, b, and c the above box plots) were determined by ANOVA on ranks test with SNK post hoc multiple pairwise comparisons (α = 0.05). (B and D) Mycoplasma CCU recovery for mutant P-130 (B) or II-3R (D). The y axis indicates the maximum number of cells recovered given the highest 10-fold serial dilution exhibiting a color shift for a given mouse.
Given the similar avirulent phenotype shared by P30 mutants II-3 and P-130, as well as the lack of hemadsorption and gliding motility of both strains, interest was raised in the virulence phenotype of II-3 revertant II-3R, which exhibits near-wild-type hemadsorption but severely reduced gliding motility (18). Again, mice were intranasally inoculated and lesions/recovery was assessed 4 days later. II-3R was not recoverable from the lungs of mice (Fig. 2D), and lesions in this group were significantly less severe than those observed in the M129-inoculated animals (Fig. 2C), indicating that this mutant is also attenuated. Thus, it appears that gliding motility, independent of hemadsorption, is essential for the virulence and in vivo survival of M. pneumoniae in BALB/c mice.
Vaccination with P-130 and challenge with PI1428.
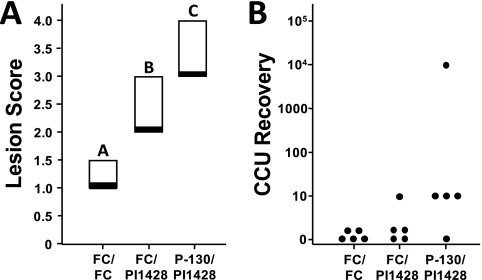
Given that a high dose of the gliding-motility and hemadsorption P30 mutant P-130 resulted in no lung lesions after intranasal inoculation in BALB/c mice, we initiated a vaccination/challenge study to explore whether this mutant might serve as a potential vaccine candidate. A standard dose of 1 × 107 CFU of P130 was intranasally administered to groups of mice, and 3 weeks later the animals were challenged with a standard dose of strain PI1428. Negative controls included animals inoculated with sterile FC medium at the vaccination and challenge time points, while sham-vaccinated (FC medium) and challenged (PI1428) mice served as positive controls. Four days postchallenge, pathological assessments and mycoplasma recovery were assessed as described above (Fig. 3A and B). Given the lack of recovery of this mutant in the virulence experiment, any recovered mycoplasmas were considered to be wild-type PI1428, so additional genotyping was not conducted. Lesion scores were compared between the vaccinated group and controls, and vaccinated animals were statistically different from both sham-vaccinated and negative controls (which were also significantly different from each other). The lesion scores in the vaccinated group were actually higher than those observed in the sham-vaccinated group, indicating that disease exacerbation had occurred upon challenge with a virulent strain. There was also more frequent mycoplasma recovery in the vaccinated group, indicating that the increased severity of the lesions may be beneficial for the in vivo survival of M. pneumoniae or that persistence of the bacterium resulted in more severe disease, perhaps as a result of immune dysregulation.
Fig 3.
Lesion scores and mycoplasma recovery from a vaccination and challenge study to determine vaccine candidate efficacy. (A) Postchallenge lesion score from negative control, sham vaccinated and challenged with virulent strain PI1428, or P-130-vaccinated and challenged groups. Box plot results represent the 25th percentile, median, and 75th percentile. Groupings for significance (denoted as a, b, and c above the box plots) were determined by ANOVA on ranks test with SNK post hoc multiple pairwise comparisons (α = 0.05). (B) Mycoplasma CCU recovery. The y axis indicates the maximum number of cells recovered given the highest 10-fold serial dilution exhibiting a color shift for a given mouse.
Differential cell count and quantitative determination of cytokines in BALF.
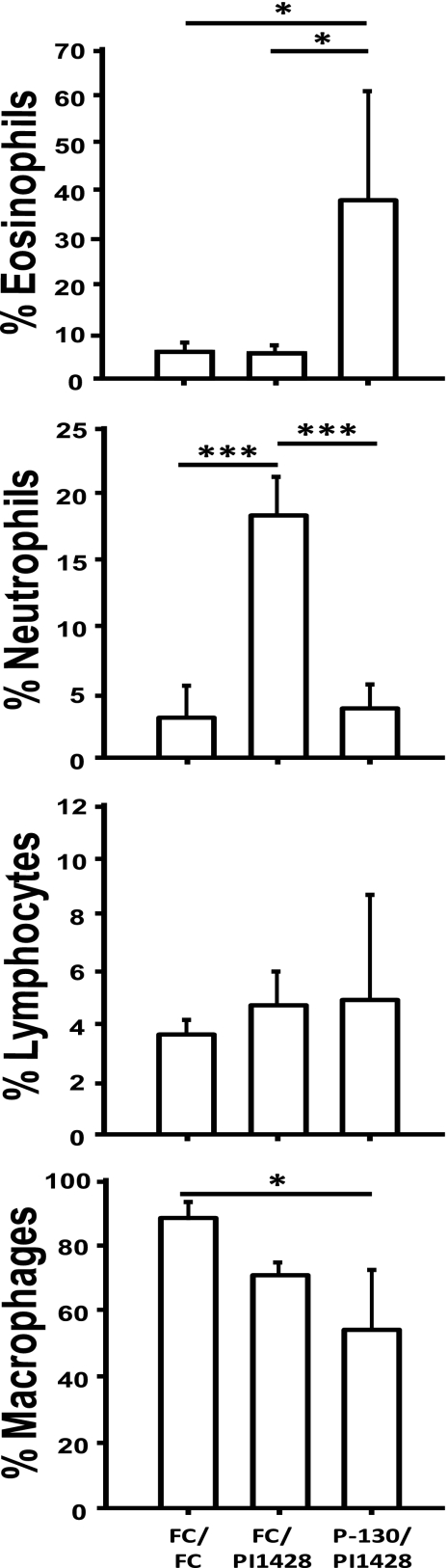
An understanding of the mechanism(s) of disease exacerbation is essential for rational design of an M. pneumoniae vaccine. Eosinophilic and neutrophilic infiltration was noted in the lesions of many animals in the vaccination study; hence, a second vaccination/challenge experiment was conducted and BALF was collected to determine the proportions of different leukocytes (Fig. 4) and cytokines (Fig. 5) in lavage samples. Differential counts (based on cell morphology and staining characteristics) of macrophages, neutrophils, eosinophils, and lymphocytes were performed with BALF samples from each animal after cytocentrifugation and cell staining. A statistically significant reduction in the percentage of macrophages was observed in the vaccinated group in part due to the significant increase in the proportion of eosinophils. Conversely, the sham-vaccinated group had a significantly higher proportion of neutrophils than all other groups. Thus, vaccination with mutant P-130 appears to dysregulate the postchallenge infiltration of neutrophils observed in sham-vaccinated controls to eosinophilic infiltration.
Fig 4.
Differential leukocyte counts in BALF from vaccinated and challenged BALB/c mice. Relative percentages of macrophages, lymphocytes, neutrophils, and eosinophils in BALF samples after cytocentrifugation and staining. Cell type was determined based on morphology and staining characteristics, as observed under a light microscope. Data represent the means and standard deviations for 500 total cells. Significance was determined by one-way ANOVA with SNK post hoc multiple pairwise comparison (*, P < 0.05; ***, P < 0.001).
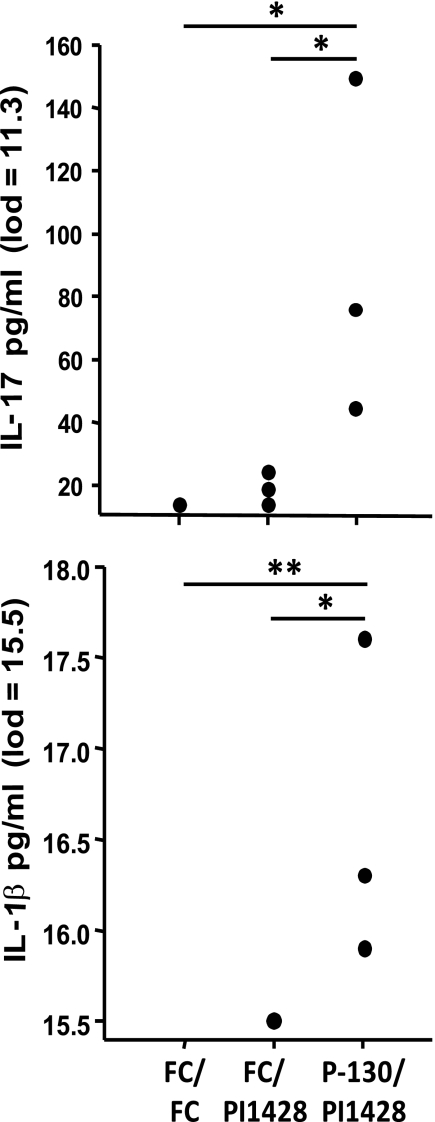
Fig 5.
Cytokine concentration in BALF as determined by Luminex bead assay. BALF supernatants were concentrated 10-fold using Amicon 3k filter devices and then assayed using a Bio-Rad Bioplex system. Sample concentrations were interpolated from a standard curve for each cytokine, and limits of detection (lod) were determined. Sample concentrations and lods were then divided by 10 to account for the concentration factor of the BALF. Dots represent concentrations for individual mice. Significance was determined by one-way ANOVA with SNK post hoc multiple pairwise comparison (*, P < 0.05; **, P < 0.01).
The concentration of various cytokines (IL-1β, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-13, IL-17, IFN-γ, and TNF-α) in BALF was determined using a Luminex bead assay. These cytokines were chosen to evaluate the potential Th1/Th2/Th17 responses that may influence the infiltration of different populations of white blood cells into the lung mucosa. There was a statistically significant increase in the presence of IL-1β and IL-17 in the BALF of vaccinated animals compared to controls. IL-4, IL-10, IL-12p70, and IFN-γ were not detected in any samples above the limit of detection. Of note, IL-6 and TNF-α were detected at higher levels in two out of three mice in the vaccinated group than in controls. The expression of IL-1β, IL-6, and TNF-α (in the absence of IL-4, IFN-γ, or IL-10) has been shown to augment IL-17 responses (reviewed in reference 29) and may contribute to the exacerbated disease observed in the vaccinated mice.
DISCUSSION
M. pneumoniae is a major human respiratory pathogen, and its virulence is dependent on its ability to glide toward, and attach to, the bronchial epithelium using a unique set of cytoskeletal and cytadhesin molecules that are unique to the Mollicutes. The previous finding that the P30 mutant II-3 is attenuated in a hamster model indicated that these phenotypes are essential for the virulence of M. pneumoniae (24). However, this isolate was obtained based on a screen for hemadsorption mutants, and while a genetic lesion in P30 would later be characterized in II-3 (32), it is possible that other mutations may have contributed to its attenuation. Therefore, we generated an isogenic transposon mutant (P-130) that disrupted 81% of the P30 gene sequence and determined that it is also attenuated and unrecoverable in a mouse model of disease, thus reaffirming a requirement for P30 in M. pneumoniae pathogenesis in vivo. The avirulent phenotype of mutants II-3 and P-130 is not surprising since attachment to host respiratory cells has been shown to be a critical component of the virulence of many other mycoplasmas, including the closely related chicken pathogen Mycoplasma gallisepticum (31).
Gliding motility is a crucial component of the pathogenesis of several human pathogens, including Plasmodium falciparum (26) and Toxoplasma gondii (19). A previous study that utilized cultured human bronchial epithelial cells infected with the M. pneumoniae P200 mutant with diminished gliding velocity but wild-type cytadherence demonstrated that gliding motility is essential for the bacterium to reach target cells in vitro (22). Mutants II-3 and P-130 harbor mutations that affect large portions of the P30 gene, and these mutants have a drastically reduced capacity for both hemadsorption and gliding motility. The natural reversion to a hemadsorption-positive/gliding-negative phenotype of P30 mutant II-3R then allowed us to determine if gliding motility is an essential component of the virulence of M. pneumoniae, independent of its ability to attach to host cells. Indeed, mutant II-3R was avirulent in mice and was not recovered from lung tissues 4 days postinoculation. The critical role of M. pneumoniae gliding in vivo has not been reported previously and has implications for gliding motility as a component of the virulence of all motile species in the genus.
Significant morbidity is associated with M. pneumoniae infection, particularly in children, and it is surprising that little recent effort has been devoted to the development of an M. pneumoniae vaccine. Several failed attempts to develop a sufficiently efficacious vaccine were conducted in the 1960s (with disease reductions as low as 11% and as high as 51%; reviewed in reference 27), and similar results were obtained from a study of a live attenuated vaccine administered to hamsters prior to challenge (45). Additionally, while not common, the literature contains anecdotal reports of disease exacerbation upon natural infection following vaccination with inactivated M. pneumoniae in individuals who elicited weak humoral responses to the vaccine (38, 39). Natural reexposure to the bacterium with resulting pulmonary disease has also been observed in military recruits (3) and in young adults (14). Moreover, clinical disease is more severe in young adults than in young children (14), suggesting that repeated exposure to the organism may enhance disease outcomes. Exacerbation of disease following M. pneumoniae repeated exposure or vaccination has also been reported in animals, particularly when early recall responses are taken into consideration (6–9). The mice in our studies were sacrificed 4 days postchallenge to examine the acute pathological response to the organism, which correlates with peak severity of lesions in BALB/c mice (17). Our findings of exacerbated disease when mice are vaccinated and challenged are in accordance with previous reports and emphasize the difficulties associated with M. pneumoniae vaccine development. Of note, Chu et al. (6) observed dramatically higher mycoplasma recovery upon rechallenge in BALB/c mice, which is in agreement with our data. This may indicate that prior sensitization by vaccination or infection with M. pneumoniae may create an environment in the pulmonary tissues that confers an advantage to the bacterium for survival in the mouse, with the bacterium then exacerbating inflammation while occupying this ecological niche.
Failure to effectively clear M. pneumoniae from the lungs of infected mice is associated with inflammation. Several studies indicate that infection with wild-type M. pneumoniae results in an infiltration of neutrophils into the respiratory tissues, and these cells can be readily found in BALF. However, results are mixed as to the importance of neutrophils in clearance of M. pneumoniae from the lungs (25, 41, 44). Consistent with these reports, we also found a significant increase in neutrophilic infiltration of the lungs in the sham-vaccinated and challenged mice. However, we also observed a significant increase in eosinophilic infiltration in BALF samples from P-130-vaccinated and challenged mice (one animal from this group had 64% eosinophils in the differential leukocyte count). Such eosinophilia resembles that found in allergic asthma, consistent with reports that M. pneumoniae exacerbates atopy in asthmatic patients (reviewed by Nisar et al. (30). Unfortunately, few reports have studied the influx of eosinophils during M. pneumoniae infection (particularly in acute models of infection), but Chu et al. (6) found no evidence of eosinophils in rechallenged BALB/c mice 3 days postinfection (which also exhibited exacerbated disease). Our results likely differ based on the virulent versus attenuated (or hemadsorption/gliding motility-negative phenotype) nature of the sensitization to M. pneumoniae used in these two studies, thereby reflecting a difference in how the immune system is stimulated upon initial exposure to different isolates.
The production of proinflammatory cytokines during M. pneumoniae infection is well established and contributes to the immunopathology of the disease. Analysis of cytokines in BALF from human patients shows a strong Th2 (IL-4) bias (23), and this appears to correlate with high antigen-specific IgE responses in serum, thereby implicating M. pneumoniae in asthma exacerbation (33, 35). Early studies in BALB/c mice indicated that these animals produce a predominant Th1 cytokine response after intranasal infection (15, 17), but more recent reports point toward a Th17 response in these mice (37, 44). It is difficult for us to interpret our data in relation to these reports, since we did not see a difference in cytokine profiles in BALF when sham-vaccinated animals were compared to the negative controls due to the high detection limit of the instrument (likely due to sample matrix interference). What is clear from our study is the correlation of exacerbated disease in P-130-vaccinated mice and significant levels of IL-17 cytokines in BALF from this group. Interestingly, these mice also exhibited marked eosinophilia, but a report from Wu et al. (44) indicates that IL-17 generated in response to M. pneumoniae results in infiltration of neutrophils but not eosinophils. Given the single time point assessed in our study, we cannot determine the dynamics of IL-17 production and eosinophilic infiltration, making a direct comparison to the work in Wu et al. (44) difficult. Regardless, human macrophages have been shown to produce IL-1β, IL-6, and TNF-α in response to IL-17, and P-130-vaccinated animals exhibited higher levels of these cytokines (in addition to IL-17) in BALF after challenge. Such a cytokine profile has been found in the BALF of acute respiratory distress syndrome patients who succumbed to their illness (28) and may stimulate fibroblasts to produce collagen, as is found in asthma (13).
In conclusion, we have reaffirmed that cytadhesin P30 is essential for the virulence of M. pneumoniae, here in a BALB/c model. Furthermore, gliding motility is a necessary component of the pathogenesis of this bacterium, independent of attachment. Vaccination with a P30 gliding and hemadsorption mutant prior to challenge produced exacerbated disease, which included an eosinophilic infiltration and IL-17 response. Taken together, these results indicate that vaccination of mice with a live attenuated strain of M. pneumoniae results in disease exacerbation upon challenge with a virulent strain and bode caution in the design of future vaccines. Future research focusing on the development of an M. pneumoniae vaccine could likely benefit from the utilization of alternative animal models, such as nonhuman primates.
ACKNOWLEDGMENT
We thank the animal care staff at the University of Connecticut for their assistance with animal husbandry.
Footnotes
Published ahead of print 17 January 2012
REFERENCES
- 1. Atkinson TP, Balish MF, Waites KB. 2008. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol. Rev. 32:956–973 [DOI] [PubMed] [Google Scholar]
- 2. Balish MF, Krause DC. 2006. Mycoplasmas: a distinct cytoskeleton for wall-less bacteria. J. Mol. Microbiol. Biotechnol. 11:244–255 [DOI] [PubMed] [Google Scholar]
- 3. Brunner H, James WD, Horswood RL, Chanock RM. 1972. Measurement of Mycoplasma pneumoniae mycoplasmacidal antibody in human serum. J. Immunol. 108:1491–1498 [PubMed] [Google Scholar]
- 4. Chang HY, Jordan JL, Krause DC. 2011. Domain analysis of protein P30 in Mycoplasma pneumoniae cytadherence and gliding motility. J. Bacteriol. 193:1726–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chanock RM, et al. 1961. Eaton agent pneumonia. JAMA 175:213–220 [DOI] [PubMed] [Google Scholar]
- 6. Chu HW, et al. 2006. Repeated respiratory Mycoplasma pneumoniae infections in mice: effect of host genetic background. Microbes Infect. 8:1764–1772 [DOI] [PubMed] [Google Scholar]
- 7. Cimolai N, Cheong AC, Morrison BJ, Taylor GP. 1996. Mycoplasma pneumoniae reinfection and vaccination: protective oral vaccination and harmful immunoreactivity after re-infection and parenteral immunization. Vaccine 14:1479–1483 [DOI] [PubMed] [Google Scholar]
- 8. Cimolai N, Mah DG, Taylor GP, Morrison BJ. 1995. Bases for the early immune response after rechallenge or component vaccination in an animal model of acute Mycoplasma pneumoniae. Vaccine 13:305–309 [DOI] [PubMed] [Google Scholar]
- 9. Cimolai N, Taylor GP, Mah D, Morrison BJ. 1992. Definition and application of a histopathological scoring scheme for an animal model of acute Mycoplasma pneumoniae pulmonary infection. Microbiol. Immunol. 36:465–478 [DOI] [PubMed] [Google Scholar]
- 10. Cloward JM, Krause DC. 2009. Mycoplasma pneumoniae J-domain protein required for terminal organelle function. Mol. Microbiol. 71:1296–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dallo SF, Chavoya A, Baseman JB. 1990. Characterization of the gene for a 30-kilodalton adhesin-related protein of mycoplasma pneumonaie. Infect. Immun. 58:4163–4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dandekar T, et al. 2000. Re-annotating the Mycoplasma pneumoniae genome sequence: adding value, function and reading frames. Nucleic Acids Res. 28:3278–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elias JA, Freundlich B, Adams S, Rosenbloom J. 1990. Regulation of human lung fibroblast collagen production by recombinant interleukin-1, tumor necrosis factor, and interferon-gamma. Ann. N. Y. Acad. Sci. 580:233–244 [DOI] [PubMed] [Google Scholar]
- 14. Fernald GW, Collier AM, Clyde WA., Jr 1975. Respiratory infections due to Mycoplasma pneumoniae in infants and children. Pediatrics 55:327–335 [PubMed] [Google Scholar]
- 15. Fonseca-Aten M, et al. 2005. Mycoplasma pneumoniae induces host-dependent pulmonary inflammation and airway obstruction in mice. Am. J. Respir. Cell Mol. Biol. 32:201–210 [DOI] [PubMed] [Google Scholar]
- 16. Hahn TW, Krebes KA, Krause DC. 1996. Expression in Mycoplasma pneumoniae of the recombinant gene encoding the cytadherence-associated protein HMW1 and identification of HMW4 as a product. Mol. Microbiol. 19:1085–1093 [DOI] [PubMed] [Google Scholar]
- 17. Hardy RD, et al. 2001. Elevated cytokine and chemokine levels and prolonged pulmonary airflow resistance in a murine Mycoplasma pneumoniae pneumonia model: a microbiologic, histologic, immunologic, and respiratory plethysmographic profile. Infect. Immun. 69:3869–3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hasselbring BM, Jordan JL, Krause DC. 2005. Mutant analysis reveals a specific requirement for protein P30 in Mycoplasma pneumoniae gliding motility. J. Bacteriol. 187:6281–6289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huynh MH, Carruthers VB. 2006. Toxoplasma MIC2 is a major determinant of invasion and virulence. PLoS Pathog. 2:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Into T, et al. 2007. Synthesis and characterization of a dipalmitoylated lipopeptide derived from paralogous lipoproteins of Mycoplasma pneumoniae. Infect. Immun. 75:2253–2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jordan JL, Berry KM, Balish MF, Krause DC. 2001. Stability and subcellular localization of cytadherence-associated protein P65 in Mycoplasma pneumoniae. J. Bacteriol. 183:7387–7391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jordan JL, et al. 2007. Protein P200 is dispensable for Mycoplasma pneumoniae hemadsorption but not gliding motility or colonization of differentiated bronchial epithelium. Infect. Immun. 75:518–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koh YY, Park Y, Lee HJ, Kim CK. 2001. Levels of interleukin-2, interferon-gamma, and interleukin-4 in bronchoalveolar lavage fluid from patients with Mycoplasma pneumonia: implication of tendency toward increased immunoglobulin E production. Pediatrics. 107:E39. [DOI] [PubMed] [Google Scholar]
- 24. Krause DC, Leith DK, Wilson RM, Baseman JB. 1982. Identification of Mycoplasma pneumoniae proteins associated with hemadsorption and virulence. Infect. Immun. 35:809–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lai JF, et al. 2010. Critical role of macrophages and their activation via MyD88-NFkappaB signaling in lung innate immunity to Mycoplasma pneumoniae. PLoS One 5:e14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leykauf K, et al. 2010. Protein kinase A dependent phosphorylation of apical membrane antigen 1 plays an important role in erythrocyte invasion by the malaria parasite. PLoS Pathog. 6:e1000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Linchevski I, Klement E, Nir-Paz R. 2009. Mycoplasma pneumoniae vaccine protective efficacy and adverse reactions—systematic review and meta-analysis. Vaccine 27:2437–2446 [DOI] [PubMed] [Google Scholar]
- 28. Meduri GU, et al. 1995. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest 108:1303–1314 [DOI] [PubMed] [Google Scholar]
- 29. Miossec P, Korn T, Kuchroo VK. 2009. Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 361:888–898 [DOI] [PubMed] [Google Scholar]
- 30. Nisar N, Guleria R, Kumar S, Chand Chawla T, Ranjan Biswas N. 2007. Mycoplasma pneumoniae and its role in asthma. Postgrad. Med. J. 83:100–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Papazisi L, et al. 2002. GapA and CrmA coexpression is essential for Mycoplasma gallisepticum cytadherence and virulence. Infect. Immun. 70:6839–6845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Romero-Arroyo CE, et al. 1999. Mycoplasma pneumoniae protein P30 is required for cytadherence and associated with proper cell development. J. Bacteriol. 181:1079–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seggev JS, Sedmak GV, Kurup VP. 1996. Isotype-specific antibody responses to acute Mycoplasma pneumoniae infection. Ann. Allergy Asthma Immunol. 77:67–73 [DOI] [PubMed] [Google Scholar]
- 34. Shimizu T, Kida Y, Kuwano K. 2007. Triacylated lipoproteins derived from Mycoplasma pneumoniae activate nuclear factor-kappaB through toll-like receptors 1 and 2. Immunology 121:473–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shimizu T, et al. 1991. Immunoglobulin levels, number of eosinophils in the peripheral blood and bronchial hypersensitivity in children with Mycoplasma pneumoniae pneumonia. Arerugi 40:21–27 (In Japanese.) [PubMed] [Google Scholar]
- 36. Siddiqui S, et al. 2008. Pulmonary eosinophilia correlates with allergen deposition to the lower respiratory tract in a mouse model of asthma. Clin. Exp. Allergy 38:1381–1390 [DOI] [PubMed] [Google Scholar]
- 37. Sieve AN, et al. 2009. A novel IL-17-dependent mechanism of cross protection: respiratory infection with mycoplasma protects against a secondary listeria infection. Eur. J. Immunol. 39:426–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith CB, Chanock RM, Friedewald WT, Alford RH. 1967. Mycoplasma pneumoniae infections in volunteers. Ann. N. Y. Acad. Sci. 143:471–483 [DOI] [PubMed] [Google Scholar]
- 39. Smith CB, Friedewald WT, Chanock RM. 1967. Inactivated Mycoplasma pneumoniae vaccine. Evaluation in volunteers. JAMA 199:353–358 [PubMed] [Google Scholar]
- 40. Szczepanek SM, et al. 2010. Identification of lipoprotein MslA as a neoteric virulence factor of Mycoplasma gallisepticum. Infect. Immun. 78:3475–3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tani K, Shimizu T, Kida Y, Kuwano K. 2011. Mycoplasma pneumoniae infection induces a neutrophil-derived antimicrobial peptide, cathelin-related antimicrobial peptide. Microbiol. Immunol. 55:582–588 [DOI] [PubMed] [Google Scholar]
- 42. Waites KB, Talkington DF. 2004. Mycoplasma pneumoniae and its role as a human pathogen. Clin. Microbiol. Rev. 17:697–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Waldo RH, III, et al. 1999. Transcriptional analysis of the hmw gene cluster of Mycoplasma pneumoniae. J. Bacteriol. 181:4978–4985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu Q, et al. 2007. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 9:78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yayoshi M, Araake M, Hayatsu E, Takezawa T, Yoshioka M. 1985. Immunogenicity and protective effect of hemolysis mutants of Mycoplasma pneumoniae. Microbiol. Immunol. 29:1029–1037 [DOI] [PubMed] [Google Scholar]