Abstract
The majority of virulence gene expression in Bordetella is regulated by a two-component sensory transduction system encoded by the bvg locus. In response to environmental cues, the BvgAS regulatory system controls expression of a spectrum of phenotypic phases, transitioning between a virulent (Bvg+) phase and a nonvirulent (Bvg−) phase, a process referred to as phenotypic modulation. We hypothesized that the ability of Bordetella bronchiseptica to undergo phenotypic modulation is required at one or more points during the infectious cycle in swine. To investigate the Bvg phase-dependent contribution to pathogenesis of B. bronchiseptica in swine, we constructed a series of isogenic mutants in a virulent B. bronchiseptica swine isolate and compared each mutant to the wild-type isolate for its ability to colonize and cause disease. We additionally tested whether a BvgAS system capable of modulation is required for direct or indirect transmission. The Bvg− phase-locked mutant was never recovered from any respiratory tract site at any time point examined. An intermediate phase-locked mutant (Bvgi) was found in numbers lower than the wild type at all respiratory tract sites and time points examined and caused limited to no disease. In contrast, colonization of the respiratory tract and disease caused by the Bvg+ phase-locked mutant and the wild-type strain were indistinguishable. The Bvg+ phase-locked mutant transmitted to naïve pigs by both direct and indirect contact with efficiency equal to that of the wild-type isolate. These results indicate that while full activation of the BvgAS regulatory system is required for colonization and severe disease, it is not deleterious to direct and indirect transmission. Overall, our results demonstrate that the Bvg+ phase is sufficient for respiratory infection and host-to-host transmission of B. bronchiseptica in swine.
INTRODUCTION
Respiratory disease in pigs is a serious concern for swine producers today. The most recent survey conducted by the National Animal Health Monitoring System (NAHMS) found that respiratory problems are a major cause of mortality in swine herds, with 53.7% of nursery pig deaths and 60.1% of grower-finisher pig deaths attributed to respiratory problems (36). Bordetella bronchiseptica is widespread in swine populations and is an important contributor to respiratory disease in pigs. In young pigs, it is a primary cause of bronchopneumonia and in older pigs contributes to secondary pneumonia. It is the primary etiologic agent of nonprogressive atrophic rhinitis, a mild to moderately severe reversible condition, and it promotes colonization by toxigenic strains of Pasteurella multocida, which leads to severe progressive atrophic rhinitis (11, 22). In pigs with pneumonia, B. bronchiseptica is often isolated in combination with other pathogens (29). Numerous studies have demonstrated that coinfection with B. bronchiseptica increases colonization and exacerbates the severity of disease caused by both viral and bacterial pathogens, including swine influenza virus (SIV), porcine reproductive and respiratory syndrome virus (PRRSV), porcine respiratory coronavirus (PRCV), Haemophilus parasuis, Pasteurella multocida, and Streptococcus suis (3, 5–8, 21, 37, 38). Additionally, there is a growing concern regarding the ability of B. bronchiseptica to inhibit the efficacy of SIV vaccines and to exacerbate enhanced pneumonia in pigs administered an inactivated SIV vaccine followed by challenge with a heterologous virus.
A universal underlying pathogenic mechanism is shared among Bordetella strains in that the majority of virulence gene expression is regulated by a two-component sensory transduction system encoded by the bvg locus. This locus comprises a histidine kinase sensor protein, BvgS, and a DNA-binding response regulator protein, BvgA. In response to environmental cues, such as temperature, MgSO4, or nicotinic acid concentrations, BvgAS controls expression of a spectrum of phenotypic phases, transitioning between a virulent (Bvg+) phase and a nonvirulent (Bvg−) phase, a process referred to as phenotypic modulation. During the virulent Bvg+ phase, the BvgAS system is fully active and many of the known virulence factors are expressed, such as filamentous hemagglutinin (FHA), pertactin, fimbriae, adenylate cyclase-hemolysin toxin, and dermonecrotic toxin (DNT), as well as a type III secretion system (TTSS) (12). Conversely, BvgAS is inactive during the Bvg− phase, resulting in the maximal expression of motility loci, virulence-repressed genes (vrg genes), and genes required for the production of urease (1, 2, 25). Previous studies involving phase-locked and ectopic expression mutants demonstrated that the Bvg+ phase promotes respiratory tract colonization by Bordetella pertussis and B. bronchiseptica (1, 13, 14, 23, 26), while the Bvg− phase of B. bronchiseptica promotes survival under conditions of nutrient deprivation, such as those potentially encountered in an environmental reservoir (13, 14). A third phenotypic phase, referred to as Bvgi or intermediate phase, can be induced by the addition of MgSO4 or nicotinic acid at concentrations lower than those needed to fully induce the Bvg− phase (14). The Bvgi phase is characterized by the expression of a subset of the Bvg+ phase genes (such as fhaB and prn) and Bvgi-specific genes (such as bipA and bcfA) (14, 33).
Many respiratory pathogens are highly contagious and can be transmitted from host to host by direct and indirect means. B. bronchiseptica is highly contagious among mammals, including swine, and experimental direct and airborne transmission of B. bronchiseptica has been documented (4, 35). A proposed role for phenotypic modulation by the BvgAS signal transduction system is to coordinate regulation of gene sets required for survival in the different environments that may be encountered by B. bronchiseptica, including those encountered during transmission between hosts. In this model, the Bvgi phase, or other phase intermediates between Bvgi and Bvg+, is proposed to be required for aerosol or indirect transmission (12, 14, 33). However, this frequently proposed hypothesis has never been experimentally addressed. In this report, we investigate the Bvg phase-dependent contribution to pathogenesis of B. bronchiseptica in swine by constructing a series of isogenic mutants in a virulent B. bronchiseptica swine isolate and comparing each mutant to the wild-type isolate for its ability to colonize and cause disease. Additionally, we test whether a BvgAS system capable of modulation is required for direct or indirect transmission. Our results demonstrate that in a natural host, phenotypic modulation of the Bvg+ phase is not required for respiratory infection and host-to-host transmission of B. bronchiseptica in swine.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. bronchiseptica strain KM22, isolated from a swine herd with atrophic rhinitis, has been used in a number of studies by our laboratory in which we have shown it is capable of causing atrophic rhinitis and pneumonia in piglets (3–9, 21, 28). KM22 was used for construction of strains TN23, TN30, and TN31. All B. bronchiseptica strains were grown at 37°C on Bordet-Gengou (BG) agar (Difco, Sparks, MD) supplemented with 10% sheep's blood or, in some cases, in Stainer-Scholte (SS) broth. Escherichia coli One Shot TOP10 (Invitrogen, Carlsbad, CA) was used for all cloning steps, and E. coli SM10λpir was used to mobilize plasmids into B. bronchiseptica KM22. When appropriate, antibiotics were included at the following concentrations: carbenicillin, 100 μg/ml; chloramphenicol, 30 μg/ml; kanamycin, 100 μg/ml; and streptomycin, 40 μg/ml.
Cloning and construction of B. bronchiseptica KM22 mutants.
For construction of TN23, the Bvg+ phase-locked derivative of KM22, the bvgS gene from KM22 was amplified by PCR using primers bvgS-for and bvgS-rev (Table 1). The resulting amplicon was cloned into pCR2.1 (Invitrogen, Carlsbad, CA) to create pSMS3. The bvgS-C3 (13) mutation R570H was introduced into pSMS3 using primers R570Hfor and R570Hrev (Table 1) and the QuikChange site-directed mutagenesis kit (Agilent Technologies, Inc., Santa Clara, CA) according to the manufacturer's instructions. The mutation was confirmed by sequencing, and the resulting plasmid was named pTN15. Flanking attB sites were added by PCR using primers bvgS-5′-attB1 and bvgS-3′-attB2 (Table 1). The modified bvgS gene was cloned into pDONR201 (Invitrogen, Carlsbad, CA), using the Gateway cloning system (Invitrogen, Carlsbad, CA), to obtain pSMS26. pSMS26 was recombined with pABB-CRS2 (31), which confers sucrose sensitivity, and digested with NcoI and NotI, to obtain pSMS29 using the Gateway cloning system. pSMS29 was introduced into E. coli SM10λpir (32) and transconjugated into KM22, as previously described (17). Transconjugates were selected on medium containing streptomycin and chloramphenicol. Cells from a second recombination event, resulting in the excision of the plasmid, were then selected on medium containing sucrose. A single colony, designated TN23, was selected for subsequent use. Confirmation that the introduced mutation resulted in the specified phase-locked phenotype was obtained by growing wild-type KM22 and TN23 on BG agar and BG agar containing MgSO4 and evaluating colony morphology and hemolytic activity. TN23 was further confirmed by PCR amplification of the bvgS gene and DNA sequence analysis.
Table 1.
Primers used for cloning and quantitative real-time PCR in this study
| Primer name or gene target | Sequence (5′ to 3′) |
|---|---|
| bvgS-for | ACCTCGCCAAACGCAACAATCTCG |
| bvgS-rev | ATCTGCTGCCATGCCGGTATCTGC |
| bvgS-FA-for | GAGCTCTTAGGTTTTGAAATCCAGCGCAGTAGTCT |
| bvgS-FA-rev | TCTAGACCGCAGGCGGTGCGGGGCGGGCAT |
| bvgS-FB-for | TCTAGATGGCTACGCGCCTGGCTGGAGCAAC |
| bvgS-FB-rev | ACTTTTCCCAATCGTACTGCGTGAAGCTT |
| TN42-for | GAGCTCTTAGGTTTTGAAATCCAGCGCAGTA |
| TN42-rev | CCTAGGAAGCTTCACGCAGTACGATTGGGAAAAG |
| R570Hfor | CCGCTCGGCCCGCTTGTGCTGGCGGATCTGGCG |
| R570Hrev | CGCCAGATCCGCCAGCACAAGCGGGCCGAGCGG |
| T733Mfor | GCCGATGATCGCGTTCATCGGCATGCGGATCTCGTGGCTCATCG |
| T733Mrev | CGATGAGCCACGAGATCCGCATGCCGATGAACGCGATCATCGGC |
| bvgS-5′-attB1 | GGGGACAAGTTTGTACAAAAAAGCAGGCTACACCTCGCCAAACGCAACAATCTCG |
| bvgS-3′-attB2 | GGGGACCACTTTGTACAAGAAAGCTGGGTAATCTGCTGCCATGCCGGTATCTGC |
| TN18-for | CCTAGGTACTACCGCCGCGAGATGCCGCCCA |
| TN18-rev | ACTAGTGCAACAATCTCGCCTAGCGCCGCGCAT |
| flhC | CTCGGTGGACTGGTTCATGA |
| CGTTGTAGAACAGCGACGAATG | |
| cheZ | TGATGGCGCAGGATTTCC |
| GCCCACCACGTCCATCAT | |
| flgB | CTGATGTACCGCCTGCCTTAC |
| GCTGTCCATGTCGACGGTATT | |
| bcfA | GCTGCACGATCCAAATTATCG |
| TGTTCATGGTGTCGAGATAGATGA | |
| cyaA | CACTGAGCAGAACAATCCTTTCC |
| CGTGAGCATCTGGCTTTCAC | |
| prn | CAGCACGGCATCCACATC |
| GCCTGACGACCGCTTACC | |
| bopN | TGCCGAGGAAAAGCATCACT |
| GCCAGAGCATCGGACGTT | |
| bopD | CGGCTCGGTGAAGACATCTAC |
| GCCTCCCGCATCTGTTGA | |
| bopB | GCTCAATTCGACGAGGCCTAT |
| TGTGCGTACTCGCCATATCG | |
| fim3 | CATGGGCACCGACGAAA |
| ACATCAGTGCCCGTGAAGGT | |
| bipA | CGCGATCACGAACATGGA |
| TTGCCGGAGACGGTAACG | |
| fimA | CAGACGAAAGCCGACAACGT |
| TGGCGGAACCATCCAGAT | |
| bvgA | TTGACGATCACCCTGTACTGAGA |
| TCGAATCCTTCCTTTTCCATCA | |
| bvgS | CGGCCGAGTCGATTCTTG |
| GAATCAGTTCTTCGAGCAATTGC | |
| bvgR | TGCTTACCGTCAGTACGTTCGA |
| GCATACATGAGTTCTGGCATCAG | |
| fimN | GCGACGCCGTTTTCCA |
| CATTGCCCAGCGATTCG | |
| fimX | CACGATCGAGGACCCGAG |
| TTGGAGATCGTGGGCAGG | |
| fim2 | TGCGATCATCGCTGCAA |
| TTCTGGTTGGAGATGCGGAT | |
| dnt | GCAGAAAGTACGGCACTACAAGGT |
| CCTGTTGTGATTTTCGATTCCA | |
| 16S | TCAGCATGTCGCGGTGAAT |
| TGTGACGGGCGGTGTGTA |
For TN30, the Bvg− phase-locked mutant, a 1,046-bp DNA fragment covering the region immediately 5′of bvgS and extending to codon 7 was amplified by PCR from KM22 genomic DNA using primers bvgS-FA-for and bvgS-FA-rev (Table 1), which were designed such that SacI and XbaI sites would be generated at the 5′ and 3′ ends, respectively. The resulting PCR product was cloned into pCR2.1 (Invitrogen, Carlsbad, CA) to create pTN31. The insert of pTN31, containing the upstream region of bvgS, was cloned into pUC19 (New England BioLabs, Ipswich, MA) via SacI and XbaI sites to obtain pTN39. A 1,097-bp DNA fragment extending from codon 1225 of bvgS into the adjacent downstream region was amplified from KM22 genomic DNA by PCR using primers bvgS-FB-for and bvgS-FB-rev (Table 1), which were designed such that XbaI and HindIII sites would be generated at the 5′ and 3′ ends, respectively. The resulting PCR product was cloned into pCR2.1 (Invitrogen, Carlsbad, CA) to obtain pTN32. The insert of pTN32, containing the downstream region of bvgS, was excised by XbaI and HindIII digestion and cloned into XbaI-HindIII-digested pTN39 to create pTN42. The 2,143-bp DNA fragment, containing the upstream and downstream regions of bvgS, was amplified from pTN42 by PCR using primers TN42-for and TN42-rev (Table 1), which were designed such that SacI and AvrII sites would be generated at the 5′ and 3′ ends, respectively. The resulting PCR product was cloned into pCR2.1 (Invitrogen, Carlsbad, CA) to create pTN46. The insert of pTN46 was excised by SacI and AvrII digestion and cloned into SacI-AvrII-digested allelic exchange vector pSS4245 (10, 19) to create pTN50. pTN50 was introduced into E. coli SM10λpir (32) and transconjugated into KM22 as previously described (10, 19). Briefly, Bvg− phase B. bronchiseptica, obtained by growth on BG agar containing 50 mM MgSO4, was coincubated with SM10λpir cells carrying pTN50 on BG agar containing 10 mM MgCl2 and 50 mM MgSO4 for 4 h at 37° C. Cointegrants were selected on BG-Sm-Km-Carb agar containing 50 mM MgSO4. Cointegrants were then streaked onto BG agar and incubated for 2 days at 37° C, allowing for the selection of B. bronchiseptica that had undergone a second recombination event resulting in a loss of the allelic exchange plasmid, pTN50. Colonies were screened for the presence of the mutant bvgS gene by growth on BG-Sm agar and lack of growth on BG-Sm-Km-Carb agar. A single colony, designated TN30, was selected for subsequent use. Confirmation that the introduced mutation resulted in the specified phase-locked phenotype was obtained by growing wild-type KM22 and TN30 on BG agar and BG agar containing MgSO4 and evaluating colony morphology and hemolytic activity. TN30 was further confirmed by PCR amplification of a DNA fragment encoding upstream and downstream regions of the bvgS gene and DNA sequence analysis.
TN31, the Bvgi phase-locked derivative of KM22, was constructed by generating the bvgS-I1 (14) mutation T733M using the QuikChange site-directed mutagenesis kit (Agilent Technologies, Inc., Santa Clara, CA) according to the manufacturer's instructions. pTN15, carrying the bvgS gene encoding the bvgS-C3 mutation R570H, served as the template for use with mutagenesis primers T733Mfor and T733Mrev (Table 1). The mutation was confirmed by sequencing, and the resulting plasmid was named pTN18. The bvgS encoding both R570H and T733M mutations was then PCR amplified from pTN18 using primers TN18-for and TN18-rev (Table 1), which were designed such that AvrII and SpeI sites would be generated at the 5′ and 3′ ends, respectively. The resulting PCR product was cloned into pCR-Blunt II-TOPO (Invitrogen, Carlsbad, CA) to create pSMS87. The insert of pSMS87 was then excised by AvrII and SpeI digestion and cloned into AvrII-SpeI-digested pSS4245 to create pSMS95. pSMS95 was introduced into E. coli SM10λpir (32) and transconjugated into KM22 as described above for TN30. A single colony, designated TN31, was selected for subsequent use. Confirmation that the introduced mutation resulted in the specified phase-locked phenotype was obtained by growing wild-type KM22 and TN31 on BG agar and BG agar containing MgSO4 and evaluating colony morphology and hemolytic activity. TN31 was further confirmed by PCR amplification of the bvgS gene and DNA sequence analysis.
Motility assay.
Motility assays were performed as previously described (2). Briefly, plates were prepared with Stainer-Scholte medium (SSM) containing 0.35% agarose and supplemented with 50 mM MgSO4. A single KM22 or TN30 colony was stab inoculated into SSM or SSM and MgSO4 and incubated at 37°C for at least 24 h. Motile organisms display outward migration from the point of inoculation.
Quantitative real-time PCR.
Mid-log-phase liquid cultures of KM22, TN23, TN30, and TN31 were diluted in SS broth containing streptomycin to obtain a starting optical density at 600 nm (OD600) of 0.02, in triplicate, and grown at 37°C with shaking at 275 rpm until an OD600 of 0.8 was reached. Bacteria were harvested, and total RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA), treated with RNase-free DNase I (Invitrogen, Carlsbad, CA), and purified using RNeasy columns (Qiagen, Valencia, CA) according to the manufacturer's instructions. DNase-treated total RNA (1 μg) from each biological replicate was reverse transcribed using 300 ng of random oligonucleotide hexamers and SuperScript III RTase (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. The resulting cDNA was diluted 1:100, and 1 μl of this dilution was used in qPCR mixtures containing 300 nM primers and 2× SYBR green PCR master mix (Applied Biosystems, Foster City, CA) using an Applied Biosystems 7300 real-time PCR detection system (Applied Biosystems, Foster City, CA). All primers were designed using Primer Express software (Applied Biosystems, Foster City, CA) and listed in Table 1. To confirm the lack of DNA contamination, reactions without reverse transcriptase were performed. Dissociation curve analysis was performed for verification of product homogeneity. Threshold fluorescence was established within the geometric phase of exponential amplification, and the cycle threshold (CT) value for each reaction was determined. The CT value from all three biological replicates for each strain was compiled, and the 16S RNA amplicon was used as an internal control for data normalization. Fold-change in transcript level was determined using the relative quantitative method (ΔΔCT) (20). Results were log2 transformed and analyzed for significance using a two-way mixed model analysis of variance (ANOVA) with a post hoc comparison of fold change in transcript level of each isogenic mutant to the wild-type isolate using the Bonferroni method. P values less than 0.001 were considered significant.
Cytotoxicity assays.
These assays were carried out as previously described (24, 40). Briefly, J774 macrophages were cultured in Dulbecco's modified Eagle's medium (DMEM) broth supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, 1% nonessential amino acids, and 1% sodium pyruvate to 85% confluence at 37°C with 5% CO2. Warmed RPMI medium lacking phenol red, with 5% FBS, 1% l-glutamine, 1% nonessential amino acids, and 1% sodium pyruvate, was then used to replace the DMEM. Bacterial infections with the indicated bacterial strains were prepared from cultures collected in mid-log phase and were carried out using a multiplicity of infection (MOI) of 10. Bacterial suspensions were centrifuged onto the macrophages at 250 × g for 5 min, and cell cultures were incubated at 37°C with 5% CO2 for 4 h. The cell culture supernatants were collected, and percent lactate dehydrogenase (LDH) release was analyzed using the Cytotox96 kit (Promega, Madison, WI) according to the manufacturer's instructions. Results were analyzed for significance using the Student's t test, and a P value of less than 0.05 was considered significant.
Bacterial adhesion assays.
L2 rat lung epithelial cells were grown to ∼80% confluence in a 24-well plate using 50/50 DMEM-F12 medium supplemented with 10% FBS and then inoculated with suspensions of the indicated bacterial strains prepared from cultures collected in mid-log phase and diluted using growth medium, resulting in an MOI of 100. Dilutions of bacterial inocula were plated on BG plates containing streptomycin to determine CFU counts and verify the MOI. Adhesion assays were carried out in triplicate and performed by removing growth medium from L2 cells and then adding either medium alone or 1 ml of bacterial inoculum. Plates were then centrifuged for 5 min at 250 × g and incubated at 37°C with 5% CO2 for 40 min. Wells were then washed four times with 1 ml of the growth medium to remove nonadherent bacteria. L2 cells were then trypsinized using 0.5 ml of 0.125% trypsin and incubated for 10 min at 37°C. The total volume of each well was then brought up to 1 ml with growth medium, and cells were homogenized by pipetting. Dilutions were then plated on BG plates containing streptomycin to determine CFU counts, which were then used to calculate the proportion of adherent bacteria, expressed as a percentage of the original inoculum. Results were analyzed for significance using Student's t test, and a P value of less than 0.05 was considered significant.
Pathogenesis experiments in swine.
B. bronchiseptica strains KM22, TN23 (Bvg+), TN31 (Bvgi), and TN30 (Bvg−) were cultured on BG agar supplemented with 10% sheep's blood at 37°C for 40 h. Suspensions of these cultures were prepared in phosphate-buffered saline (PBS) to contain approximately 2 × 109 CFU/ml, and a 1:2,000 dilution of this suspension was made in PBS for inoculation of the pigs. An aliquot of inoculum for each strain was plated on BG agar plates, with and without 50 mM MgSO4, to determine CFU/ml and to confirm the expected colony morphology and hemolytic phenotype. All colonies from all inocula displayed the expected colony morphology and hemolytic characteristics. A single isolation room in BL2 barn facilities was used to house each experimental group. No B. bronchiseptica was isolated from nasal swabs prior to the start of either experiment. For the first pathogenesis trial, 56 pigs were divided into 3 experimental groups of 16 pigs each and 1 control group of 8. Pigs were inoculated intranasally at 1 week of age with 1 ml (0.5 ml/nostril) of a bacterial suspension containing 106 CFU of KM22, TN23 (Bvg+), TN30 (Bvg−) or with 1 ml of sterile PBS. For the second pathogenesis trial, 48 pigs were divided into 2 experimental groups of 16 pigs each and 1 control group of 16. Pigs were inoculated intranasally at 1 week of age with 1 ml (0.5 ml/nostril) of a bacterial suspension of strains KM22, TN31 (Bvgi), or 1 ml of sterile PBS. For both trials, bacterial colonization of the nasal cavity was quantified by nasal swabs on days 1, 3, and 5 and by nasal washes at the indicated days postinoculation. Animals were euthanized and bacterial load in the trachea and the lungs was quantified at the indicated days postinoculation. At necropsy, snouts were transected and removed at the level of the first premolar tooth. A 1-cm cross-section was cut from the caudal portion of the snout and used for scoring turbinate atrophy. The trachea was then severed just below the larynx, and the trachea and lung were removed. All housing, husbandry, and experiments performed with pigs were in accordance with the law and approved by the Institutional Animal Care and Use Committee.
Determination of colonization.
Nasal swabs were placed into tubes containing 500 μl PBS and vortexed. Nasal washes were performed by injecting 5 ml of PBS into the nasal cavity through one nostril and collecting the effluent into a beaker. Tracheal washes were performed by placing a segment of trachea approximately 8 cm in length in a 15-ml centrifuge tube with 5 ml of PBS and shaking vigorously. Lung lavage was performed by filling the lungs with 100 ml of sterile PBS, gently massaging, and aspirating; approximately 50 ml of the PBS was recovered. Serial 10-fold dilutions were made from nasal swab fluids, nasal and tracheal washes, and lung lavages, and the number of CFU of B. bronchiseptica per ml was determined by plating 100 μl of the dilutions on duplicate selective blood agar plates containing 20 μg/ml penicillin, 10 μg/ml amphotericin B, 10 μg/ml streptomycin, and 10 μg/ml spectinomycin. The limit of detection was 10 CFU/ml. B. bronchiseptica was identified on the basis of colony morphology. Colonization levels in the TN31 (Bvgi)-inoculated pigs was confirmed using colony lifts hybridized with a probe for the Bordetella adenylate cyclase toxin gene, cyaA. Colonies from plates representing each respiratory tract site (nasal cavity, trachea, and lung) from each experimental group were randomly screened by PCR amplification of the bvgS gene and DNA sequence analysis to confirm the expected bvgS genotype. Statistical analyses of the nasal colonization data were performed using a mixed linear model (SAS 9.2 for Windows XP; SAS Institute Inc., Cary, NC) for repeated measures and a spatial spherical covariance structure to best account for unequal study day intervals. Linear combinations of the least squares means estimates for log10 CFU were used in a priori comparisons to test for a significant (P < 0.05) effect of the bacterial challenge strains. Comparisons were made between challenge groups for each isolate at each time point using a 5% level of significance (P < 0.05) to assess statistical differences. Endpoint data for tracheal and lung bacterial loads were analyzed using analysis of variance using a general linear model for unbalanced data that included treatment group and study day, with CFU as the dependent variable. A 5% level of significance (P < 0.05) was used to assess statistical differences.
Pathological evaluation of the lung.
Sections of lung taken at necropsy for microscopic evaluation were fixed in 10% neutral buffered formalin for 24 h and then placed in 70% ethanol. All sections were routinely processed and embedded in paraffin, sectioned, and stained with hematoxylin and eosin. At necropsy, an estimate of gross lung involvement was assigned based on the percentage of each lung lobe affected and the percentage of total lung volume represented by each lobe. Percentage of total lung volume of each lobe was estimated as 10% for the left cranial, 10% for the left middle, 25% for the left caudal, 10% for the right cranial, 10% for the right middle, 25% for the right caudal, and 10% for the intermediate lung lobes.
Turbinate atrophy scores.
Each of the four scrolls of the ventral turbinate in snout cross-sections was assigned an atrophy score that ranged from 0 to 4: 0 = normal, 1 = more than half of turbinate remaining, 2 = half or less of turbinate remaining, 3 = turbinate is straightened with only a small portion left, and 4 = total atrophy. The atrophic rhinitis score is the sum of the four turbinate atrophy scores and ranges from 0 to 16.
Transmission experiments in swine.
B. bronchiseptica strains KM22 and TN23 (Bvg+) were cultured on BG agar supplemented with 10% sheep's blood at 37°C for 40 h. Suspensions of these cultures were prepared in PBS to contain approximately 2 × 109 CFU/ml, and a 1:2,000 dilution of this suspension was made in PBS for inoculation of the pigs. An aliquot of inoculum for each strain was plated on BG agar plates, with and without 50 mM MgSO4, to determine CFU/ml and to confirm the expected colony morphology and hemolytic phenotype. All colonies from both inocula displayed the expected characteristics. A single isolation room in BL2 barn facilities was used to house each experimental group. For the direct transmission study, 2 experimental groups of 16 1-week-old piglets and 1 control group of 8 were intranasally inoculated with 106 CFU of KM22 or TN23 (Bvg+) or 1 ml sterile PBS. At 3 days postinoculation, two naïve pigs were added to each room to serve as direct contacts. Bacterial colonization of the nasal cavity was assessed by nasal washes on days 2, 8, 18, 39, 46, 69, 76, and 84 postcontact. Four primary-challenged pigs were removed on days 10, 17, 34, and 60 postcontact for necropsy, leaving only direct contacts in the room beyond day 60 postcontact. On day 84 postcontact, necropsies were performed and pigs were euthanized with an overdose of barbiturate. For the direct and indirect transmission study, three isolation rooms in BL2 barn facilities were used to house each experimental group. Two isolation rooms were identically set up with two pens placed 18 inches apart. Two pigs were intranasally inoculated with 106 CFU of KM22 and placed in a pen together in one isolation room, and two pigs were intranasally inoculated with 106 CFU of TN23 (Bvg+) and placed in a pen together in a second isolation room. These pigs served as the “primary-challenged” pigs in the study. A control group of 8 pigs were intranasally inoculated with 1 ml sterile PBS and housed together in a third isolation room with no pens. Two days postinoculation of primary-challenged pigs, 3 naïve pigs, serving as a direct contacts, were placed in the pen containing the primary-challenged pigs, and 5 naïve pigs, serving as indirect contacts, were placed in the second pen. Nasal colonization of all pigs was monitored by periodical nasal swabbing on days 4, 7, 12, 18, and 25 postcontact. Nasal swabs were obtained from indirect contacts first, followed by direct contacts, and then nasal swabs were obtained from primary-challenged pigs. Necropsies were performed on day 25 postcontact, and all pigs were euthanized with an overdose of barbiturate. For all transmission experiments, the trachea was then severed just below the larynx, and the trachea and lung were removed for determination of colonization. All housing, husbandry, and experiments performed with pigs were in accordance with the law and approved by the Institutional Animal Care and Use Committee.
RESULTS
Phenotype and gene expression pattern of each KM22 isogenic mutant (Bvg−, Bvgi, and Bvg+).
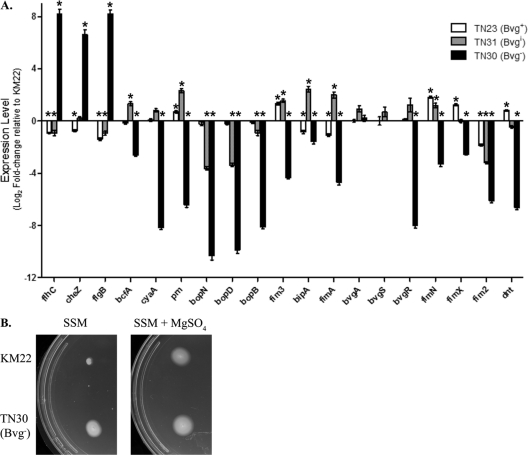
To investigate the role of phenotypic modulation by B. bronchiseptica during infection of swine, we constructed a series of isogenic phase-locked mutants in the virulent swine strain KM22. Since regulation of virulence gene expression by the bvg locus is highly conserved among Bordetella strains, we hypothesized that isogenic mutants (Bvg−, Bvgi, and Bvg+) of KM22 would have similar phenotypes as those reported for each corresponding isogenic mutant in the well-characterized laboratory strain, RB50. Gene expression in TN23 (Bvg+), TN31 (Bvgi), and TN30 (Bvg−) was analyzed by quantitative real-time PCR and compared to the wild-type KM22 strain. The phase-locked mutants and the wild-type KM22 were grown under nonmodulating conditions at 37°C. Nineteen genes that have been shown in RB50 to be differentially expressed in response to phase modulation were chosen for analysis. These included genes for regulatory proteins (bvgA, bvgS, bvgR), adhesins (prn, fimA, fim2, fim3, fimN, fimX), toxins (cyaA, dnt), the type III secretion system (bopB, bopD, bopN), motility (flhC, cheZ, flgB), and the intermediate-phase-expressed genes bipA and bcfA (15, 27, 34). As shown in Fig. 1A, the three isogenic mutant strains have unique patterns of gene expression. The expression of prn, fim3, fimN, fimX, and dnt were significantly increased in the Bvg+ phase-locked mutant, TN23, while the expression of flhC, cheZ, flgB, bipA, fimA, and fim2 were significantly decreased relative to the wild-type KM22 (Fig. 1A). The expression levels of the other eight genes analyzed did not differ significantly from the wild-type KM22 (Fig. 1A). In contrast, the expression of all the virulence-associated genes analyzed was significantly decreased in the Bvg− phase-locked mutant, TN30, relative to wild-type KM22 (Fig. 1A). The expression of bcfA, prn, fim3, bipA, fimA, and fimN were significantly increased in the Bvgi phase-locked mutant, TN31, while the expression of flhC, flgB, bopN, bopD, bopB, and fim2 were significantly decreased relative to the wild-type KM22 (Fig. 1A). In summary, expression of all 19 genes analyzed in each isogenic mutant of KM22 was found to be similar to that reported for the corresponding isogenic mutants in the well-characterized laboratory strain, RB50.
Fig 1.
(A) Gene expression changes in B. bronchiseptica TN23 (Bvg+), TN31 (Bvgi), and TN30 (Bvg−) evaluated by qRT-PCR. The x axis indicates the genes analyzed. The y axis indicates the log2-fold change in gene expression of each strain relative to KM22 using the relative quantitative method (ΔΔCT). Values for bvgS in TN30 (Bvg−) were not attainable since the region targeted by the primers used in the assay is missing in this strain. Error bars represent ±standard errors. An asterisk indicates that the P value is <0.001. (B) Soft-agar motility of B. bronchiseptica KM22 and TN30 (Bvg−). A single colony of either KM22 or TN30, as indicated on the left, was stab inoculated into SSM or SSM supplemented with 50 mM MgSO4, as indicated above. Motile organisms display outward migration from the point of inoculation.
Perhaps the best-characterized phenotypes of the Bvg− phase are motility and the expression of flagellar synthesis genes, which are tightly regulated through a complex hierarchy requiring the presence of the FrlAB regulatory locus, and the production of the flagellin monomer, FlaA (1, 2). Relative to B. bronchiseptica RB50, the expression of the flagellar structural gene flaA has been reported to increase in RB54, the Bvg− phase-locked derivative of RB50 (16, 33). To test whether TN30 is motile under both modulating and nonmodulating conditions, a single colony of either wild-type KM22 or TN30 was stab inoculated into soft-agar plates containing SSM or SSM supplemented with 50 mM MgSO4. As shown in Fig. 1B, TN30 is motile in both nonmodulating SSM and modulating SSM supplemented with 50 mM MgSO4 conditions, while KM22 is motile only in modulating conditions and SSM supplemented with 50 mM MgSO4. These data demonstrate that the Bvg− phase-locked mutant, TN30, is expressing the Bvg− phase phenotype motility during both modulating and nonmodulating conditions.
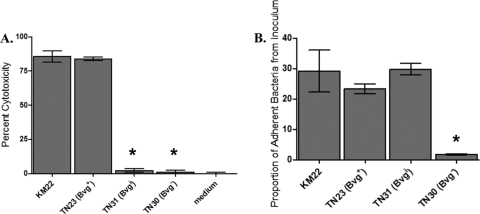
A well-characterized function of the Bordetella type III secretion system (TTSS) is killing of mammalian cells (30, 39, 40). Since the BvgAS system regulates the TTSS in B. bronchiseptica RB50, we hypothesized that the BvgAS system would also regulate TTSS in KM22. Therefore, we assessed the ability of KM22 and the isogenic phase-locked mutants to mediate cytotoxicity in J774 murine macrophages in vitro. Both KM22 and the Bvg+-locked mutant, TN23, displayed a high level of cytotoxicity (Fig. 2A). In contrast, the cytotoxic activity of the Bvgi-locked strain, TN31, and Bvg− phase-locked strain, TN30, was statistically lower than KM22 and similar to medium alone (Fig. 2A). These data correlate with the gene expression data and suggest that both KM22 and TN23 produce a functional TTSS, while the decreased expression of some TTSS genes in strains TN31 and TN30 may have resulted in the decreased cytotoxicity, a TTSS-dependent function.
Fig 2.
(A) TTSS-mediated cytotoxicity of B. bronchiseptica KM22, TN23 (Bvg+), TN31 (Bvgi), and TN30 (Bvg−). Cytotoxicity as measured by percent LDH release in J774 macrophages following 4 h of exposure to medium alone or to the indicated strain, using an MOI of 10. The error bars represent ±standard deviations. An asterisk indicates that the P value is <0.005. (B) Adherence of B. bronchiseptica KM22, TN23 (Bvg+), TN31 (Bvgi), and TN30 (Bvg−). Adherence in vitro to L2 cells is expressed as the proportion of bacteria in the original inoculum found to be adherent after centrifugation and a 40-min incubation period, using an MOI of 100. Adherence of bacteria to L2 cells is expressed as the proportion of adherent bacteria to the original inoculum. The error bars represent ±standard deviation. An asterisk indicates that the P value is <0.005.
The ability of B. bronchiseptica to adhere to epithelial surfaces is required for the initiation and establishment of infection. The BvgAS system regulates a number of genes whose products have been postulated or, in some cases, directly shown to function as adhesins. To determine if the BvgAS system is involved in regulating cellular adherence by KM22, the ability of the phase-locked mutants to adhere to L2 rat lung epithelial cells in vitro was compared to that of the wild-type strain. KM22, TN23 (Bvg+), and TN31 (Bvgi) had a similar level of adherence to L2 cells, whereas TN30 (Bvg−) was unable to adhere (Fig. 2B). These results indicate that Bvg− B. bronchiseptica does not express factors necessary for adhesion to L2 cells, supporting the hypothesis that Bvg− phase gene products are not involved in colonization and establishment of infection.
Full activation of the BvgAS regulatory system is required for colonization and maximal disease severity.
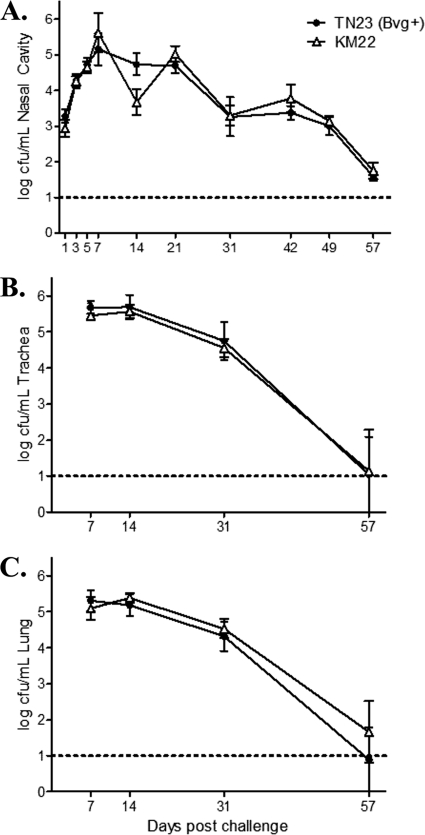
To investigate the Bvg phase-dependent contribution to pathogenesis of B. bronchiseptica in swine, we assessed, in two separate trials, the ability of TN23 (Bvg+), TN31 (Bvgi), and TN30 (Bvg−) to colonize and cause disease compared to the wild-type isolate. In the first trial, groups of 1-week-old piglets were intranasally inoculated with KM22, TN23 (Bvg+), TN30 (Bvg−) or 1 ml sterile PBS. Clinical signs were noted only in piglets from groups inoculated with KM22 or TN23 (Bvg+) and consisted of sneezing and coughing. No clinical signs were observed in piglets from groups inoculated with TN30 (Bvg−) or PBS.
Colonization of the nasal cavity was evaluated on days 1, 3, 5, 7, and weekly thereafter until day 57 postinfection (Fig. 3A). No B. bronchiseptica CFU were recovered from any site in the respiratory tract of piglets inoculated with TN30 (Bvg−) or PBS. The Bvg+ phase-locked mutant, TN23, was recovered from the nasal cavity in numbers similar to those for the wild-type KM22 at all days examined (Fig. 3A). Colonization of sites in the lower respiratory tract was also assessed at days 7, 14, 31, and 57 postinfection. Similar to nasal colonization, there was no difference in the number of CFU recovered from TN23 (Bvg+) compared to KM22 in both the trachea and the lung (Fig. 3B and C).
Fig 3.
Colonization of the swine respiratory tract by wild-type B. bronchiseptica strain KM22 or TN23, a KM22 Bvg+ isogenic mutant. Groups of 16 pigs were inoculated intranasally with KM22 (open triangles) or TN23, a KM22 Bvg+ isogenic mutant (closed circles). Bacterial load in the nasal cavity (A) was quantified on days 1, 3, 5, 7, 14, 21, 31, 42, 49, and 57 postinoculation. Bacterial load in the trachea (B) and the lungs (C) was quantified on days 7, 14, 31, and 57 postinoculation. The x axis indicates days postinoculation, and the y axis indicates the mean CFU expressed as the log10 mean ± standard errors (error bars). The dashed line indicates the lower limit of detection (log101).
The degree of atrophic rhinitis was evaluated by determining the mean turbinate atrophy score from piglets inoculated with KM22, TN23 (Bvg+), TN30 (Bvg−), or PBS on days 7, 14, 31, and 57 postinfection. No significant turbinate atrophy was seen in the pigs inoculated with either PBS or TN30 (Bvg−) on any of the days examined (Table 2). Only piglets infected with either KM22 or TN23 (Bvg+) had any evidence of significant nasal turbinate atrophy, reflective of an atrophic rhinitis score greater than 2 (Table 2). Pigs infected with TN23 (Bvg+) showed a similar degree of turbinate atrophy as pigs infected with the wild-type KM22 on all days except day 31, where an increased degree of turbinate atrophy was observed in KM22-infected pigs (Table 2).
Table 2.
Mean turbinate atrophy scores and percentages of the lungs affected by pneumonia in pigs inoculated with B. bronchiseptica KM22, TN23 (Bvg+ isogenic mutant), or TN30 (Bvg− isogenic mutant)
| Day of necropsy | Group | Mean turbinate atrophy score (range) | Mean % pneumonia (range) |
|---|---|---|---|
| 7 | PBS control | 0 (0) | 0 (0) |
| KM22 (BvgWT) | 2.25 (1–4) | 1.15 (0.1–2) | |
| TN23 (Bvg+) | 1.5 (0–4) | 1.275 (0.1–3) | |
| TN30 (Bvg−) | 0 (0) | 0 (0) | |
| 14 | PBS control | 0 (0) | 0 (0) |
| KM22 (BvgWT) | 2.25 (0–4) | 2.375 (0–9) | |
| TN23 (Bvg+) | 3.75 (2–5) | 2.25 (0–9) | |
| TN30 (Bvg−) | 0 (0) | 0 (0) | |
| 31 | PBS control | 1.5 (1–2) | 0 (0) |
| KM22 (BvgWT) | 4.25 (1–8) | 0.625 (0–1.5) | |
| TN23 (Bvg+) | 2.0 (0–3) | 0.875 (0–2.5) | |
| TN30 (Bvg−) | 0 (0) | 0 (0) | |
| 57 | PBS control | 1.5 (1–2) | 0 (0) |
| KM22 (BvgWT) | 1.67 (0–3) | 2.75 (0–11) | |
| TN23 (Bvg+) | 2.25 (2–3) | 5.625 (0–18) | |
| TN30 (Bvg−) | 0 (0) | 0 (0) |
Lung pathology was assessed on days 7, 14, 31, and 57 postinfection by determining the mean percentage of the lung affected by pneumonia. Overall, no pneumonia was observed in TN30 (Bvg−)- or PBS-infected piglets, whereas a similar degree of pneumonia was observed in TN23 (Bvg+)- and KM22-infected pigs on days 7, 14, and 31 (Table 2). On day 57 postinfection, the mean percentage of lung affected by pneumonia was slightly higher in TN23 (Bvg+)-challenged pigs, with 5.625% of lungs affected compared to 2.75% of lungs affected in KM22-infected pigs (Table 2). Additionally, only one out the four pigs infected with KM22 exhibited pneumonia, whereas three out the four pigs infected with TN23 (Bvg+) exhibited pneumonia. The pneumonias exhibited in TN23 (Bvg+)- and KM22-infected pigs were similar and consisted of areas of red, seen on days 7 and 14 postinfection, to tan, seen on days 31 and 57 postinfection, consolidation with well-demarcated borders, and a cranial ventral distribution. Microscopically, the lesions in both TN23 (Bvg+)- and KM22-challenged pigs were typical of B. bronchiseptica pneumonia, characterized by suppurative bronchopneumonia that is gradually replaced by fibroplasia.
In a second trial, we compared the ability of TN31 (Bvgi) and the wild-type KM22 isolate to colonize and cause disease in swine following intranasal inoculation of groups of 1-week-old piglets with KM22, TN31 (Bvgi), or PBS. No clinical signs were observed in piglets inoculated with TN31 (Bvgi) or in piglets receiving PBS. Clinical signs, consisting of sneezing and coughing, were noted only in piglets inoculated with KM22.
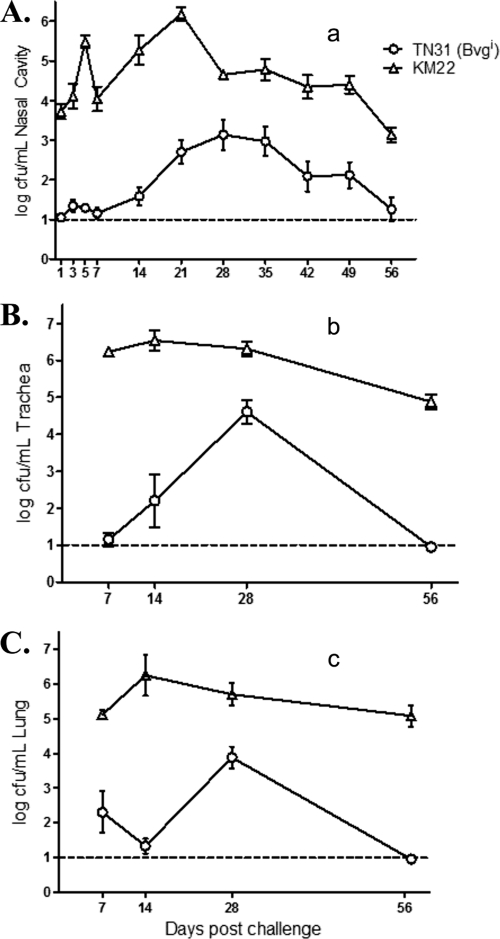
Nasal cavity colonization was assessed at days 1, 3, 5, and 7 and then weekly through day 56 postinfection. The Bvgi phase-locked mutant, TN31, was recovered at levels significantly lower than those of wild-type KM22 on all days (Fig. 4A). Throughout the course of the experiment, the number of CFU recovered from pigs infected with TN31 (Bvgi) was approximately two logs less than that from pigs infected with KM22. Additionally, the peak in the number of CFU recovered from the nasal cavity of TN31 (Bvgi)-infected pigs was delayed compared to that of KM22-infected pigs, with CFU levels barely rising above detectable limits through day 14 and reaching a peak at day 28, and by day 56 CFU levels fell to barely detectable levels (Fig. 4A). In contrast, the numbers of CFU recovered from the nasal cavity of KM22-infected pigs were significantly higher on day 1, reached a peak on day 21, and remained high throughout the course of the study (Fig. 4A).
Fig 4.
Colonization of the swine respiratory tract by wild-type B. bronchiseptica strain KM22 or TN31, a KM22 Bvgi isogenic mutant. Groups of 16 pigs were inoculated intranasally with KM22 (open triangles) or TN31, a KM22 Bvgi isogenic mutant (open circles). Bacterial load in the nasal cavity (A) was quantified on days 1, 3, 5, 7, 14, 21, 28, 35, 42, 49, and 56 postinoculation. Bacterial load in the trachea (B) and the lungs (C) was quantified on days 7, 14, 28, and 56 postinoculation. The x axis indicates days postinoculation, and the y axis indicates the mean CFU expressed as the log10 mean ± standard errors (error bars). The dashed line indicates the lower limit of detection (log101). Statistical difference is indicated as P values of <0.0001 for TN31 (Bvgi) compared to KM22, assessed as repeated measurements over all time points (a), P values of <0.0001 for TN31 (Bvgi) compared to KM22, assessed as repeated measurements over all time points (b), and P values of <0.0001 for TN31 (Bvgi) compared to KM22, assessed as repeated measurements over all time points (c). A P value of less than 0.05 was considered significant.
Colonization of the trachea and lung was evaluated on days 7, 14, 28, and 56 postinfection (Fig. 4B and C). In general, significantly lower CFU levels were recovered from the trachea and lungs of TN31 (Bvgi)-inoculated pigs than from the trachea and lungs of pigs infected with KM22 (Fig. 4B and C). Similar to nasal colonization, peak CFU recovered from the trachea and lungs was delayed until day 28 in pigs infected with TN31 compared to day 14 in KM22-inoculated pigs. Additionally, KM22 was able to persist in significantly high numbers through day 56 in both the trachea and lung, whereas the number of TN31 (Bvgi) decreased more dramatically, approaching the lower limit of detection by day 56 (Fig. 4B and C).
No significant turbinate atrophy was seen in the pigs inoculated with either PBS or TN31 (Bvgi) at any of the days examined (Table 3). Only piglets infected with KM22 had any evidence of significant nasal turbinate atrophy, reflective of an atrophic rhinitis score greater than 2 (Table 3). Pigs inoculated with TN31 (Bvgi) displayed little to no pneumonia (Table 3), with the range of lung percentage affected as well as the total number of pigs displaying evidence of pneumonia similar to those of the PBS-inoculated control group. Despite isolation of significantly lower numbers of B. bronchiseptica from the lung, two TN31 (Bvgi)-infected pigs displayed mild lesions on days 7 and 56. Microscopically, the lesions on day 7 were consistent with Bordetella infection and included small areas of consolidated interstitial pneumonia, with septal infiltrates consisting mainly of macrophages and neutrophils. Lesions detected on day 56 were suppurative bronchopneumonia, also consistent with Bordetella infection. The colonization and pathology data from each of the two trials together suggest that the full activation of the BvgAS regulatory system is required for maximal colonization and disease severity in swine.
Table 3.
Mean turbinate atrophy scores and percentages of the lungs affected by pneumonia in pigs inoculated with B. bronchiseptica KM22 or TN31 (Bvgi isogenic mutant)
| Day of necropsy | Group | Mean turbinate atrophy score (range) | Mean % pneumonia (range) |
|---|---|---|---|
| 7 | PBS control | 1.5 (0–2) | 0.875 (0–2) |
| KM22 (BvgWT) | 3.75 (1–8) | 3.625 (0–10) | |
| TN31 (Bvgi) | 1.25 (0–2) | 1.0 (0–3.5) | |
| 14 | PBS control | 0.5 (0–1) | 0.75 (0–3) |
| KM22 (BvgWT) | 4.5 (2–8) | 7.5 (2–20) | |
| TN31 (Bvgi) | 1.0 (0–2) | 0.25 (0–1) | |
| 28 | PBS control | 0.25 (0–1) | 0.25 (0–1) |
| KM22 (BvgWT) | 5.75 (3–8) | 9.75 (0–17) | |
| TN31 (Bvgi) | 1.5 (1–4) | 0 (0) | |
| 56 | PBS control | 1.25 (1–2) | 0 (0) |
| KM22 (BvgWT) | 4.75 (4–6) | 5.0 (2–13) | |
| TN31 (Bvgi) | 1.5 (0–4) | 0.875 (0–3.5) |
Phenotypic modulation of the Bvg+ phase is not required for direct or indirect transmission of B. bronchiseptica in swine.
An attractive and frequently proposed hypothesis is that a BvgAS system capable of modulation between the Bvg+ and Bvg− phases is required for transmission (12, 14, 33). To experimentally address this hypothesis, we undertook two separate studies designed to test the ability of the Bvg+ phase-locked mutant, TN23, to be transmitted, either directly or indirectly, from infected pigs to naïve pigs.
To assess direct transmission, groups of 16 1-week-old piglets were intranasally inoculated with KM22, TN23 (Bvg+), or 1 ml sterile PBS. At 3 days postinoculation two naïve pigs were added to each room to serve as direct contacts. Nasal washes were performed on the direct contact pigs to test for colonization by each respective B. bronchiseptica strain. As shown in Table 4, the direct-contact pigs in groups inoculated with KM22 or TN23 (Bvg+) were rapidly colonized by each respective strain and remained colonized for the duration of the trial. No B. bronchiseptica was recovered from any site in the respiratory tract of piglets in the group inoculated with PBS. Colonization of the respiratory tract was evaluated in the direct-contact pigs from each group 84 days postcontact with primary-infected pigs. Both direct-contact pigs were positive for nasal colonization from the group inoculated with KM22 and the group inoculated with TN23 (Bvg+) (Table 4 and Table 5). B. bronchiseptica was recovered from the trachea in one direct-contact pig from the group inoculated with KM22 and both direct-contact pigs from the group inoculated with TN23 (Bvg+) (Table 5). B. bronchiseptica was recovered from the lung of one direct-contact pig from the group inoculated with KM22 but neither of the direct-contact pigs from the group inoculated with TN23 (Bvg+) (Table 5). Together, the data demonstrate that a Bvg+ phase-locked mutant can transmit directly from pig-to-pig when animals are comingled and suggest that phenotypic modulation of the Bvg+ phase is not required for direct transmission in swine.
Table 4.
Direct transmission to naïve pigsa
| No. of daysb | No. of contact pigs positive/total no. of pigsc |
|
|---|---|---|
| KM22 (BvgWT) | TN23 (Bvg+) | |
| 2 | 0/2 | 1/2 |
| 8 | 2/2 | 2/2 |
| 84 | 2/2 | 2/2 |
Three days after 16 primary pigs were challenged by the intranasal route with 106 CFU of the contact strain, two naïve pigs were added to each room. Four primary-challenged pigs were removed on days 7, 14, 31, and 57 for necropsy, leaving only transmission pigs in the room beyond day 57.
Days after initial contact with primary-challenged pigs.
Data are reported as the number of contact pigs positive out of a possible of two pigs for respective strain of B. bronchiseptica.
Table 5.
Respiratory tract colonization in direct-transmission pigs 84 days after initial contact with primary-challenged pigsa
| Sample site | No. of contact pigs positive/total no. of pigsb |
|||
|---|---|---|---|---|
| KM22 (BvgWT) |
TN23 (Bvg+) |
|||
| Positive | CFU/ml | Positive | CFU/ml | |
| Nasal wash | 2/2 | 2.10/2.32 | 2/2 | 3.64/2.51 |
| Tracheal wash | 1/2 | 2.24/0 | 2/2 | 2.88/1.74 |
| Lung lavage | 1/2 | 2.04/0 | 0/2 | 0/0 |
Pigs were euthanized on day 87 of the experiment, 84-days after first contact with primary challenged pigs.
Data are reported as the number of contact pigs positive out of a possible of two pigs for a strain of B. bronchiseptica. CFU are reported as the log10.
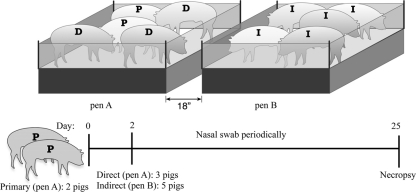
A second trial was designed to test whether the Bvg+ phase is sufficient for both direct and indirect transmission in swine. In this experiment, two pigs were intranasally inoculated with KM22 and placed in a pen within an isolator room (Fig. 5). These two pigs served as primary-challenged pigs. After 2 days, three naïve pigs, serving as direct contacts, were added to the same pen, and five naïve pigs were placed in a second pen placed at least 18 inches away from the first pen (Fig. 5). These five pigs served as indirect-contact pigs. The exact experimental procedure was repeated in a second isolation room, except that the two pigs serving as primary-challenged pigs were intranasally inoculated with TN23 (Bvg+). In a third isolator room with no pens, pigs were intranasally inoculated with sterile PBS and allowed to comingle.
Fig 5.
Experimental design of direct and indirect contact transmission experiment. Two pens, indicated as pen A and pen B, were placed 18 inches apart in one isolator room in a BL2 barn facility. Two pigs were intranasally inoculated with the B. bronchiseptica strain tested, placed in pen A, and served as primary-challenged pigs, indicated by “P.” After 2 days, three naïve pigs, serving as a direct contacts, indicated by “D,” were added to pen A, and five naïve pigs were placed in pen B, serving as indirect contacts, indicated by “I.” Nasal colonization of all pigs was monitored by periodical nasal swabbing, and necropsy of all pigs was performed on day 25.
Nasal colonization of all pigs was monitored by nasal swabbing. B. bronchiseptica was not recovered from any site in piglets inoculated with PBS. Similar to the first trial, direct transmission occurred rapidly for both KM22 and TN23 (Bvg+), with the majority of direct-contact pigs from each group becoming colonized by day 4 postcontact (Table 6). Transmission by indirect contact took longer; however, for both strains, an indirect-contact pig demonstrated nasal colonization 12 days after initial contact (Table 6). By day 25 postcontact, B. bronchiseptica was recovered from the nasal cavity of all pigs (primary-challenged, direct-contact, and indirect-contact pigs) from both the KM22 and the TN23 (Bvg+) groups. Colonization of the trachea and lungs was determined at necropsy 25 days postcontact. The B. bronchiseptica strain given to the primary-challenged pigs from each group was isolated from the trachea and lungs in all pigs (primary, direct contact, and indirect contact) from both the KM22 (wild-type) and TN23 (Bvg+) groups (Table 7). These results show that both the wild type and the Bvg+ phase-locked mutant were equally capable of transmission in swine by both direct and indirect contact. Additionally, these data demonstrate that phenotypic modulation of Bvg+ B. bronchiseptica is not required for direct or indirect transmission in swine.
Table 6.
Direct and indirect transmission to naïve pigsa
| No. of daysb | No. of contact pigs positive/total no. of pigsc |
|||||
|---|---|---|---|---|---|---|
| KM22 (BvgWT) |
TN23 (Bvg+) |
|||||
| Primary | Direct | Indirect | Primary | Direct | Indirect | |
| 4 | 2/2 | 2/3 | 0/5 | 2/2 | 3/3 | 0/5 |
| 7 | 2/2 | 2/3 | 0/5 | 2/2 | 3/3 | 0/5 |
| 12 | 2/2 | 2/3 | 1/5 | 2/2 | 3/3 | 1/5 |
| 18 | 2/2 | 3/3 | 2/5 | 2/2 | 3/3 | 3/5 |
| 25 | 2/2 | 3/3 | 5/5 | 2/2 | 3/3 | 5/5 |
Two days after 2 primary pigs were challenged by the intranasal route with 106 CFU of the contact strain, three naïve pigs were added to the same pin and represent direct-contact pigs. On the same day, five naïve pigs were added to a separate pin and represent indirect-contact pigs. One isolator room was used per contact strain studied, and the experimental procedure was duplicated in the two isolator rooms used.
Number of days after initial contact with primary-challenged pigs.
Contact strain is reported as the name (phenotype). Data are reported as the number of contact pigs positive out of the total number possible for a strain of B. bronchiseptica.
Table 7.
Respiratory tract colonization in direct- and indirect-transmission pigs 25 days after initial contact with primary-challenged pigsa
| Sample site | No. of contact pigs positive/total no. of pigsb |
|||||
|---|---|---|---|---|---|---|
| KM22 (BvgWT) |
TN23 (Bvg+) |
|||||
| Primary | Direct | Indirect | Primary | Direct | Indirect | |
| Nasal wash | 2/2 | 3/3 | 5/5 | 2/2 | 3/3 | 5/5 |
| Tracheal wash | 2/2 | 3/3 | 5/5 | 2/2 | 3/3 | 5/5 |
| Lung lavage | 2/2 | 3/3 | 5/5 | 2/2 | 3/3 | 5/5 |
Pigs were euthanized on day 27 of the experiment, 25 days after initial contact with primary-challenged pigs.
Data are reported as the number of contact pigs positive out of a possible of two pigs for a strain of B. bronchiseptica.
DISCUSSION
Despite widespread use of B. bronchiseptica vaccines by swine producers throughout the world, Bordetella-associated respiratory diseases remain a significant problem for the industry (18, 36). The development of vaccines with improved efficacy is hindered by the absence of definitive data related to virulence mechanisms of B. bronchiseptica in swine. Evaluation of B. bronchiseptica-specific virulence factors involved in pathogenesis and transmission in swine provides data directly relevant and necessary for designing improved vaccines and therapeutic interventions. We hypothesized that the ability of B. bronchiseptica to undergo phenotypic modulation is required at some point during the infectious cycle in swine. To investigate the Bvg phase-dependent contribution to pathogenesis of B. bronchiseptica in swine, we constructed a series of isogenic mutants in a virulent B. bronchiseptica swine isolate and first evaluated the in vitro-associated phenotypes of each KM22 isogenic mutant. We found the in vitro phenotypes associated with each KM22 isogenic mutant (Bvg−, Bvgi, and Bvg+) to be similar to those reported for each corresponding RB50 isogenic mutant.
To investigate the Bvg phase-dependent contribution to pathogenesis of B. bronchiseptica in swine, we compared TN23 (Bvg+), TN31 (Bvgi), and TN30 (Bvg−) to the wild-type swine isolate for their ability to colonize and cause disease. The Bvg− phase-locked mutant, TN30, was never recovered from any respiratory tract site at any time point examined. Except for the degree of pneumonia observed late in the infection, the Bvg+ phase-locked mutant, TN23, was indistinguishable from the wild type and caused similar disease severity. These results parallel previous studies involving phase-locked and ectopic expression mutants, which demonstrated that the Bvg+ phase promotes respiratory tract colonization by B. pertussis and B. bronchiseptica (1, 13, 14, 23, 26), while the Bvg− phase of B. bronchiseptica does not promote colonization or disease in a host (13, 14). The increased pneumonia exhibited by the TN23 (Bvg+)-infected pigs late in the infection suggests that the ability to modulate pathology during acute infection may be biologically beneficial for B. bronchiseptica. For example, while not evaluated here, the ability to modulate pathology could increase the possibility that a host remains more active and mobile, thereby increasing transmission dynamics within host populations. The Bvg− phase of B. bronchiseptica has been demonstrated to promote bacterial survival under conditions of nutrient deprivation (13, 14). Therefore, while not tested here, the Bvg− phase of B. bronchiseptica may promote increased survival in containment rooms, pens, and water sources in swine production facilities, thereby increasing the incidence of indirect transmission between animals. The inability to monitor such events illustrates a limitation of studying transmission in a confined experimental setting.
As we followed colonization levels throughout the infection, the Bvgi phase-locked mutant, TN31, was recovered at significantly lower levels than the wild-type KM22 at all respiratory tract sites and time points examined. This is in direct contrast to the in vitro data, where we found no difference in the capacity of TN31 and KM22 to adhere to L2 cells. While it may be possible to identify a difference in the adherence capability between KM22 and TN31 using different cell types, we found significantly increased transcription levels for adhesion-associated genes, bcfA, prn, fim3, bipA, fimA, and fimN, in TN31 compared to those in KM22 and similar transcription levels for fimX in both TN31 and KM22. The decreased bacterial burdens throughout the respiratory tract of TN31-infected pigs observed late in the infection demonstrate the ability of swine to clear Bvgi phase B. bronchiseptica more rapidly than wild-type B. bronchiseptica. In addition to the decreased in vivo adherence ability, this may be due to the lack of maximal expression and production of characterized immune suppressors and protective proteins. One example includes the TTSS, which is capable of modulating the host immune response to aid in survival and persistence in vivo (30, 39, 40). The gene expression and cytotoxicity data correlate with the in vivo colonization data and suggest that TN31 does not produce a functional TTSS; however, we cannot exclude the potential contribution of other factors as well. The decreased colonization of TN31-infected pigs compared to wild-type KM22 at all respiratory tract sites and time points examined demonstrates that in order to adhere, initiate infection, and persist to levels similar to those of the wild type in a natural host, Bvg+ phase factors are required. Further, pigs infected with TN31 displayed limited to no disease and few turbinate and lung lesions. Only pigs infected with KM22 or TN23 (Bvg+) displayed coughing and sneezing, which are mechanisms of transmission. These data suggest that a critical threshold of colonization is required to cause clinical signs of disease, such as sneezing and coughing, as well as degenerative pathology, such as turbinate atrophy, and are in agreement with previous findings (14). It is difficult to discern if pathological differences in the TN31-inoculated pigs were due to decreased colonization levels, lack of virulence factor expression, or both. Given that the number of CFU recovered from the nasal cavity of TN31-infected pigs barely rose above detectable limits through the first 2 weeks of infection, we were unable to test the ability of this strain to transmit to naïve pigs.
To test whether bacteria in the Bvg+ phase are readily transmitted, we used the Bvg+ phase-locked mutant, TN23, in direct and indirect transmission studies. Data from the first trial showed that a Bvg+ phase-locked mutant can transmit directly from pig to pig when animals are comingled and demonstrates that phenotypic modulation of the Bvg+ phase is not required for direct transmission in swine. The experimental design of the second trial is set up to specifically address whether or not a Bvg+ phase-locked strain can transmit both directly and indirectly to naïve pigs. Data from the second trial showed that a Bvg+ phase-locked mutant can transmit directly to naïve pigs and indirectly to naïve pigs physically separated by pens. It is important to note that once B. bronchiseptica was recovered from one naïve pig from the pen containing indirect-contact pigs, it was no longer possible to discern the route of transmission, direct or indirect, that led to the B. bronchiseptica colonization of the other four naïve pigs housed in the same pen. In any case, the isolation of TN23 from the nasal cavity of an indirect-contact pig in a separate pen demonstrates that a Bvg+ phase-locked mutant is capable of transmission by an indirect route.
We observed a difference in the timing of when B. bronchiseptica was recovered from the nasal cavity of direct-contact pigs from these studies. By day 2 postcontact, B. bronchiseptica was recovered from direct-contact pigs in the first study, whereas in the second study, B. bronchiseptica was not recovered until 7 days postcontact. The increase in the number of days for direct transmission to occur likely reflects the ratio of primary-infected pigs to naïve pigs, where in the first trial 16 primary-infected pigs to 3 naïve pigs were used and in the second trial there were 2 primary-infected pigs to 3 naïve pigs. These studies provide experimental data supporting conventional practices of decreasing the number of animals housed or comingled together in efforts to delay or decrease transmission.
It was not unexpected to find no significant differences in colonization between KM22-inoculated and TN23 (Bvg+)-inoculated pigs. However, given that the hypothesis that some degree of phenotypic modulation is required for transmission of B. bronchiseptica between animals is frequently proposed (12, 14, 33), it was surprising to find that the Bvg+ phase-locked mutant transmitted to naïve pigs by both direct and indirect contact with equal efficiency as the wild-type isolate. Our results demonstrate that in a natural host, phenotypic modulation of the fully active Bvg+ phase is not required for respiratory infection and host-to-host transmission of B. bronchiseptica.
ACKNOWLEDGMENTS
We thank Kim Driftmier, William Boatwright, and Gwen Nordholm for excellent technical support, and Brian Pottebaum, Jason Huegel, Jason Crabtree, and Dalene Whitney for animal care assistance.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Footnotes
Published ahead of print 12 December 2011
REFERENCES
- 1. Akerley BJ, Cotter PA, Miller JF. 1995. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell 80:611–620 [DOI] [PubMed] [Google Scholar]
- 2. Akerley BJ, Monack DM, Falkow S, Miller JF. 1992. The bvgAS locus negatively controls motility and synthesis of flagella in Bordetella bronchiseptica. J. Bacteriol. 174:980–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brockmeier SL. 2004. Prior infection with Bordetella bronchiseptica increases nasal colonization by Haemophilus parasuis in swine. Vet. Microbiol. 99:75–78 [DOI] [PubMed] [Google Scholar]
- 4. Brockmeier SL, Lager KM. 2002. Experimental airborne transmission of porcine reproductive and respiratory syndrome virus and Bordetella bronchiseptica. Vet. Microbiol. 89:267–275 [DOI] [PubMed] [Google Scholar]
- 5. Brockmeier SL, Loving CL, Nicholson TL, Palmer MV. 2008. Coinfection of pigs with porcine respiratory coronavirus and Bordetella bronchiseptica. Vet. Microbiol. 128:36–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brockmeier SL, Palmer MV, Bolin SR. 2000. Effects of intranasal inoculation of porcine reproductive and respiratory syndrome virus, Bordetella bronchiseptica, or a combination of both organisms in pigs. Am. J. Vet. Res. 61:892–899 [DOI] [PubMed] [Google Scholar]
- 7. Brockmeier SL, Palmer MV, Bolin SR, Rimler RB. 2001. Effects of intranasal inoculation with Bordetella bronchiseptica, porcine reproductive and respiratory syndrome virus, or a combination of both organisms on subsequent infection with Pasteurella multocida in pigs. Am. J. Vet. Res. 62:521–525 [DOI] [PubMed] [Google Scholar]
- 8. Brockmeier SL, Register KB. 2007. Expression of the dermonecrotic toxin by Bordetella bronchiseptica is not necessary for predisposing to infection with toxigenic Pasteurella multocida. Vet. Microbiol. 125:284–289 [DOI] [PubMed] [Google Scholar]
- 9. Brockmeier SL, et al. 2002. Role of the dermonecrotic toxin of Bordetella bronchiseptica in the pathogenesis of respiratory disease in swine. Infect. Immun. 70:481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buboltz AM, Nicholson TL, Weyrich LS, Harvill ET. 2009. Role of the type III secretion system in a hypervirulent lineage of Bordetella bronchiseptica. Infect. Immun. 77:3969–3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chanter N, Magyar T, Rutter JM. 1989. Interactions between Bordetella bronchiseptica and toxigenic Pasteurella multocida in atrophic rhinitis of pigs. Res. Vet. Sci. 47:48–53 [PubMed] [Google Scholar]
- 12. Cotter PA, Jones AM. 2003. Phosphorelay control of virulence gene expression in Bordetella. Trends Microbiol. 11:367–373 [DOI] [PubMed] [Google Scholar]
- 13. Cotter PA, Miller JF. 1994. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect. Immun. 62:3381–3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cotter PA, Miller JF. 1997. A mutation in the Bordetella bronchiseptica bvgS gene results in reduced virulence and increased resistance to starvation, and identifies a new class of Bvg-regulated antigens. Mol. Microbiol. 24:671–685 [DOI] [PubMed] [Google Scholar]
- 15. Cummings CA, Bootsma HJ, Relman DA, Miller JF. 2006. Species- and strain-specific control of a complex, flexible regulon by Bordetella BvgAS. J. Bacteriol. 188:1775–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deora R, Bootsma HJ, Miller JF, Cotter PA. 2001. Diversity in the Bordetella virulence regulon: transcriptional control of a Bvg-intermediate phase gene. Mol. Microbiol. 40:669–683 [DOI] [PubMed] [Google Scholar]
- 17. Donnenberg MS, Kaper JB. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guerrero RJ. 1990. Respiratory disease: an important global problem in the swine industry, p 98 Proc. 11th Int. Pig Vet. Soc., Lausanne, Switzerland [Google Scholar]
- 19. Inatsuka CS, et al. 2010. Pertactin is required for Bordetella species to resist neutrophil-mediated clearance. Infect. Immun. 78:2901–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 21. Loving CL, et al. 2010. Influenza virus coinfection with Bordetella bronchiseptica enhances bacterial colonization and host responses exacerbating pulmonary lesions. Microb. Pathog. 49:237–245 [DOI] [PubMed] [Google Scholar]
- 22. Magyar T, Lax AJ. 2002. Atrophic rhinitis, p 169–197 In Brogden KA, Guthmiller J. (ed), Polymicrobial diseases. ASM Press, Washington, DC: [PubMed] [Google Scholar]
- 23. Martinez de Tejada G, et al. 1998. Neither the Bvg- phase nor the vrg6 locus of Bordetella pertussis is required for respiratory infection in mice. Infect. Immun. 66:2762–2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mattoo S, Yuk MH, Huang LL, Miller JF. 2004. Regulation of type III secretion in Bordetella. Mol. Microbiol. 52:1201–1214 [DOI] [PubMed] [Google Scholar]
- 25. McMillan DJ, Shojaei M, Chhatwal GS, Guzman CA, Walker MJ. 1996. Molecular analysis of the bvg-repressed urease of Bordetella bronchiseptica. Microb. Pathog. 21:379–394 [DOI] [PubMed] [Google Scholar]
- 26. Merkel TJ, Stibitz S, Keith JM, Leef M, Shahin R. 1998. Contribution of regulation by the bvg locus to respiratory infection of mice by Bordetella pertussis. Infect. Immun. 66:4367–4373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nicholson TL. 2007. Construction and validation of a first-generation Bordetella bronchiseptica long-oligonucleotide microarray by transcriptional profiling the Bvg regulon. BMC Genomics 8:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nicholson TL, Brockmeier SL, Loving CL. 2009. Contribution of Bordetella bronchiseptica filamentous hemagglutinin and pertactin to respiratory disease in swine. Infect. Immun. 77:2136–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palzer A, Ritzmann M, Wolf G, Heinritzi K. 2008. Associations between pathogens in healthy pigs and pigs with pneumonia. Vet. Rec. 162:267–271 [DOI] [PubMed] [Google Scholar]
- 30. Pilione MR, Harvill ET. 2006. The Bordetella bronchiseptica type III secretion system inhibits gamma interferon production that is required for efficient antibody-mediated bacterial clearance. Infect. Immun. 74:1043–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sekiya K, et al. 2001. Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA-sheath-like structure. Proc. Natl. Acad. Sci. U. S. A. 98:11638–11643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Simon R, Priefer U, Puhler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Biotechnology 1:784–791 [Google Scholar]
- 33. Stockbauer KE, Fuchslocher B, Miller JF, Cotter PA. 2001. Identification and characterization of BipA, a Bordetella Bvg-intermediate phase protein. Mol. Microbiol. 39:65–78 [DOI] [PubMed] [Google Scholar]
- 34. Sukumar N, Mishra M, Sloan GP, Ogi T, Deora R. 2007. Differential Bvg phase-dependent regulation and combinatorial role in pathogenesis of two Bordetella paralogs, BipA and BcfA. J. Bacteriol. 189:3695–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trahan CJ, Stephenson EH, Ezzell JW, Mitchell WC. 1987. Airborne-induced experimental Bordetella bronchiseptica pneumonia in strain 13 guinea pigs. Lab Anim. 21:226–232 [DOI] [PubMed] [Google Scholar]
- 36. U. S. Department of Agriculture 2008. Swine 2006. III. Reference of swine health and health management in the United States. USDA/APHIS/VS CEAH N478.0308 USDA, Fort Collins, CO [Google Scholar]
- 37. Vecht U, Arends JP, van der Molen EJ, van Leengoed LA. 1989. Differences in virulence between two strains of Streptococcus suis type II after experimentally induced infection of newborn germ-free pigs. Am. J. Vet. Res. 50:1037–1043 [PubMed] [Google Scholar]
- 38. Vecht U, Wisselink HJ, van Dijk JE, Smith HE. 1992. Virulence of Streptococcus suis type 2 strains in newborn germfree pigs depends on phenotype. Infect. Immun. 60:550–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yuk MH, Harvill ET, Cotter PA, Miller JF. 2000. Modulation of host immune responses, induction of apoptosis and inhibition of NF-kappaB activation by the Bordetella type III secretion system. Mol. Microbiol. 35:991–1004 [DOI] [PubMed] [Google Scholar]
- 40. Yuk MH, Harvill ET, Miller JF. 1998. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol. Microbiol. 28:945–959 [DOI] [PubMed] [Google Scholar]