Abstract
The Bordetella adenylate cyclase toxin-hemolysin (CyaA; also called ACT or AC-Hly) targets CD11b-expressing phagocytes and translocates into their cytosol an adenylyl cyclase (AC) that hijacks cellular signaling by conversion of ATP to cyclic AMP (cAMP). Intriguingly, insertion of large passenger peptides removes the enzymatic activity but not the cell-invasive capacity of the AC domain. This has repeatedly been exploited for delivery of heterologous antigens into the cytosolic pathway of CD11b-expressing dendritic cells by CyaA/AC− toxoids, thus enabling their processing and presentation on major histocompatibility complex (MHC) class I molecules to cytotoxic CD8+ T lymphocytes (CTLs). We produced a set of toxoids with overlapping deletions within the first 371 residues of CyaA and showed that the structure of the AC enzyme does not contain any sequences indispensable for its translocation across target cell membrane. Moreover, replacement of the AC domain (residues 1 to 371) with heterologous polypeptides of 40, 146, or 203 residues yielded CyaAΔAC constructs that delivered passenger CTL epitopes into antigen-presenting cells (APCs) and induced strong antigen-specific CD8+ CTL responses in vivo in mice and ex vivo in human peripheral blood mononuclear cell cultures. This shows that the RTX (repeats in toxin) hemolysin moiety, consisting of residues 374 to 1706 of CyaA, harbors all structural information involved in translocation of the N-terminal AC domain across target cell membranes. These results decipher the extraordinary capacity of the AC domain of CyaA to transport large heterologous cargo polypeptides into the cytosol of CD11b+ target cells and pave the way for the construction of CyaAΔAC-based polyvalent immunotherapeutic T cell vaccines.
INTRODUCTION
The 1,706-residue-long adenylate cyclase toxin hemolysin (ACT; also called AC-Hly or CyaA) secreted by the whooping cough agent Bordetella pertussis primarily targets the phagocytic myeloid cells expressing the αMβ2 integrin receptor CD11b/CD18, such as macrophages, neutrophils, and dendritic cells (16). The toxin directly penetrates the cytoplasmic membrane of cells, without the need for endocytosis (13), and delivers its N-terminal adenylyl cyclase (AC) enzyme domain, which consists of the first 373 residues, to the cytosol (18). Inside cells, the AC binds calmodulin and catalyzes unregulated conversion of cellular ATP to the key signaling molecule cyclic AMP (cAMP), thereby disrupting signaling and bactericidal functions of CD11b+ phagocytes and promoting host colonization by Bordetella (50).
The 1,333 carboxy-proximal residues of CyaA constitute an Hly moiety belonging to the RTX (repeats in toxin) family of pore-forming hemolysins and leukotoxins of Gram-negative pathogens (29, 51). Hly accounts for the receptor binding, membrane insertion, and pore-forming activities of CyaA (6, 40). It contains a hydrophobic domain (residues 500 to 700 of CyaA) that forms small cation-selective pores in target cell membranes with a diameter of only 0.6 to 0.8 nm (1, 3, 35, 47). The Hly further harbors two posttranslational palmitoylation sites at lysine residues 860 and 983 (19, 20), where acylation of at least one of them confers on CyaA the capacity to bind its receptor, CD11b/CD18, and penetrate cells (6, 32). Finally, the C-terminal RTX domain of Hly harbors ∼40 calcium-binding sites that are formed by glycine- and aspartate-rich nonapeptide repeats. Loading of these sites with Ca2+ structures the toxin into the active conformation for target cell interactions (23, 39).
The structure of Hly has not been determined, and the mechanistic details of AC domain penetration across target cytoplasmic membrane remain poorly understood. AC translocation into cells depends on negative plasma membrane potential (36) and does not appear to proceed through the cation-selective pore formed by CyaA (34, 49). It depends, however, on structural integrity of the four predicted transmembrane amphipathic α-helices located between residues 502 to 522 and 565 to 591 of the hydrophobic domain of CyaA (35). These harbor pairs of negatively charged glutamate residues (Glu509 plus Glu516 and Glu570 plus Glu581) that have been found to be directly involved in AC domain translocation across target cell membranes (1, 35).
It has repeatedly been demonstrated that substitution of catalytic residues, or disruption of the ATP-binding site of the AC by dipeptide insertions, does not affect the capacity of the resulting CyaA/AC− toxoids to translocate the enzymatically inactive AC polypeptide across the cell membrane (45). Moreover, the cell-invasive capacity of CyaA was found to be mostly conserved even upon insertion of a broad range of heterologous antigenic polypeptides up to 200 residues in length into defined permissive sites within the AC domain (12, 30, 33). This has been successfully exploited for delivery of numerous AC-inserted passenger antigens into the cytosol of antigen-presenting cells (APCs) for processing by proteasomes and subsequent presentation of the excised major histocompatibility complex (MHC) class I-restricted epitopes on the surface of APCs to cytotoxic CD8+ T lymphocytes (CTL) (9). Immunization with CyaA/AC− bearing appropriate CD8+ T-cell epitopes, indeed, induced strong and epitope-specific CTL responses, which conferred full prophylactic protection against a lethal lymphocytic choriomeningitis virus challenge (41) or were efficient in tumor immunotherapy in mice (8, 30, 37).
It has previously been shown that the AC domain has to unfold during translocation across the cellular membrane (12). Moreover, the versatility of the AC domain in accommodating and delivering a broad range of heterologous polypeptides into cells suggested that no particular tertiary structure of the AC domain was involved in its cell-invasive capacity. However, it remained unclear whether the AC domain harbors any specific segments playing a mechanistic role in the AC translocation process through interactions with the Hly moiety or the target cell membrane. Here we used a systematic deletion analysis to show that no segment of the AC polypeptide (residues 1 to 371) is essential for its translocation into cells. We found that the AC domain is passively entrained across the cellular membrane by the Hly moiety, which can also translocate large artificial polypeptides, instead of the AC domain, into cells.
MATERIALS AND METHODS
Mice.
Male C57BL/6 (Ly 5.2) mice were obtained from a breeding colony at the Institute of Physiology of the ASCR in Prague, Czech Republic. Transgenic OT-I mice and B6.SJL (Ly5.1) mice were bred and kept at the GMO facility of the Institute of Molecular Genetics of the ASCR in Prague. Transgenic OT-I mice were mated with B6.SJL (Ly5.1) mice, and OT-I positive Ly5.1+ littermates were identified. The mice were used at 12 to 20 weeks of age. All animal experiments were approved by the competent Animal Welfare Committee of the Institute of Microbiology of the ASCR, v.v.i., in Prague, Czech Republic. Handling of animals was performed according to the Guidelines for the Care and Use of Laboratory Animals, the Act of the Czech National Assembly, Collection of Laws No. 149/2004, inclusive of the amendments, on the Protection of Animals against Cruelty, and Public Notice of the Ministry of Agriculture of the Czech Republic, Collection of Laws No. 207/2004, on care and use of experimental animals.
Cell cultures, growth conditions, and handling of cells.
DC2.4 and B3Z cell cultures were maintained in RPMI medium containing 10% fetal calf serum (FCS; Gibco) and antibiotic-antimycotic solution (0.1 mg/ml streptomycin, 1,000 units/ml penicillin, and 0.25 μg/ml amphotericin; Sigma-Aldrich). Only adherent cells of the semiadherent B3Z hybridoma were used for the next passage. HLA-A*0201-expressing T2 cells (ATCC CRL-1992) were cultured in standard Dulbecco's modified Eagle's medium (DMEM) with l-glutamine (Lonza, Verviers, Belgium), 10% bovine fetal serum, and 1% penicillin-streptomycin (both from Sigma-Aldrich, Prague, Czech Republic).
Peripheral blood mononuclear cells (PBMCs) from 10 cytomegalovirus (CMV)-seropositive donors (anti-CMV IgG antibodies detectable in serum) were used. Healthy donors were positive for human leukocyte antigen A*0201 (HLA-A*0201) and were recruited at the transfusion unit from blood donors that signed an informed-consent form approved by the Ethical Committee of the University Hospital of Brno. The PBMCs were isolated by Histopaque (Sigma-Aldrich, Prague, Czech Republic) gradient centrifugation of anticoagulated blood buffy coats. Viable cells were quantified, cryopreserved in Hanks' balanced salt solution (HBSS) containing 40% fetal bovine serum (FBS) and 10% dimethyl sulfoxide (all from Sigma-Aldrich, Prague, Czech Republic), and stored in liquid nitrogen.
Deletion mutagenesis in the AC domain of CyaA.
Deletions in the AC domain were performed according to standard protocols using the plasmid pT7CT7ACT1/E5-mutNdeI. This was derived from pT7CACT1 (33) by placing the ribosome binding site RBS10 from gene 10 of bacteriophage T7 upstream of the cyaA open reading frame, thus allowing increased production of CyaA protein in Escherichia coli. Insertion of a synthetic BamHI linker (5′-GGATCC) between codons 188 and 189 of cyaA was used to generate alleles for production of detoxified CyaA/AC− toxoids, in which the ATP binding site was disrupted by a GlySer dipeptide insert at position 188 of the AC domain as previously described (9). Next, the NdeI restriction site sequence (5′-CATATG), comprising the ATG initiation codon of the cyaC gene, was removed by a T-to-A mutation, generating the 5′-CAAATG sequence, to yield the pT7CT7ACT1/E5-mutNdeI plasmid with a unique NdeI restriction site comprising the ATG initiation codon of the cyaA gene. Unique BsrGI restriction sites were then introduced at four different permissive sites within the cyaA gene, between codons 107 and 108, codons 232 and 233, codons 335 and 336, and codons 423 and 424 (33). Deletions in the 5′ portion of cyaA encoding the AC domain were next performed by using combinations of the unique NdeI, BsrGI, BclI, AgeI, and BstBI restriction sites. At the same time, double-stranded synthetic oligonucleotides encoding the OVA257-264 epitope SIINFEKL, corresponding to residues 257 to 264 of ovalbumin and surrounded by unique KpnI and NheI restriction sites, were inserted in frame into the truncated cyaA alleles. The generated deletions in the AC domain are summarized in Table 1, where the sequences of the modified portions of CyaA toxoids are given. Further details will be provided upon request.
Table 1.
CyaA/AC− toxoids
| Toxoida | Deletion | Sequenceb |
|---|---|---|
| A1 | Δ233–271 | SEATGG232VHGTSIINFEKLASA272ITDFEL |
| A2 | Δ274–319 | MNIGVIT273GTSIINFEKLASS320GESQML |
| A3 | Δ321–335 | VVSATG320GTSIINFEKLASGVH336QQRGEG |
| A4 | Δ108–271 | SSLAHG107VHGTSIINFEKLASA272ITDFEL |
| A5 | Δ108–319 | SSLAHG107VHGTSIINFEKLASS320GESQML |
| A6 | Δ233–319 | SEATGG232VHGTSIINFEKLASS320GESQML |
| A7 | Δ274–335 | MNIGVIT273GTSIINFEKLASGVH336QQRGEG |
| A8 | Δ336–371 | LKEYIG335VRGTSIINFEKLAS372RSKFSP |
| A9 | Δ321–371 | VVSATG320GTSIINFEKLAS372RSKFSP |
| A10 | Δ274–371 | MNIGVIT273GTSIINFEKLAS372RSKFSP |
| A11 | Δ233–371 | SEATGG232VHGTSIINFEKLAS372RSKFSP |
| A12 | Δ108–371 | SSLAHG107VHGTSIINFEKLAS372RSKFSP |
| A13 | Δ2–107 | M1GTSIINFEKLASGVH108HTAVDL |
| A14 | Δ2–232 | M1GTSIINFEKLASGVH233LDRERI |
| A15 | Δ2–271 | M1GTSIINFEKLASA272ITDFEL |
| A16 | Δ2–319 | M1GTSIINFEKLASS320GESQML |
| A17 | Δ2–335 | M1GTSIINFEKLASGVH336QQRGEG |
| A18 | Δ2–371 | M1GTSIINFEKLAS372RSKFSP |
| B18 | Δ2–371 | M1GTVNGLEQLESIINFEKLTEWTSSNVMEERKIKVYLPRAS372RSKFSP |
| B19 | Δ2–424 | M1VNGLEQLESIINFEKLTEWTSSNVMEERKIKVYLPRMYT425AVEAAE |
| A+C18 | Δ2–371 | M1EEKAFSPEVIPMFTALSEGATPQDLNTMLNTVGGHQAAMQMLKDTINEEAAEWDRIYKRWIILGLNKIVRMYSPVSILDIRQGPKEPFRDYVDRFARNSGSIINFEKLRNCRAPRKKGCWKCGKEGHQMKDCTERQANFLGKIWPS372RSKFSP |
| B+D18 | Δ2–371 | M1GTVNGLEQLESIINFEKLTEWTSSNVMEERKIKVYLPRASRRTSLNIPSINVHHYPSFVFPTKDVALRHVIGDQYVKVYLESFCEDVPSGKLFMKPGKISHIMLDVAFTSHEHYSEHPTFTSQYRIQGKLEYVTTERKTPRVTGGGAMAGASTSATWPPWQAGILARNLVPMVATVQGQNLKYQEFFWDANDIYRIFAERRAS372RSKFSP |
| 108A/233D/AC− | SSLAHG107VLSIINFEKLVH108HTAVDL......SEATGG232VQRRTSLNIPSINVHHYPSFVFPTKDVALRHVIGDQYVKVYLESFCEDVPSGKLFMKPGKISHIMLDVAFTSHEHYSEHPTFTSQYRIQGKLEYVTTERKTPRVTGGGAMAGASTSATWPPWQAGILARNLVPMVATVQGQNLKYQEFFWDANDIYRIFAERRDVH233LDRERI | |
| 108B/AC− | SSLAHG107VQLTGLEQLESIINFEKLTEWTSSNVMEERKIKVYLPRIVH108HTAVDL | |
| 233A+C/AC− | SEATGG232MEEKAFSPEVIPMFTALSEGATPQDLNTMLNTVGGHQAAMQMLKDTINEEAAEWDRIYKRWIILGLNKIVRMYSPVSILDIRQGPKEPFRDYVDRFARNSGSIINFEKLRNCRAPRKKGCWKCGKEGHQMKDCTERQANFLGKIWPS233LDRERI | |
| 233B/AC− and 233B–KP/AC− | SEATGG232VQLTGLEQLESIINFEKLTEWTSSNVMEERKIKVYLPRIVH233LDRERI | |
| 336B/AC− | LKEYIG335VQLTGLEQLESIINFEKLTEWTSSNVMEERKIKVYLPRIVH336QQRGEG |
The constructs were enzymatically inactive either due to a GlySer dipeptide insert between residues 188 and 189 of the AC domain (AC−) or due to deletions abolishing the AC enzyme activity.
Each entry includes the insertion point, insert sequence, and flanking sequence. The sequences of CD8+ epitopes of OVA (SIINFEKL) and CMV (NLVPMVATV) are underlined, and the inserted flanking residues are in bold. In the A+C18 and 233A+C/AC− constructs, the 135-residue artificial sequence assembled from the conserved portions of HIV Gag protein, also comprising T cell epitopes (not used), is in italics. In the B+D18 and 108A/233D/AC− constructs, the 155-residue sequence composed of conserved CD4+ and CD8+ epitopes of the CMV pp65 phosphoprotein is in italics. The numbers indicate the corresponding residue positions in the intact CyaA polypeptide sequence.
To extend the flanking sequence of the OVA257-264 epitope (SIINFEKL) in the A18 construct (Table 1), and to introduce a VNGLEQLESIINFEKLTEWTSSNVMEERKIKVYLPR insert, corresponding to residues 249 to 284 of ovalbumin (OVA249-284), a corresponding double-stranded synthetic oligonucleotide was inserted using the KpnI and NheI restriction sites, yielding construct B18 (Table 1).
The control toxoids 108B/AC−, 233B/AC−, and 336B/AC− were prepared by the insertion of the corresponding double-stranded synthetic oligonucleotide, encoding the OVA249-284 peptide, into the permissive BsrGI restriction sites inserted at codons 108, 233, and 336, respectively. The negative control 233B-KP/AC− was prepared from a doubly mutated CyaA/E570K+E581P/AC− toxoid variant that was previously obtained in frame from extensive mutagenesis of the CyaA toxin gene (1).
To generate the A+C18 and 233A+C/AC− constructs (Table 1), the open reading frame encoding a polypeptide composed of conserved segments of the HIV Gag protein (28) was PCR amplified from pGA15 HIVCON (kind gift of T. Hanke, University of Oxford, Oxford, United Kingdom).
To generate constructs B+D18 and 108A/233D/AC− (Table 1), the immunodominant epitopes of the human cytomegalovirus (CMV) phosphoprotein 65 (pp65) (46, 52) were assembled into one polyepitope polypeptide (Table 1), and the corresponding codon-optimized synthetic open reading frame was purchased from GenScript (Piscataway, NJ).
The exact sequence of relevant portions of all plasmids used for protein production was systematically verified by DNA sequencing.
Production and purification of CyaA proteins.
CyaA toxins and CyaA/AC− toxoids were produced in Escherichia coli BL21/pMM100 (lac repressor gene lacIq carried on pMM100) and purified as described earlier (33). During hydrophobic chromatography, the resin with bound CyaA was repeatedly washed with several bed volumes of 60% isopropanol to remove bacterial endotoxin (48). In the final step, the proteins were eluted with 8 M urea, 2 mM EDTA, 50 mM Tris-HCl (pH 8.0) and stored at −20°C. The endotoxin content of the samples was determined by the Limulus amebocyte lysate assay (QCL-1000; Cambrex) according to the manufacturer's instructions and was below 100 endotoxin units (EU)/mg of purified protein.
On-column biotinylation of CyaA/AC− toxoids was performed after the DEAE-Sepharose purification step as previously described (49).
Cell binding and cAMP elevation activity of enzymatically active CyaA toxins.
For determination of cell binding of enzymatically active CyaA toxins, DC2.4 cells (2 × 106/ml) were incubated in the presence of 30 nM CyaA in Dulbecco's modified Eagle's medium (DMEM) without FCS for 30 min on ice. Cells were washed to remove unbound toxin, and CyaA binding was assessed by determining cell-associated adenylate cyclase enzyme activity in the presence of 1 μM calmodulin as described previously (26). The cAMP-elevating capacity of CyaA was assessed on DC2.4 cells (3 × 105 per well) incubated with various concentrations of CyaA in DMEM without FCS at 37°C for 30 min. The reaction was stopped by the addition of 0.2% Tween 20 in 50 mM HCl, and the intracellular cAMP concentration was determined by an antibody competition immunoassay, as described elsewhere (2).
Cell binding of enzymatically inactive CyaA/AC− toxoids.
DC2.4 cells (5 × 105 per well) in 100 μl of DMEM with 1% FCS were incubated with biotinylated CyaA/AC− toxoids for 30 min on ice. Unbound toxoid was removed by repeated washing, and cells were stained with phycoerythrin (PE)-conjugated streptavidin (Exbio) at an 1:400 dilution (50 μl/well) for 30 min on ice. CyaA/AC− binding was analyzed by flow cytometry using a FACS LSR II instrument (BD Biosciences) and FlowJo version 7.2.1 (Treestar, Inc.). To block CyaA/AC− binding to the CD11b/CD18 receptor, DC2.4 cells were preincubated for 30 min on ice with 10 μg/ml of the anti-mouse CD11b-specific monoclonal antibody M1/70 (eBioscience) prior to addition of CyaA (16).
Presentation of OVA257-264 epitope to the B3Z hybridoma in vitro.
For in vitro presentation of antigens, the immortalized dendritic cell line DC2.4, generated from bone marrow cells of C57BL/6 mice, was used (44). OVA257-264 presentation was determined as β-galactosidase production in B3Z hybridoma CD8+ T cells, which carry a lacZ reporter gene under the control of the interleukin 2 (IL-2) promoter NF-AT elements, activated upon TCR recognition of the OVA257-264 peptide (SIINFEKL) on the murine H-2Kb MHC class I molecules (25). DC2.4 cells (105/well) were seeded in DMEM with 10% FCS in 96-well plates and pulsed for 4 h at 37°C with various concentrations of toxoids, OVA peptide (purity ≥ 95%; Sigma-Genosys), or ovalbumin protein (albumin from chicken egg white; Sigma-Aldrich). Alternatively, toxoids were preincubated at the indicated concentration in 200 μl of DMEM containing 10% FCS with 5 μg/ml of 3D1 or the control isotype TU-01 monoclonal antibody (MAb) (Exbio, Prague, Czech Republic) at room temperature for 15 min, before 150 μl of the medium with toxoids and antibody was used to cover 105 adherent DC2.4 cells in a 96-well plate for 4 h. The cells were then washed with phosphate-buffered saline (PBS) and further cultured with B3Z hybridoma (105 cells/well) in RPMI medium. After 18 h of incubation at 37°C in a humidified 5% CO2 atmosphere, cultures were lysed by addition of 100 μl per well of PBS containing 100 μM 2-mercaptoethanol, 9 mM MgCl2, 0.125% Nonidet P-40, and 0.15 mM chlorophenol red-β-d-galactopyranoside (CPRG; Sigma-Aldrich), and the β-galactosidase activity was determined as described previously (24). Absorbance was read at 570 nm.
Measuring of intracellular concentration of Ca2+ ions.
Calcium influx into cells was measured as previously described (10).
Isolation of detergent-resistant membrane and Western blotting.
Detergent-resistant membranes (coalesced lipid rafts) were separated by flotation in discontinuous sucrose density gradients, and toxoids were detected in Western blots using the 9D4 antibody as described previously (4).
CD8+ T cell activation in vivo.
Purified CD8+ T cells from OT-I Ly5.1+ mice were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) and injected intravenously (i.v.) into C57BL/6 (Ly5.2) mice at 1.1 × 106 to 1.5 × 106 cells per mouse. Next day the mice were injected intraperitoneally (i.p.) with PBS and 2 nmol of OVA257-264 peptide (SIINFEKL; MBL International, Woburn, MA) plus poly(I·C) (75 μg) and i.v. with tested purified toxoids (0.3 nmol). Each dose of tested purified toxoids in 8 M urea, 2 mM EDTA, and 50 mM Tris-HCl (pH 8.0) was placed into a separate tube, kept on ice in a small volume (25 to 30 μl), and diluted up to 300 μl with ice-cold PBS immediately before administration. Mice were euthanized 4 days after injection of tested samples, and their spleen cells were harvested.
To determine the expansion of adoptively transferred CD8+ Ly5.1+ cells, spleen cells were resuspended in FACS buffer (PBS with 2% FCS, 2 mmol EDTA, and 0.05% sodium azide), blocked with 10% mouse serum for 30 min on ice, and stained with fluorochrome-labeled MAbs CD8-A700, CD8-PerCP (peridinin chlorophyll protein), CD45.1-allophycocyanin, and CD25-PE (eBioscience, San Diego, CA) for 30 min on ice in the dark. Cells were then washed twice in FACS buffer and transferred into buffer with 4% paraformaldehyde.
For determination of functionality of expanded CD8+ T cells, spleen cells were restimulated in vitro with 1 μM SIINFEKL peptide for 2 h, followed by the addition of brefeldin A (1 μg/ml) to block the extracellular release of gamma interferon (IFN-γ) for 4 h. Cells were stained as described above, fixed, and permeabilized in fixation and permeabilization buffer (eBioscience, San Diego, CA), blocked with 2% mouse serum for 10 min on ice, and incubated with IFN-γ–PE and granzyme B–PE (eBioscience) for 30 min on ice in the dark. Flow cytometry was performed using an LSRII instrument (BD Biosciences, Mountain View, CA), and data were analyzed with FlowJo software (Tree Star, Ashland, OR).
Activation of CMV-specific CD8+ T cells.
To monitor CMV-specific T cell activation, PBMCs from CMV-seropositive donors were incubated with various toxoids (5 nM) in high-glucose DMEM with l-glutamine (Lonza, Verviers, Belgium), 8% male human AB+ serum, and 1% penicillin-streptomycin (both from Sigma-Aldrich, Prague, Czech Republic) at a concentration of 2 × 106 per ml in a 10% CO2 atmosphere at 37°C for 6 h. After 3 h, 1 μl/ml of BD GolgiPlug (containing brefeldin A; BD Biosciences, San Jose, CA) was added. PBMCs were stained with the fluorochrome-labeled MAbs CD3-PeCy7 (Immunotech, Marseille, France) and CD8-allophycocyanin (Exbio, Prague, Czech Republic) for 20 min on ice. Cells were washed and fixed with a Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's instructions. Cells were stained intracellularly with anti-IFN-γ–PE MAb (BD Biosciences) for 30 min on ice. The percentage of CD8+ IFN-γ+ T cells was determined by flow cytometry using FACS Canto II (BD Biosciences). Data were processed using BD FACS DIVA software, version 5.0.1 (2006) (BD Biosciences). In parallel, PBMCs were also incubated with toxoids in the absence of BD GolgiPlug for 18 h, and the concentration of IFN-γ released into in culture medium was determined by cytometric bead array (CBA) using a human IFN-γ Flex set (BD Biosciences) according to the manufacturer's instructions. Data were analyzed with FCAP array software (2007) and BD FACS array software (2007) (BD Biosciences).
Clonal expansion of CMV-specific CD8+ T cells was assessed after 7 days of PBMC stimulation with 5 nM toxoids by staining with allophycocyanin-labeled HLA-A*0201 NLVPMVATV-loaded MHC I pentamers according to the manufacturer's instructions (Proimmune, Oxford, United Kingdom). PBMCs were further stained with anti-CD3–PeCy7 (Immunotech, Marseille, France) and anti-CD8–PE (Exbio, Prague, Czech Republic) monoclonal antibodies for 20 min on ice. The percentage of pentamer-specific CD8+ T cells was determined by flow cytometry using FACS Canto II and FACS DIVA software, version 5.0.1 (2006).
Functional characterization of clonally expanded CMV-specific CD8+ T cells.
PBMC suspensions from CMV-seropositive donors were subjected to antigenic stimulation with various toxoids (5 nM) for 7 days. T2 target cells were loaded with 10 μg/ml of CMV peptide (HLA*A0201 restricted; NLVPMVATV) or HIV-1 reverse transcriptase (Pol) peptide (ILKEPVHGV) for 2 h, washed, and used for restimulation of PBMCs at an effector-to-target ratio of 10:1 for 4.5 h. After 1.5 h, 1 μl/ml of BD GolgiPlug (containing brefeldin A; BD Biosciences) was added. Cells were stained with anti-CD3–PeCy7 (Immunotech, Marseille, France) and anti-CD8–allophycocyanin (Exbio, Prague, Czech Republic) MAbs for 25 min on ice, and CD3+ T cells were sorted using a FACS ARIA instrument (BD Biosciences). The sorted cells were fixed with a Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's instructions and stained with anti-IFN-γ–PE MAb (BD Biosciences) for 30 min at 4°C. The percentage of CD8+ IFN-γ+ T cells was determined by flow cytometry using FACS Canto II and BD FACS DIVA software. To determine percentage of CMV-specific CD8+ IFN-γ+ T cells, the percentage of CD8+ IFN-γ+ T cells restimulated by T2 target cells loaded with an irrelevant HIV-1 Pol peptide (ILKEPVHGV) was subtracted from the percentage of CD8+ IFN-γ+ T cells restimulated by T2 target cells loaded with the CMV peptide (NLVPMVATV). In parallel, effector and target cells were cultivated without addition of BD GolgiPlug for 9 h, and the concentration of IFN-γ released into culture supernatants was measured using a cytometric bead array, as described above. The background production of IFN-γ resulting from restimulation with T2 target cells loaded with irrelevant HIV-1 Pol peptide was subtracted.
For determination of cytotoxic activity of the clonally expanded CD8+ T cells, T2 cells were labeled with 1 μM CFSE in PBS with 0.1% bovine serum albumin (BSA) for 10 min at 37°C. Labeling was stopped by addition of male human AB+ serum (Sigma-Aldrich, Prague, Czech Republic), and cells were washed twice and resuspended in DMEM with 2% male human AB+ serum. CFSE-labeled T2 cells were loaded with 10 μg/ml of CMV peptide (HLA*A0201 restricted; NLVPMVATV), or the control HIV-1 Pol peptide (ILKEPVHGV) for 2 h and washed prior to use. Antigenic stimulation of PBMCs with CyaA toxoids (5 nM) was performed for 7 days, and cells were washed and coincubated with 4 × 104 of CFSE-labeled and peptide-loaded T2 targets at effector-to-target ratios of 1:1, 5:1, and 10:1. After 3 h of coincubation, cells were stained with 1 μg/ml of propidium iodide (PI) (Sigma-Aldrich, Prague, Czech Republic), and the percentage of dead PI+ CFSE+ cells was determined by flow cytometry using FACS Canto II and BD FACS DIVA software. Specific cytotoxicity was determined as the percentage of dead target cells loaded with the CMV peptide minus the percentage of dead target cells loaded with HIV peptide.
Statistical analysis.
The data were analyzed with STATISTICA 8 software (StaSoft, Prague, Czech Republic). The Mann-Whitney test was used for comparison of two groups, and the Kruskal-Wallis test was used for comparison of three or more groups. Experimental data were compared to those obtained with the corresponding controls, and a P value of 0.05 or less was considered statistically significant.
RESULTS
All information for translocation of the AC domain is contained within the Hly moiety of CyaA.
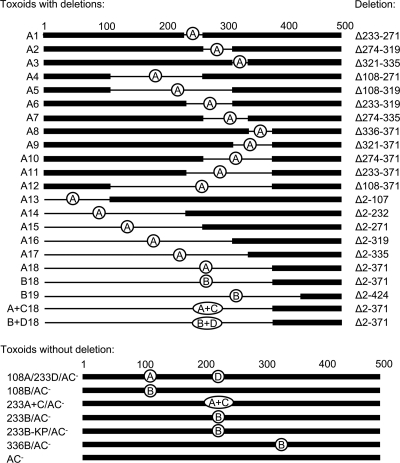
To map the segments of the AC domain that might be involved in its translocation across the cytoplasmic membrane of CD11b+ target cells, we generated a comprehensive set of 18 overlapping deletions within the AC domain of CyaA. As schematically depicted in Fig. 1 and summarized in Table 1, this construct series, designated A1 to A18, comprised toxoids with limited internal deletions of up to 46 residues (A1, A2, and A3), as well as constructs with rather extensive internal deletions of up to 212 residues (A4, A5, A6, and A7). Moreover, deletions systematically extending from either the carboxy-terminal (A8 to A12) or the amino-terminal (A13 to A18) extremity of the 371-residue AC domain of CyaA were engineered.
Fig 1.
Schematic depiction of toxoid constructs. Deletions in toxoids in which the removed portions were replaced by the inserted antigens are indicated by thin lines (only the first 500 residues of CyaA are depicted). The individual inserts are labeled A to D, corresponding to the names of the constructs. Construct sequences are summarized in Table 1, where exact sequences of all cargo inserts are given in full. Briefly, A stands for the CD8+ CTL epitope OVA257-264 (SIINFEKL), used as a marker, B stands for the OVA249-284 (SIINFEKL with extended flanking sequence), C stands for the artificial cargo polypeptide of 135 residues assembled from conserved segments of the HIV Gag protein (28), and D stands for a 155-residue artificial polypeptide composed of CD8+ and CD4+ T cell epitopes from the pp65 protein of CMV.
Since these deletions eliminated the AC enzyme activity of CyaA, the capacity to elevate cAMP concentration in cells could not be used for assessment of the cell-invasive capacity of such constructs, and a surrogate assay had to be used. To this end, the A1 to A18 toxoids were tagged by the OVA257-264 epitope (SIINFEKL) from ovalbumin (Table 1), inserted in place of the deleted AC domain portions. It has previously been established that CyaA delivers its AC domain directly across the cytoplasmic membrane into the cytosol of CD11b+ antigen-presenting cells, such as dendritic cells, where the AC is processed by the cytosolic proteasome. The excised OVA257-264 epitope can then enter the classical pathway for MHC class I-restricted antigenic presentation (17), which can be measured as the extent of stimulation of the B3Z CD8+ T hybridoma cells recognizing H-2Kb molecules loaded with the OVA257-264 epitope (17, 33, 42). Therefore, the capacity of CD11b+ dendritic cells to stimulate B3Z cells in vitro can be used as a measure of the capacity of CyaA to deliver the OVA-tagged AC polypeptide into cytosol of APCs.
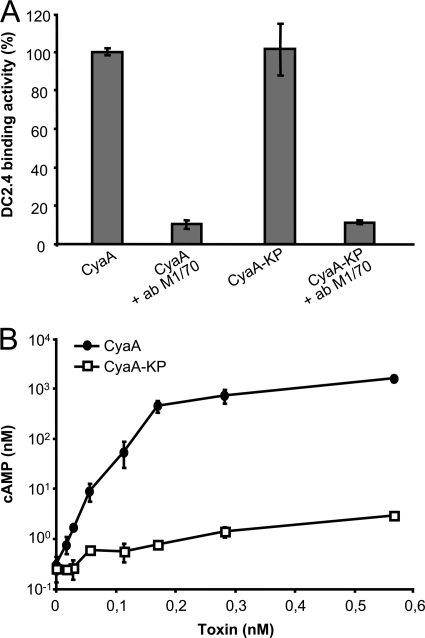
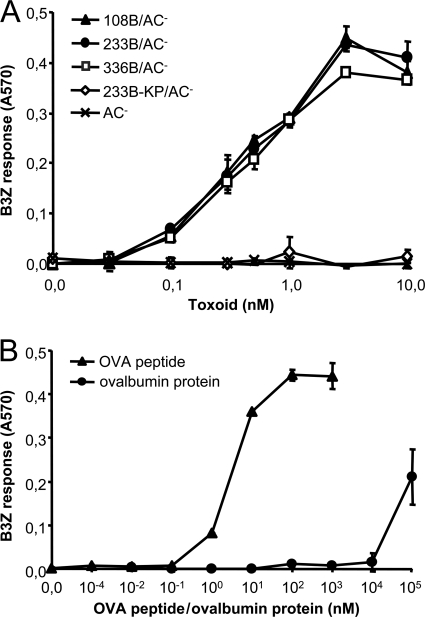
To verify that this assay can be specifically used for determination of translocation of the OVA-tagged AC segments into cytosol of APCs, we compared the B3Z-stimulatory capacity of DC2.4 cells incubated with an intact cell-invasive 233B/AC− toxoid or with a doubly mutated 233B/E570K+E581P/AC− (233B-KP/AC−) toxoid variant. The latter harbored a debilitating E570K+E581P combination of residue substitutions in the pore-forming domain, which was previously shown to preserve full receptor-binding capacity of the toxin while abolishing its capacity to deliver the AC domain into cytosol of cells and form pores in cellular membrane (4, 49). As documented in Fig. 2A, the enzymatically active (AC+) CyaA/E570K+E581P (CyaA-KP) construct exhibited the full capacity to bind to DC2.4 cells through CD11b/CD18, and this interaction was blocked by the CD11b-specific antibody M1/70 (17). In contrast to intact CyaA, however, CyaA-KP exhibited only a residual capacity (<2%) to translocate the AC domain into the cytosol of DC2.4 cells and elevate cytosolic cAMP (Fig. 2B) (4). As shown in Fig. S1 in the supplemental material, the enzymatically inactive AC− toxoid constructs 233B/AC− and 233B-KP/AC−, bearing a GlySer dipeptide insert between residues 188 and 189 to disrupt the ATP-binding site (9), also exhibited an identical capacity to bind DC2.4 cells in an M1/70-inhibitable manner. As shown in Fig. 3A, 233B-KP/AC− failed to deliver the OVA257-264 epitope into DC2.4 cells and failed to promote stimulation of B3Z cells, in contrast to the three cell-invasive 108B/AC−, 233B/AC−, and 336B/AC− toxoids bearing the OVA epitope inserted at residues 108, 233, and 336 of the AC domain, respectively (33). These control toxoids promoted a readily detectable B3Z response at a <0.1 nM concentration, while no B3Z stimulation was observed even at 100-fold-higher concentrations of 233B-KP/AC−. As further shown in Fig. 3B, a molar concentration of free soluble ovalbumin protein (0.1 mM) over five orders of magnitude higher had to be used to induce a detectable B3Z hybridoma response. Therefore, cross presentation of OVA257-264 due to macropinocytic uptake or receptor-mediated antigen endocytosis was undetectable under the conditions used and did not interfere with the assay. It can hence be concluded that mere toxoid binding to the CD11b/CD18-expressing APCs did not result in presentation of the OVA257-264 epitope and that stimulation of a B3Z T cell response strictly depended on the specific capacity of the used toxoids to translocate the epitope across the cellular membrane into the cytosol of DC2.4 cells.
Fig 2.
Combination of the E570K and E581P substitutions selectively abolishes the AC domain translocation capacity of CyaA without affecting toxin binding to CD11b/CD18-expressing cells. (A) Mouse CD11b+ DC2.4 cells (2 × 106/ml) were incubated in the presence of 30 nM CyaA (AC+) in DMEM without FCS for 30 min on ice. After removal of the unbound toxin, the cell-associated adenylate cyclase enzyme activity was determined. To block the CD11b/CD18 receptor, cells were preincubated for 30 min on ice with 10 μg/ml of the CD11b-specific antibody M1/70 prior to addition of CyaA. DC2.4 binding activities are expressed as percentages of wild-type CyaA binding activity and are means ± standard deviations from two independent determinations performed in duplicate (n = 4). (B) cAMP levels in DC2.4 cells were determined upon 30 min of incubation of DC2.4 cells (3 × 105 per well) with the indicated toxin concentrations. Values are means ± standard deviations from two independent determinations performed in triplicate (n = 6).
Fig 3.
The capacity to translocate the AC domain into the cytosol is required for efficient delivery of the OVA257-264 epitope for presentation on MHC class I molecules. DC2.4 cells were incubated with the indicated concentrations of toxoids (A), the OVA257-264 peptide, or free soluble ovalbumin (B) for 4 h. After being washed with PBS, the DC2.4 cells were further cultured for 18 h with the B3Z CD8+ T-hybridoma cells selectively recognizing cell surface-presented complexes of the H-2Kb MHC class I molecules with bound OVA257-264 peptide. Stimulation of B3Z cells was assayed as the amount of accumulated β-galactosidase. The means ± standard errors (SE) of duplicate samples, representative of three independent determinations, are shown.
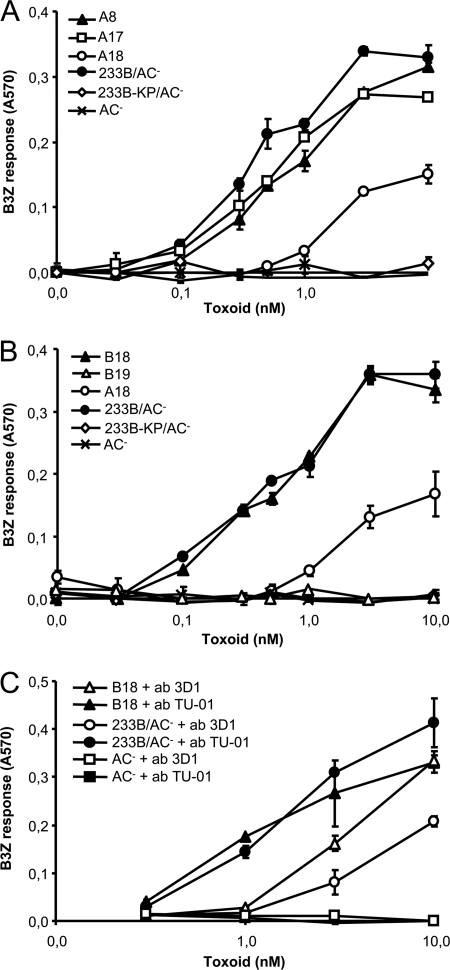
Once the robustness and specificity of the used OVA/AC translocation assay had been demonstrated, the capacity of the A1 to A18 deletion constructs to deliver the OVA257-264 epitope into the cytosol of DC2.4 cells could be assessed. As shown in Fig. 4A and Fig. S2 in the supplemental material, all but one of the 18 tested constructs (A1 to A17) reproducibly exhibited a comparable capacity to deliver the OVA257-264 epitope for presentation on MHC class I molecules, as the 233B/AC− control toxoid, which was unaffected by the size and location of the various deletions within the AC domain. The only construct with impaired capacity to deliver the epitope was A18 protein, in which the residues 1 to 371 were replaced by the OVA257-264 epitope peptide. As shown in Fig. 4A and Fig. S2 in the supplemental material, ∼10-fold more toxoid A18 (10 nM) than A1 to A17 was needed for induction of a B3Z response.
Fig 4.
The entire AC domain polypeptide sequence is dispensable for CyaA-mediated delivery of the OVA257-264 epitope for MHC class I-restricted antigenic presentation. DC2.4 cells were incubated with the indicated concentrations of the toxoids, and their capacity to deliver the OVA257-264 for antigenic presentation was assessed as described above. (C) Toxoids were preincubated with 5 μg/ml of 3D1 or the control isotype TU-01 antibody at room temperature for 15 min before addition to DC2.4 cells. The means ± SE of duplicate samples, representative of three independent determinations, are shown.
Interestingly, while the A17 construct had the first 335 residues of CyaA deleted, A18 carried a larger deletion, comprising 371 of the 373 N-terminal residues that form the minimal functional structure of the AC enzyme (18). The segment between residues 336 and 371 of the AC was, however, unlikely to be specifically required for AC domain translocation and OVA257-264 delivery into DC2.4 cytosol. The same segment was missing in several constructs that exhibited a full capacity to deliver the OVA epitope into DC2.4 cells, such as A8 (Fig. 4A) and A9 to A12 (see Fig. S2 in the supplemental material). Hence, it appeared more likely that when delivered by the A18 construct, the OVA257-264 epitope remained buried in the plasma membrane and was inefficiently excised by intracellular proteases.
To test this hypothesis, a 36-residue OVA249-284 polypeptide (VNGLEQLESIINFEKLTEWTSSNVMEERKIKVYLPR) comprising the natural flanking regions from ovalbumin was used to derive from the A18 construct the B18 protein, in which the number of residues separating the OVA257-264 epitope from the fusion point at position 372 of the CyaA moiety was extended from 2 to 22 residues. As shown in Fig. 4B, this extension restored the antigen delivery capacity of the truncated CyaA moiety, as the B18 toxoid exhibited a capacity to deliver OVA257-264 at a level comparable to that of the 233B/AC− control. As further shown in Fig. 4B, however, when the N-terminal deletion in CyaA was extended up to residue 424 in a B19 construct, still exhibiting a nearly full (∼60%) capacity to bind cells through interaction with CD11b/CD18 (see Fig. S1 in the supplemental material), the B19 protein was unable to deliver the OVA257-264 epitope for presentation to B3Z T hybridoma cells by APCs.
To further verify that the OVA257-264 epitope delivered by B18 reached the cytosol of APC due to translocation across the cellular membrane, the antigen delivery capacity of the B18 protein complexed with the 3D1 monoclonal antibody was determined. 3D1 was previously shown to block translocation of the AC domain across the cell membrane because of specific binding to the segment delimited by residues 373 to 400, which links the AC domain to the Hly moiety of CyaA (27). Moreover, we found recently that 3D1 binding locks CyaA in a translocation-intermediate conformation, in which the AC domain remains outside the cytosol and is inaccessible to processing by cytosolic proteases, while still being able to conduct extracellular Ca2+ ions into cells (4). As shown in Fig. 4C, by comparison to a control isotype antibody TU-01, preincubation with the 3D1 MAb reproducibly inhibited the capacity of the B18 and 233B/AC− toxoids to deliver the OVA257-264 epitope for presentation by DC2.4 cells to B3Z CD8+ T cells. It can hence be concluded that extension of the N-terminal sequence of the A18 construct by an artificial 20-residue peptide spacer conferred on the B18 construct the capacity to deliver the OVA257-264 epitope across the cytoplasmic membrane of APCs and rendered the cargo SIINFEKL peptide accessible for excision and presentation on APCs.
Collectively, the above-discussed results show that of the 373 residues in the AC polypeptide, the first 371 residues do not contain any segments specifically involved in AC domain translocation across cell membrane. All structural information required in this process appears to be confined within the residues 372 to 1706 of CyaA, which consists of the linker segment between residues 374 to 400 and the 1,306 C-terminal residues of the Hly moiety of CyaA. Indirectly, these data also suggest that the linker segment between residues 373 to 400 is inserted into the cell membrane or may even translocate across it.
CyaAΔAC, which has the AC domain replaced by an unrelated polypeptide, conducts Ca2+ ions into cells and relocates into the lipid rafts.
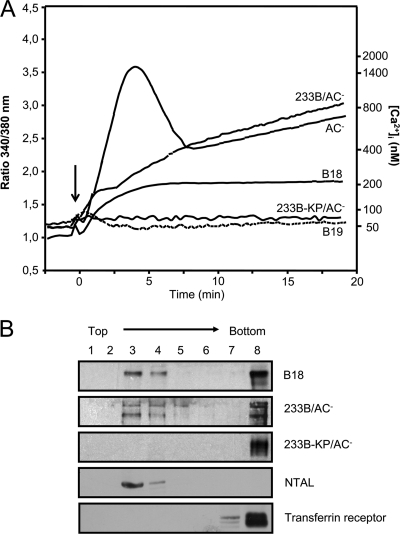
Recently we showed that upon interaction with its receptor CD11b/CD18, the CyaA toxin is inserted into macrophage membranes as a translocation intermediate that forms a novel path conducting extracellular Ca2+ ions across the cytoplasmic membrane (10). The incoming Ca2+ was then shown to activate the cytosolic protease calpain, which by processing the talin tether liberates the toxin-receptor complex for relocation into lipid rafts, from which the translocation of the AC polypeptide across membrane is completed (4). It was therefore important to examine whether the truncated B18 protein, lacking 371 of the 373 residues of the AC enzyme polypeptide, was still able to conduct Ca2+ ions into cells and relocate into lipid rafts. As shown by comparison to 233B/AC− in Fig. 5A, the truncated B18 toxoid exhibited a severalfold-lower but clearly detectable capacity to conduct extracellular Ca2+ ions into cells. In contrast, the B19 construct, which was unable to deliver the epitope for presentation, was as unable to promote Ca2+ influx as the construct 233B-KP/AC−, which is defective in formation of the translocation precursor and delivery of the AC domain into cytosol. These results are consistent with our previous report showing that segments of the AC domain inserted into and translocating across the cell membrane participate in formation of the conduit enabling influx of extracellular Ca2+ ions (10). Moreover, the structural and conformational alterations of the AC domain due to peptide inserts were previously shown to also affect the calcium-conducting properties of CyaA (10). This is also corroborated here by the observation of different amplitudes and time courses of the Ca2+ influx curves promoted by AC−, 233B/AC−, and B18 constructs.
Fig 5.
The CyaAΔAC toxoid delivering OVA257-264 epitope conducts Ca2+ ions into cells and associates with lipid rafts. (A) J774A.1 cells were loaded with the calcium probe Fura-2/AM at a 3 μM final concentration at 25°C for 30 min. After cells were washed in HBSS medium, toxoid variants (17 nM) were added at time zero (arrow), and the time course of calcium entry into the cytosol (elevation of intracellular [Ca2+]) was monitored by spectrofluorimetry (ratiometric measurement) as described elsewhere (10). (B) J774A.1 cells were incubated for 10 min with the indicated toxoids (1 nM). Detergent-resistant membrane microdomains were extracted with cold Triton X-100 and separated by flotation in a sucrose density gradient. The toxoids were detected in gradient fractions with the 9D4 antibody using Western blots. Results of one representative experiment of two are shown.
Consistent with its capacity to promote Ca2+ influx, the B18 toxoid still relocated into lipid rafts in cell membranes and floated in sucrose density gradient separations with the detergent-resistant membrane fraction (coalesced lipid rafts), like the intact 233B/AC− and the lipid raft marker NTAL (Fig. 5B). In contrast, the 233B-KP/AC− mutant remained in the bulk membrane phase together with the marker CD71 (transferrin receptor). However, the processing of the B18 protein present in the raft fraction could not be assessed due to poor resolution of the full-length ∼150-kDa (1,375-residue) B18 protein and its processed form in the 7.5% acrylamide gels used for Western blot analysis.
CyaAΔAC toxoids with the AC domain replaced by a CTL polyepitope sequence prime specific mouse and human CTL responses.
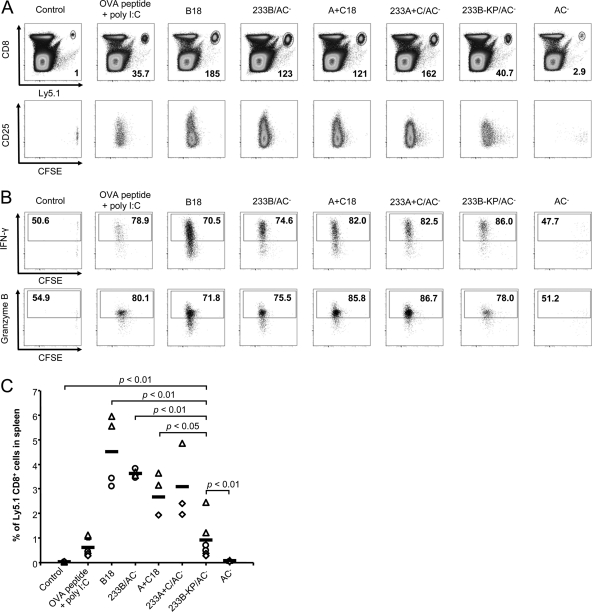
It was next important to examine whether the CyaAΔAC toxoids, having the first 371 residues of the AC domain replaced by large artificial polypeptides, were still able to deliver the cargo CTL epitopes for presentation to specific CD8+ T cells. Therefore, an A+C18 construct was generated, in which the OVA257-264 epitope was integrated into an artificial 135-residue antigen sequence derived from the HIV Gag protein (28). The capacity of the A+C18 construct (for the sequence, see Table 1) to deliver the OVA257-264 marker epitope for antigenic presentation was next assessed in vivo as the capacity of the A+C18 protein to expand naïve OT-I CD8+ T cells expressing the T cell receptor specifically recognizing the OVA257-264 epitope in complex with H-2Kb MHC class I molecules. To this end, 1.5 × 106 purified CFSE-labeled CD8+ T cells from OT-I (Ly5.1) transgenic mice were injected i.v. into syngeneic C57BL/6 (Ly5.2) mice, and 24 h after the transfer, the mice were immunized by i.v. injection of selected CyaA toxoids. Presentation of toxoid-delivered epitope to OT-I cells was then measured as specific stimulation of OT-I cells. As shown by comparison to PBS-injected mice (Fig. 6A), 4 days after administration of 0.3 nmol of full-length control toxoids (233B/AC− and 233A+C/AC−) or of the toxoids lacking the AC domain (B18 and A+C18), a very strong, 100- to 200-fold in vivo expansion (mean CFSE label intensity decrease per cell) of the adoptively transferred Ly5.1+ CD8+ T cells was observed. Moreover, the expanded OT-I CD8+ T cells were functional in terms of IFN-γ and granzyme B production after restimulation (Fig. 6B).
Fig 6.
The CyaAΔAC toxoid delivering the OVA257-264 epitope induces expansion of functional antigen-specific CD8+ T lymphocytes in vivo. CFSE-labeled CD8+ T cells (1.5 × 106) were purified from OT-I mice on a Ly5.1 background and injected i.v. into C57BL/6 mice (Ly5.2 background). Twenty-four hours later, the mice were immunized i.v. with the indicated toxoids (0.3 nmol) or i.p. with 2 nmol of OVA257-264 peptide with 75 μg of poly(I·C). Control mice received PBS only. Mice were euthanized 4 days after immunization, and their spleens were analyzed by flow cytometry. (A) Expansion of Ly5.1+ CD8+ T cells induced by immunization with the toxoids or OVA257-264 peptide plus poly(I·C) relative to the control is shown in bottom right corner of each dot plot. Data from one representative mouse of two immunized mice per experimental condition are shown. Data are representative of two independent experiments. (B) In vivo-expanded OVA257-264-specific OT-I CD8+ T cells produce IFN-γ and granzyme B. Spleen cells were incubated with 1 μM OVA257-264 peptide for 6 h in vitro, and brefeldin A (1 μM) was added for the last 4 h. The percentage of Ly5.1+ cells producing IFN-γ and granzyme B was determined by flow cytometry and is shown inside the positive gate in each dot plot. Data are from one representative mouse out of two mice per experimental condition and are representative of two independent experiments. (C) Percentage of Ly5.1+ CD8+ T cells in spleens of immunized mice. Data from repeated experiments involving two mice per group were pooled, and the horizontal bars represent the mean values. Symbols correspond to individual experiments.
The expanded CFSElow cells represented 2.5% to 4.5% of all cells in the spleens on average (Fig. 6C). Importantly, 2- to 4-fold-higher levels of expansion of Ly5.1+ CD8+ T cells were observed in mice that received the ΔAC toxoids B18 (P < 0.01) and A+C18 (P < 0.05) than in mice receiving 0.3 nmol of the nontranslocating 233B-KP/AC− toxoid or 2 nmol of free synthetic OVA257-264 peptide with poly(I·C). These results show that B18 and the A+C18 toxoid, which had the AC domain replaced by an artificial polypeptide sequence, were still able to deliver the OVA257-264 epitope into the cytosolic antigen presentation pathway of APCs in vivo, thus priming a strong OVA257-264-specific CD8+ T cell immune response in mice.
It is noteworthy that despite being impaired in translocation of the OVA257-264 epitope into cytosol of APCs for presentation in vitro, the control 233B-KP/AC− toxoid still exhibited a low but statistically significant capacity to stimulate proliferation of OT-I cells in vivo, compared to the PBS control and immunization with mock toxoid (P < 0.01). This indicates that the capacity of the 233B-KP/AC− toxoid to specifically bind to CD11b+ APCs enabled sufficient levels of cross presentation to promote induction of detectable CD8+ T cell response in vivo.
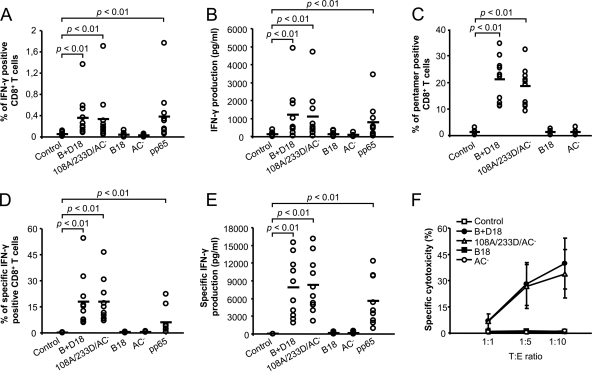
It was next important to establish the polyepitope delivery and CD8+ CTL-priming capacity of the ΔAC toxoids in a clinically relevant system. To this end, advantage was taken of the facts that 50 to 85% of healthy adults are latently infected with cytomegalovirus (CMV) and that several strong and highly conserved CD8+ CTL epitopes of the pp65 phosphoprotein of CMV have been identified (46, 52). Moreover, efficient tools for ex vivo expansion of CMV-specific CD8+ cytotoxic T lymphocytes exhibit a clear clinical potential for use in the treatment of CMV disease in recipients of bone marrow transplants (15, 43). Therefore, a B+D18 construct was prepared, in which the AC domain was replaced by a synthetic 203-residue polypeptide composed of conserved CD4+ and CD8+ CTL epitope sequences (Table 1). In parallel, the same polyepitope was placed adjacent to the OVA257-264 epitope in the control 108A/233D/AC− toxoid, and it was verified that both constructs retained the capacity to deliver the OVA257-264 epitope for presentation by APCs in vitro (data not shown). The capacity of the two constructs to activate and expand human pp65-specific CD8+ CTLs could then be compared. As shown in Fig. 7A, incubation of PBMCs from CMV-seropositive healthy HLA.A2 donors with 5 nM B+D18 or 108A/233D/AC− toxoids for 6 h resulted in a significant increase in the percentage of IFN-γ-secreting CD8+ T cells (P < 0.01). Consequently, a statistically significant (P < 0.01) increase in overall IFN-γ production by PBMCs from seropositive donors was detected upon 18 h of stimulation with B+D18 or 108A/233D/AC− toxoids or a 118-fold-higher molar amount of the pp65 protein, used as a positive control (Fig. 7B). In contrast, no such increase in percentage of CD8+ T cells secreting IFN-γ or in total secreted amounts of IFN-γ was observed for cells treated with the mock toxoids lacking the pp65 epitopes, such as the B18 protein and AC− toxoid (Fig. 7A and B). It can hence be concluded that the ΔAC protein B+D18 delivered the inserted pp65 polyepitope for antigenic presentation by primary human APCs as efficiently as the full-length 108A/233D/AC− toxoid and that both proteins were able to induce specific ex vivo stimulation of human CMV-specific CD8+ T cells in cultures of PBMCs of CMV-seropositive donors with a 100-fold-higher molar efficiency than the free pp65 protein.
Fig 7.
CyaAΔAC toxoid delivering a CMV pp65 polyepitope effectively activates and expands human CMV-specific CD8+ cytotoxic T lymphocytes. (A) Ex vivo activation of pp65-specific CD8+ T lymphocytes. PBMCs (5 × 105) from CMV-seropositive donors were stimulated in vitro in the presence of the indicated toxoids (5 nM) or with the pp65 protein (590 nM) for 6 h. The percentage of CD8+ T cells producing IFN-γ was determined by flow cytometry. The horizontal bars represent means of pooled values obtained for PBMC samples from 10 donors. (B) Levels of IFN-γ secreted into culture supernatants by cells stimulated for 18 h as described above were determined using a cytometric bead array. (C) Clonal expansion of pp65-specific CD8+ T lymphocytes in PBMC from 10 CMV-seropositive donors cultured for 7 days with 5 nM toxoids and stained with MHC I pentamers loaded with the 495-503 peptide of pp65. (D) Frequency of clonally expanded IFN-γ-producing pp65-specific CD8+ T lymphocytes. Washed PBMCs cultured for 7 days in the presence of 5 nM toxoids were restimulated by coincubation with T2 cells pulsed with 10 μg/ml of CMV pp65 peptide (amino acids 495 to 503) at a 10:1 ratio for 4.5 h. Background levels of IFN-γ production resulting from restimulation with T2 cells loaded with an irrelevant HIV-1 Pol peptide were subtracted. (E) Levels of IFN-γ secreted into culture supernatants by clonally expanded pp65-specific CD8+ T cells following restimulation for 9 h. (F) Clonally expanded pp65-specific CD8+ T lymphocytes were coincubated at the indicated target/effector (T:E) ratios with 4 × 104 CFSE-labeled T2 cells pulsed with 10 μg/ml CMV pp65 peptide (amino acids 495 to 503). T2 cell permeabilization was determined after PI staining by FACS analysis. Background lytic activity on T2 cells pulsed with the irrelevant HIV-1 Pol peptide was subtracted. The data were obtained with PBMCs from nine CMV-seropositive donors.
Therefore, we next examined the capacity of the B+D18 and 108A/233D/AC− toxoids to expand CMV-specific CD8+ T cells recognizing the HLA-A*0201-restricted pp65 epitope NLVPMVATV (residues 495 to 503). As documented in Fig. 7C by comparison to mock-treated or control PBMCs, after 7 days of culture of PBMC with 5 nM B+D18 and 108A/233D/AC− toxoids, a significant expansion of CMV pp65-specific CD8+ T lymphocytes was detected using MHC I pentamers loaded with the 495 to 503 pp65 peptide. Indeed, IFN-γ-secreting CD8+ T cells represented 10 to 35% of all CD8+ T cells present (Fig. 7D), and this pp65-specific stimulation was accompanied by a 10-fold increase (up to 18,000 pg/ml) of levels of secreted IFN-γ (Fig. 7E). As further shown in Fig. 7F, the expanded CD8+ T cells exhibited a cytotoxic capacity and permeabilized T2 target cells pulsed with the 495 to 503 peptide of pp65, compared to control cells, which were cultured with mock toxoid (P < 0.01). The ex vivo stimulation with the B+D18 and 108A/233D/AC− toxoids therefore resulted in comparably high levels of activation and expansion of functional CMV pp65-specific human cytotoxic CD8+ T lymphocytes (CTLs), which were able to specifically permeabilize T2 cells pulsed with the CMV pp65 peptide.
DISCUSSION
We show here that the AC domain of Bordetella adenylate cyclase toxin is entrained across the membrane of CD11b-expressing target cells as a mere passenger in a delivery step accomplished entirely by the RTX Hly moiety of the toxin (last 1,333 residues of CyaA). Unexpectedly, the deletion mapping results showed that the AC enzyme domain does not contain any segments that would play any active structural or mechanistic role in the process of AC polypeptide translocation across the cytoplasmic membrane of target cells. Indeed, the Hly moiety was also able to deliver to the cytosol two unrelated, 146- and 203-residue long artificial polypeptides extending from the N-terminal end of the Hly moiety in place of the AC domain. Delivery of the OVA257-264 epitope into cells by CyaAΔAC constructs was, however, inhibited upon binding of the 3D1 antibody to the segment located between residues 372 and 400 of CyaA (22, 27), which links the AC domain to the Hly moiety. This linker segment was preserved in all CyaAΔAC constructs able to deliver to the cytosol the N-terminally attached artificial polypeptides, and its deletion with the first 424 residues abolished the capacity of CyaAΔAC to translocate the attached polypeptides. Collectively, these results suggest that the segment linking the AC and Hly moieties plays a structural role and needs to be inserted across the cell membrane during the AC domain delivery.
Recently, processing of the CyaA molecule upon translocation of the AC domain across the target membrane was found to liberate the AC for action inside cells (4, 38, 49). This would suggest some resemblance of the mode of AC domain penetration into cells to the mechanism employed by A-B toxins. These typically consist of an enzymatic A component that is either noncovalently associated with the receptor-binding B moiety or linked to it by a disulfide bond that is reduced within the cytosol (7). Classical A-B toxins, however, deliver the enzymatic A subunit to the cytosol only upon receptor-mediated endocytosis. In sharp contrast, the AC domain of CyaA reaches the cytosol by direct translocation across the cytoplasmic membrane. Inhibitors of the known endocytic pathways do not interfere with translocation of the AC domain of CyaA into cells and elevation of cytosolic cAMP or with the AC domain-mediated delivery of inserted CD8+ T cell epitopes into the cytosol of antigen-presenting cells (4, 14, 17, 42).
Recently, we showed that AC translocation into cells occurs in two steps, where the membrane-inserted translocation precursor of CyaA first generates a calcium-conducting path across the cell membrane (4). The incoming extracellular Ca2+ next activates cytosolic calpain for processing of the talin tether of CD11b/CD18 to the actin cytoskeleton, thereby mobilizing the toxin-receptor complexes for relocation into cholesterol-rich membrane lipid rafts (4). Indeed, as documented in this study, a reduced but readily detectable capacity to conduct calcium ions into CD11b-expressing J774A.1 cells was still associated with the B18 construct able to deliver the N-terminally fused OVA249-284 polypeptide into cells. Such calcium-conducting activity is likely to also play an important role in the capacity of the toxoids to deliver the larger artificial polyepitopes (i.e., A+C18 and B+D18) into cells. This is suggested by our recent observation that toxoid-mediated influx of calcium ions into cells results in deceleration of the endocytic uptake (removal) of CyaA with cell membrane. As a result, a positive feedback loop of exacerbated cell permeabilization is initiated, where the efflux of potassium ions from permeabilized cells through toxin pores further decelerates the clathrin-mediated endocytosis of the toxin-receptor complexes (R. Fiser et al., submitted for publication).
Moreover, we recently observed that the CyaA-AC− toxoid can promote maturation of bone marrow derived dendritic cells in vitro, and work in progress indicates that this is due to the capacity of the toxoid to conduct calcium ions and permeabilize antigen-presenting cells for K+ efflux (M. Kosova, I. Adkins, R. Fiser, J. Masin, and P. Sebo, unpublished data). Such an adjuvant effect of the toxoid moiety is likely to contribute to the efficacy of induction of CTL responses to the delivered epitopes. A recent study showed that by eliciting K+ efflux from dendritic cells, and perhaps some other CD11b-expressing phagocytes, the pore-forming activity of CyaA contributes to activation of the NALP3 inflammasome (5) and thereby to induction of innate IL-1β response, which may also be important for expansion of anti-tumor CTL responses (11).
It will hence be interesting to explore in more detail the relation between the capacity of CyaA toxoids to promote influx of calcium and efflux of potassium ions and their capacity to deliver CTL epitopes for MHC class I-restricted presentation to CD8+ T lymphocytes. Enzymatically inactive but pore-forming CyaA-AC− toxoids have been extensively used over the past 18 years for delivery of AC-inserted CD8+ and CD4+ T cell epitopes from viruses, mycobacteria, and tumors into the MHC class I- and II-restricted antigen presentation pathways of CD11b-expressing dendritic cells (8, 9, 21, 31, 41). Promising CyaA-derived vaccines delivering within the AC domain a defined CD8+ CTL epitope from human tyrosinase or two large segments of the E7 oncoprotein of human papillomavirus 16 (HPV16) and HPV18 have entered phase I clinical studies and are being evaluated as experimental immunotherapeutic treatments for metastatic melanoma and cervical cancer, respectively. The results presented here provide the proof of concept for construction of a second generation of CyaA-based antigen delivery tools having the entire AC domain replaced by large artificial CTL polyepitope strings or entire tumor-specific antigens. This provides grounds for the design of polyvalent CTL vaccines.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Research Concept AV0Z50200510 and AS and CSF grants IAA500200914 (P.S.), P207/11/0717 (L.B.), P301/11/0325 (M.K.), NS9871 from IGA MZCR (J.M.), 1M0506 (P.S.), and 2B06161 (J.H.) of the Ministry of Education, Youth and Sports of the Czech Republic.
We thank Erik L. Hewlett (University of Virginia, Charlottesville, VA) for the generous gift of the 3D1 monoclonal antibody and Darren E. Higgins (Harvard Medical School, Boston, MA) for the gift of the B3Z hybridoma. Excellent technical help from Eva Pospisilova, Hana Kubinova, and Sona Charvatova is gratefully acknowledged.
ADDENDUM IN PROOF
After our paper was published ahead of print, it was brought to our attention that the hemolysin moiety of CyaA (residues 373 to 1,706) was able to deliver across the cytoplasmic membrane of erythrocytes and into cytosol of target cells also the enzymatically active C180 fragment of the pertussis toxin subunit S1 (Iwaki M, Kamachi K, Sato H. 1998. Biological activities of the subunit of pertussis toxin: analysis of PTS1-ACT fusion. Zentbl. Bakteriol. Suppl. 29:64–65). This work by Iwaki et al. is in line with conclusions of our study.
Footnotes
Published ahead of print 3 January 2012
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Basler M, et al. 2007. Segments crucial for membrane translocation and pore-forming activity of Bordetella adenylate cyclase toxin. J. Biol. Chem. 282:12419–12429 [DOI] [PubMed] [Google Scholar]
- 2. Basler M, Masin J, Osicka R, Sebo P. 2006. Pore-forming and enzymatic activities of Bordetella pertussis adenylate cyclase toxin synergize in promoting lysis of monocytes. Infect. Immun. 74:2207–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benz R, Maier E, Ladant D, Ullmann A, Sebo P. 1994. Adenylate cyclase toxin (CyaA) of Bordetella pertussis. Evidence for the formation of small ion-permeable channels and comparison with HlyA of Escherichia coli. J. Biol. Chem. 269:27231–27239 [PubMed] [Google Scholar]
- 4. Bumba L, Masin J, Fiser R, Sebo P. 2010. Bordetella adenylate cyclase toxin mobilizes its beta2 integrin receptor into lipid rafts to accomplish translocation across target cell membrane in two steps. PLoS Pathog. 6:e1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dunne A, et al. 2010. Inflammasome activation by adenylate cyclase toxin directs Th17 responses and protection against Bordetella pertussis. J. Immunol. 185:1711–1719 [DOI] [PubMed] [Google Scholar]
- 6. El-Azami-El-Idrissi M, et al. 2003. Interaction of Bordetella pertussis adenylate cyclase with CD11b/CD18: role of toxin acylation and identification of the main integrin interaction domain. J. Biol. Chem. 278:38514–38521 [DOI] [PubMed] [Google Scholar]
- 7. Falnes PO, Sandvig K. 2000. Penetration of protein toxins into cells. Curr. Opin. Cell Biol. 12:407–413 [DOI] [PubMed] [Google Scholar]
- 8. Fayolle C, Ladant D, Karimova G, Ullmann A, Leclerc C. 1999. Therapy of murine tumors with recombinant Bordetella pertussis adenylate cyclase carrying a cytotoxic T cell epitope. J. Immunol. 162:4157–4162 [PubMed] [Google Scholar]
- 9. Fayolle C, et al. 2001. Delivery of multiple epitopes by recombinant detoxified adenylate cyclase of Bordetella pertussis induces protective antiviral immunity. J. Virol. 75:7330–7338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fiser R, et al. 2007. Third activity of Bordetella adenylate cyclase (AC) toxin-hemolysin. Membrane translocation of AC domain polypeptide promotes calcium influx into CD11b+ monocytes independently of the catalytic and hemolytic activities. J. Biol. Chem. 282:2808–2820 [DOI] [PubMed] [Google Scholar]
- 11. Ghiringhelli F, et al. 2009. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nat. Med. 15:1170–1178 [DOI] [PubMed] [Google Scholar]
- 12. Gmira S, Karimova G, Ladant D. 2001. Characterization of recombinant Bordetella pertussis adenylate cyclase toxins carrying passenger proteins. Res. Microbiol. 152:889–900 [DOI] [PubMed] [Google Scholar]
- 13. Gordon VM, Leppla SH, Hewlett EL. 1988. Inhibitors of receptor-mediated endocytosis block the entry of Bacillus anthracis adenylate cyclase toxin but not that of Bordetella pertussis adenylate cyclase toxin. Infect. Immun. 56:1066–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordon VM, et al. 1989. Adenylate cyclase toxins from Bacillus anthracis and Bordetella pertussis. Different processes for interaction with and entry into target cells. J. Biol. Chem. 264:14792–14796 [PubMed] [Google Scholar]
- 15. Grigoleit GU, et al. 2007. Dendritic cell vaccination in allogeneic stem cell recipients: induction of human cytomegalovirus (HCMV)-specific cytotoxic T lymphocyte responses even in patients receiving a transplant from an HCMV-seronegative donor. J. Infect. Dis. 196:699–704 [DOI] [PubMed] [Google Scholar]
- 16. Guermonprez P, et al. 2001. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the αMβ2 integrin (CD11b/CD18). J. Exp. Med. 193:1035–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guermonprez P, Ladant D, Karimova G, Ullmann A, Leclerc C. 1999. Direct delivery of the Bordetella pertussis adenylate cyclase toxin to the MHC class I antigen presentation pathway. J. Immunol. 162:1910–1916 [PubMed] [Google Scholar]
- 18. Guo Q, et al. 2005. Structural basis for the interaction of Bordetella pertussis adenylyl cyclase toxin with calmodulin. EMBO J. 24:3190–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hackett M, Guo L, Shabanowitz J, Hunt DF, Hewlett EL. 1994. Internal lysine palmitoylation in adenylate cyclase toxin from Bordetella pertussis. Science 266:433–435 [DOI] [PubMed] [Google Scholar]
- 20. Hackett M, et al. 1995. Hemolytic, but not cell-invasive activity, of adenylate cyclase toxin is selectively affected by differential fatty-acylation in Escherichia coli. J. Biol. Chem. 270:20250–20253 [DOI] [PubMed] [Google Scholar]
- 21. Hervas-Stubbs S, et al. 2006. High frequency of CD4+ T cells specific for the TB10.4 protein correlates with protection against Mycobacterium tuberculosis infection. Infect. Immun. 74:3396–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hewlett EL, Donato GM, Gray MC. 2006. Macrophage cytotoxicity produced by adenylate cyclase toxin from Bordetella pertussis: more than just making cyclic AMP! Mol. Microbiol. 59:447–459 [DOI] [PubMed] [Google Scholar]
- 23. Hewlett EL, et al. 1991. Adenylate cyclase toxin from Bordetella pertussis. Conformational change associated with toxin activity. J. Biol. Chem. 266:17503–17508 [PubMed] [Google Scholar]
- 24. Higgins DE, Shastri N, Portnoy DA. 1999. Delivery of protein to the cytosol of macrophages using Escherichia coli K-12. Mol. Microbiol. 31:1631–1641 [DOI] [PubMed] [Google Scholar]
- 25. Karttunen J, Sanderson S, Shastri N. 1992. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc. Natl. Acad. Sci. U. S. A. 89:6020–6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ladant D. 1988. Interaction of Bordetella pertussis adenylate cyclase with calmodulin. Identification of two separated calmodulin-binding domains. J. Biol. Chem. 263:2612–2618 [PubMed] [Google Scholar]
- 27. Lee SJ, Gray MC, Guo L, Sebo P, Hewlett EL. 1999. Epitope mapping of monoclonal antibodies against Bordetella pertussis adenylate cyclase toxin. Infect. Immun. 67:2090–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Letourneau S, et al. 2007. Design and pre-clinical evaluation of a universal HIV-1 vaccine. PLoS One 2:e984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Linhartova I, et al. 2010. RTX proteins: a highly diverse family secreted by a common mechanism. FEMS Microbiol. Rev. 34:1076–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mackova J, et al. 2006. Prime/boost immunotherapy of HPV16-induced tumors with E7 protein delivered by Bordetella adenylate cyclase and modified vaccinia virus Ankara. Cancer Immunol. Immunother. 55:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Majlessi L, et al. 2006. An increase in antimycobacterial Th1-cell responses by prime-boost protocols of immunization does not enhance protection against tuberculosis. Infect. Immun. 74:2128–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Masin J, et al. 2005. Acylation of lysine 860 allows tight binding and cytotoxicity of Bordetella adenylate cyclase on CD11b-expressing cells. Biochemistry 44:12759–12766 [DOI] [PubMed] [Google Scholar]
- 33. Osicka R, et al. 2000. Delivery of CD8+ T-cell epitopes into major histocompatibility complex class I antigen presentation pathway by Bordetella pertussis adenylate cyclase: delineation of cell invasive structures and permissive insertion sites. Infect. Immun. 68:247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Osickova A, et al. 2010. Adenylate cyclase toxin translocates across target cell membrane without forming a pore. Mol. Microbiol. 75:1550–1562 [DOI] [PubMed] [Google Scholar]
- 35. Osickova A, Osicka R, Maier E, Benz R, Sebo P. 1999. An amphipathic alpha-helix including glutamates 509 and 516 is crucial for membrane translocation of adenylate cyclase toxin and modulates formation and cation selectivity of its membrane channels. J. Biol. Chem. 274:37644–37650 [PubMed] [Google Scholar]
- 36. Otero AS, Yi XB, Gray MC, Szabo G, Hewlett EL. 1995. Membrane depolarization prevents cell invasion by Bordetella pertussis adenylate cyclase toxin. J. Biol. Chem. 270:9695–9697 [DOI] [PubMed] [Google Scholar]
- 37. Preville X, Ladant D, Timmerman B, Leclerc C. 2005. Eradication of established tumors by vaccination with recombinant Bordetella pertussis adenylate cyclase carrying the human papillomavirus 16 E7 oncoprotein. Cancer Res. 65:641–649 [PubMed] [Google Scholar]
- 38. Rogel A, Hanski E. 1992. Distinct steps in the penetration of adenylate cyclase toxin of Bordetella pertussis into sheep erythrocytes. Translocation of the toxin across the membrane. J. Biol. Chem. 267:22599–22605 [PubMed] [Google Scholar]
- 39. Rose T, Sebo P, Bellalou J, Ladant D. 1995. Interaction of calcium with Bordetella pertussis adenylate cyclase toxin. Characterization of multiple calcium-binding sites and calcium-induced conformational changes. J. Biol. Chem. 270:26370–26376 [DOI] [PubMed] [Google Scholar]
- 40. Sakamoto H, Bellalou J, Sebo P, Ladant D. 1992. Bordetella pertussis adenylate cyclase toxin. Structural and functional independence of the catalytic and hemolytic activities. J. Biol. Chem. 267:13598–13602 [PubMed] [Google Scholar]
- 41. Saron MF, et al. 1997. Anti-viral protection conferred by recombinant adenylate cyclase toxins from Bordetella pertussis carrying a CD8+ T cell epitope from lymphocytic choriomeningitis virus. Proc. Natl. Acad. Sci. U. S. A. 94:3314–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schlecht G, Loucka J, Najar H, Sebo P, Leclerc C. 2004. Antigen targeting to CD11b allows efficient presentation of CD4+ and CD8+ T cell epitopes and in vivo Th1-polarized T cell priming. J. Immunol. 173:6089–6097 [DOI] [PubMed] [Google Scholar]
- 43. Schmitt A, et al. 2009. Cytomegalovirus vaccination of leukemia and lymphoma patients after allogeneic stem cell transplantation—validation of a peptide vaccine. J. Immunol. Methods 343:140–147 [DOI] [PubMed] [Google Scholar]
- 44. Shen Z, Reznikoff G, Dranoff G, Rock KL. 1997. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J. Immunol. 158:2723–2730 [PubMed] [Google Scholar]
- 45. Simsova M, Sebo P, Leclerc C. 2004. The adenylate cyclase toxin from Bordetella pertussis—a novel promising vehicle for antigen delivery to dendritic cells. Int. J. Med. Microbiol. 293:571–576 [DOI] [PubMed] [Google Scholar]
- 46. Slezak SL, et al. 2007. CMV pp65 and IE-1 T cell epitopes recognized by healthy subjects. J. Transl. Med. 5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Szabo G, Gray MC, Hewlett EL. 1994. Adenylate cyclase toxin from Bordetella pertussis produces ion conductance across artificial lipid bilayers in a calcium- and polarity-dependent manner. J. Biol. Chem. 269:22496–22499 [PubMed] [Google Scholar]
- 48. Tartz S, et al. 2006. Immunization with a circumsporozoite epitope fused to Bordetella pertussis adenylate cyclase in conjunction with cytotoxic T-lymphocyte-associated antigen 4 blockade confers protection against Plasmodium berghei liver-stage malaria. Infect. Immun. 74:2277–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vojtova-Vodolanova J, et al. 2009. Oligomerization is involved in pore formation by Bordetella adenylate cyclase toxin. FASEB J. 23:2831–2843 [DOI] [PubMed] [Google Scholar]
- 50. Vojtova J, Kamanova J, Sebo P. 2006. Bordetella adenylate cyclase toxin: a swift saboteur of host defense. Curr. Opin. Microbiol. 9:69–75 [DOI] [PubMed] [Google Scholar]
- 51. Welch RA. 1991. Pore-forming cytolysins of gram-negative bacteria. Mol. Microbiol. 5:521–528 [DOI] [PubMed] [Google Scholar]
- 52. Wills MR, et al. 1996. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J. Virol. 70:7569–7579 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.