Abstract
Acetic acid bacteria were previously considered nonpathogenic in humans. However, over the past decade, five genera of Acetobacteraceae have been isolated from patients with inborn or iatrogenic immunodeficiencies. Here, we describe the first studies of the interactions of the human innate immune system with a member of this bacterial family, Granulibacter bethesdensis, an emerging pathogen in patients with chronic granulomatous disease (CGD). Efficient phagocytosis of G. bethesdensis by normal and CGD polymorphonuclear leukocytes (CGD PMN) required heat-labile serum components (e.g., C3), and binding of C3 and C9 to G. bethesdensis was detected by immunoblotting. However, this organism survived in human serum concentrations of ≥90%, indicating a high degree of serum resistance. Consistent with the clinical host tropism of G. bethesdensis, CGD PMN were unable to kill this organism, while normal PMN, in the presence of serum, reduced the number of CFU by about 50% after a 24-h coculture. This finding, together with the observations that G. bethesdensis was sensitive to H2O2 but resistant to LL-37, a human cationic antimicrobial peptide, suggests an inherent resistance to O2-independent killing. Interestingly, 10 to 100 times greater numbers of G. bethesdensis were required to achieve the same level of reactive oxygen species (ROS) production induced by Escherichia coli in normal PMN. In addition to the relative inability of the organism to elicit production of PMN ROS, G. bethesdensis inhibited both constitutive and FAS-induced PMN apoptosis. These properties of reduced PMN activation and resistance to nonoxidative killing mechanisms likely play an important role in G. bethesdensis pathogenesis.
INTRODUCTION
Granulibacter bethesdensis is a Gram-negative pathogen first isolated from a patient with chronic granulomatous disease (CGD) in 2003 (13). G. bethesdensis has since been cultured from at least 5 patients with CGD in the United States (15, 16) and 1 in Spain (23), justifying its classification as an emerging pathogen in this primary immunodeficiency. Although the bacterial family to which G. bethesdensis belongs–the Acetobacteraceae–was previously considered nonpathogenic in humans, case reports of human infections by other members of this family are being published with increasing frequency (1, 2, 5, 6, 9, 12, 17, 22, 26, 27). Common elements of infection by these other Acetobacteraceae species appear to include intravenous drug administration (for either medicinal or recreational purposes), organ transplant, or chronic medical problems requiring dialysis. In contrast, G. bethesdensis has been reported only in patients with CGD, a disease resulting from inadequate superoxide anion production required for normal myeloid host defenses. While G. bethesdensis is the only member of this family for which Koch's postulates have been fulfilled (13), there is scant information about the mechanisms of pathogenesis of infection by these organisms. Although mice with CGD are significantly more susceptible than normal mice to infection by G. bethesdensis, viable organisms could be recovered from the spleens of wild-type mice more than 70 days after infection. These findings suggest that the organism is capable of long-term persistence in vivo and has a significant degree of resistance to normal murine innate host defenses (13), a phenomenon possibly related to the repeated isolation of genetically identical isolates from the same patient with CGD over several years (15). Given that G. bethesdensis in particular and the Acetobacteraceae in general appear to represent an emerging group of pathogens, we investigated the interaction of G. bethesdensis with components of the innate immune system as a first step toward elucidating the mechanisms of G. bethesdensis virulence.
MATERIALS AND METHODS
Ethics statement and isolation of human PMN.
Blood samples were obtained after informed consent from donors enrolled at the NIH Clinical Center under institutional review board-approved protocols 93-I-0119, 05-I-0213, or 99-CC-0168. Human polymorphonuclear leukocytes (PMN) were isolated from venous blood samples as previously described (20, 31). Sera were prepared using Becton Dickenson serum separator tubes according to manufacturer's instructions and stored on ice for immediate use or frozen at −80°C in single-use aliquots.
Bacterial strains and culture.
G. bethesdensis strain NIH1.1 (strain CGDNIH1T; ATCC BAA-1260) was grown to mid-log phase at 37°C in YPG medium (5 g yeast extract, 3 g peptone, and 10 g glucose per liter). Escherichia coli TOP10 and K1/r were grown in Luria-Bertani (LB) broth or Trypticase soy broth (TSB) at 37°C or as indicated. For each strain, A600 was used for enumeration of washed bacteria after appropriate conversion factors (no. of CFU/optical density [OD]) were determined.
Luminol-enhanced neutrophil chemiluminescence.
PMN were suspended at 1.1 × 106/ml in RPMI 1640 medium with 5.5% autologous serum, 27.5 mM HEPES (pH 7.4), and 55 μM luminol, and 90 μl was aliquoted into a 96-well polypropylene white plate. After a 30-min incubation period at 37°C in a 5% CO2 incubator, 10 μl of washed bacteria (multiplicities of infection [MOI] of 100:1, 10:1, 1:1) was suspended in saline and added to the plate. Saline alone was added as a control to some wells of the plate, and luminometry was performed in an Anthos Zenyth 3100 set at 37°C, with readings of 1 s per well taken every 2.4 min. Results are expressed kinetically or converted to area under the concentration-time curve (AUC) by the GraphPad Prism 5.0 software package.
Bacterial internalization assays.
Bacteria were labeled with pHrodo according to the manufacturer's instructions (Invitrogen, Carlsbad, CA) and kept at 4°C until use. For flow cytometry experiments, bacteria and PMN (MOI = 10 bacteria:1 PMN) were added to a 96-well plate with RPMI 1640 containing 25 mM HEPES (pH 7.2) and 10% autologous serum for a final volume of 200 μl. The plate was incubated at 37°C, and phagocytosis was stopped at each time point by addition of 50 μl of 10% neutralized buffered formaldehyde to each well. For the 0-min time point, formaldehyde was added to the wells immediately prior to addition of bacteria. After the final time point, the plate was stored at 4°C and read within 24 h on a FACSCalibur flow cytometer (BD Biosciences). Cells were gated by forward scatter and side scatter to exclude debris and extracellular bacteria. The pHrodo signal was measured in the FL2 channel, and the pHrodo+ threshold was set based on the 0-min time point and applied to all samples. For microscopy studies, EtOH-washed coverslips were placed in each well of a 24-well culture plate (Corning, NY) and dried. Assays were performed in RPMI 1640 containing 25 mM HEPES (pH 7.2), and the indicated numbers of PMN with or without additives were added. After thorough mixing, plates were centrifuged at 500 × g and placed in a humidified incubator at 5% CO2 and 37°C. At the indicated times, plates were centrifuged as indicated above, and the supernatant was aspirated, replaced with buffered formalin fixative, and placed at 4°C for at least 30 min. Coverslips were then mounted and observed using a Leica AF 6000 LX fluorescence microscope. A minimum of 10 fields (>100 cells) were imaged, and the numbers of PMN per field and the numbers of G. bethesdensis organisms in each PMN were enumerated in a blinded fashion.
Complement binding studies.
Saline-washed bacteria (2 × 108) were suspended in 1 ml RPMI and incubated at 37°C with the supplements and durations indicated in the figure legends. After 3 washes in 1 ml ice-cold PBS each, the bacteria were boiled in SDS-PAGE sample buffer with reducing agent and then subjected to Western blotting using standard methods. C3 and C3-deficient sera and goat anti-human C3 and C9 antisera were obtained from Complement Technology, Inc. (Tyler, TX). Primary antibodies were used at 1:2,000 and detected with an anti-goat horseradish peroxidase (HRP) conjugate (Santa Cruz) and Pierce West Pico chemiluminescent detection reagents.
Neutrophil killing assays.
All assays were performed as previously described (14).
Neutrophil apoptosis studies.
Apoptosis of normal PMN or CGD PMN (CGD PMN) was estimated by nuclear morphology, DNA fragmentation, or flow cytometry for active caspase-3. PMN were incubated in RPMI 1640 buffered with 10 mM HEPES containing 10% autologous human serum (where indicated) in the presence or absence of G. bethesdensis and/or 1 μg/ml anti-FAS antibody (clone CH-11; MBL) for the indicated times. Freshly isolated PMN were also assessed by these methods and typically displayed <5% apoptosis. For morphological analysis, cells were subjected to cytospin, stained with the HARLECO Hemacolor stain set (EMD Chemicals), and imaged, and at least 200 cells were scored as healthy (typical multilobed nuclei) or apoptotic (condensed nuclei or “ghost” cell membranes lacking nuclei). To assess DNA fragmentation, a modified terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay (APO-BRDU kit; BD Pharmingen) was used. Briefly, cells were washed with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for 15 min, washed with PBS, and stored in 70% ethanol at −20°C. For the staining procedure, control cells and experiment samples were washed with kit wash buffer, incubated with DNA labeling solution for 1.5 h at 37°C with agitation every 15 min, washed twice with kit rinse buffer, and incubated with antibody labeling solution for 30 min at room temperature (RT) in the dark before fluorescence-activated cell sorting (FACS) analysis (conducted within 3 h). All centrifugations were conducted at 400 × g for 3 min at 4°C. Active caspase-3 was measured after washing cells once in 1% heat-inactivated fetal calf serum (FCS) and 1% bovine serum albumin (BSA) in PBS and twice with permeabilization buffer (1% heat-inactivated FCS, 0.1% saponin in PBS), followed with incubation for 15 min on ice to complete permeabilization. Cells were then stained with anti-active caspase-3–phycoerythrin (PE) antibody (BD Biosciences) for 30 min on ice in the dark, washed with permeabilization buffer, and fixed with 2% paraformaldehyde before FACS analysis.
Antimicrobial activity assays.
Bacteria were grown to mid-logarithmic phase, washed in 150 mM NaCl, and enumerated as described above. Antimicrobial activity was determined in RPMI 1640 containing 10 mM HEPES (pH 7.4). A peptide corresponding to the mature human cathelicidin peptide (LL-37) was synthesized commercially (Biosynthesis, Inc., Lewisville, TX), dissolved in 20 mM sodium acetate buffer at pH 4.0, and stored at 4°C. Hydrogen peroxide was freshly diluted from a 30% stock solution. Growth inhibition of inocula of 106/ml was determined after the indicated time points by plating (using sterile beads to achieve a uniform distribution on each plate) saline dilutions of cultures on appropriate media (either YPG or LB agar) and is expressed as the number of CFU/ml or percent control (buffer alone, plated at indicated times). Data and statistical analyses were performed using Microsoft Excel and GraphPad Prism for Mac, version 5.0.
RESULTS AND DISCUSSION
G. bethesdensis is highly resistant to serum complement.
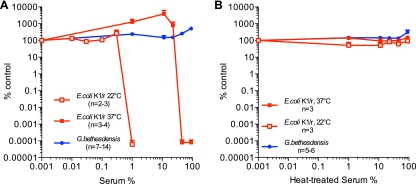
The complement system forms an important front line of innate host defense, and microbial evasion of the complement is a virulence determinant (32). We therefore compared the effect of pooled human serum on the viabilities of G. bethesdensis and E. coli K1/r, a serum-resistant encapsulated bacteremia isolate (11). As previously reported, the polysialic acid capsular polysaccharide of E. coli K1/r confers serum resistance following growth at 37°C but not at 22°C (7). While E. coli K1/r cultured at 22°C is killed by as little as 1% (vol/vol) serum, the organism grown at 37°C is resistant to killing by serum (up to 45% serum) (Fig. 1A). In comparison, G. bethesdensis survived in 90% serum, indicating a greater degree of serum resistance than E. coli K1/r. Both serum-sensitive and serum-resistant bacteria survived for 24 h in 90% heat-treated serum (Fig. 1B). Gluconobacter oxydans, a related member of the Acetobacteraceae, was serum sensitive following culture and testing at 25°C (it will not grow at 37°C), indicating that serum resistance is not a general attribute of this family (data not shown).
Fig 1.
Granulibacter bethesdensis is serum resistant. (A, B) Bacteria were cultured for 24 h in the presence of the indicated concentrations of pooled human serum (A) or serum treated at 56°C for 30 min (B). Results are expressed as the mean percentages of untreated control at 24 h ± standard errors of the means (SEM) for the indicated number of experiments.
Phagocytosis of G. bethesdensis by PMN.
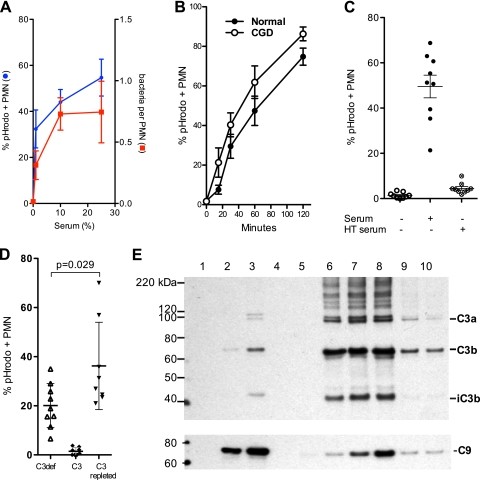
Another role of serum is opsonization, the coating of microbes with heat-labile components such as complement and/or heat-stable antibodies that significantly enhance their phagocytosis (29). We therefore measured the effect of serum on internalization by PMN by labeling G. bethesdensis with pHrodo, a pH-sensitive dye that fluoresces more intensely as the pH decreases, and counting the number of PMN with fluorescent bacteria. Internalized bacteria were fluorescent and easily counted by microscopy, while extracellular bacteria were not fluorescent in this medium and could not be counted. Internalization of the bacteria was confirmed by overlaying the fluorescent signal with the differential interference contrast (DIC) image. Phagocytosis of G. bethesdensis at an MOI of 1:1 was serum dependent (e.g., in the presence of 10% autologous serum, 40% of PMN contained bacteria by 30 min, whereas uptake was ∼1% or less for nonopsonized bacteria) (Fig. 2A), with both the number of PMN with bacteria and the number of bacteria per cell increasing with an increasing serum dose. There also were similar rates of phagocytosis between PMN from healthy individuals and those from patients with CGD, although there was a nonsignificant trend toward increased internalization by CGD cells (Fig. 2B). Serum-dependent internalization was blocked by heat inactivation (56°C) of serum (Fig. 2C). Since C3 is a major serum opsonin, we obtained commercially prepared C3 and C3-depleted human serum to test whether C3 is critical for phagocytosis of G. bethesdensis. As shown in Fig. 2D, C3 alone failed to promote uptake of G. bethesdensis, whereas C3-depleted serum supported some internalization (∼50% of that supported by fresh serum). However, the combination of C3 and C3-depleted serum (C3-repleted serum) promoted internalization significantly greater than that promoted by either component alone (a similar significant enhancement of phagocytosis was found using 1% serum [data not shown]).
Fig 2.
G. bethesdensis is internalized by human PMN in a time- and serum-dependent manner. (A) Internalization of pHrodo-labeled bacteria was assessed microscopically by blinded scorers at 30 min and an MOI of 1:1. The percentage of all cells that contained at least 1 G. bethesdensis organism is plotted as a function of the serum dose (mean ± SEM; n = 9 healthy donors over 6 separate experiments) and the number of bacteria per PMN (mean ± SEM; n = 4 to 6 subjects). (B) Flow cytometry studies demonstrate internalization by normal or CGD PMN incubated with G. bethesdensis at an MOI of 10:1 in the presence of 10% autologous serum (mean ± SEM; n = 8 healthy patients and 4 patients with CGD). (C) Internalization of G. bethesdensis was assessed microscopically in the absence of serum or in the presence of either 10% serum or 10% heat-treated (HT; 56°C, 30 min) serum (mean ± SEM; n = 9). (D) Internalization of G. bethesdensis was measured microscopically in the presence of 10% C3-depleted serum alone, purified C3 at a concentration of 10 μg/ml, and both together (mean ± SEM of 9 healthy donors). Repletion of C3-depleted serum with C3 resulted in a significant increase in internalization (paired t test, 2 tailed). (E) Immunodetection of C3 and C9 bound to G. bethesdensis. Lane 1, bacteria alone; lane 2, 10% C3-deficient serum; lane 3, 10% C3-deficient serum plus 1 μg C3/ml; lane 4, bacteria alone; lane 5, 10% serum at 0 min; lane 6, 10% serum at 5 min; lane 7, 10% serum at 10 min; lane 8, 10% serum at 30 min; lane 9, 10% serum with EGTA plus MgCl2; lane 10, 10% serum previously heat treated at 50°C for 20 min. Blots are representative of at least 3 different binding experiments.
C3 and/or C9 bound G. bethesdensis after incubation of bacteria with human sera (Fig. 2E). Similar to what is seen in Fig. 2D, some deposition of C3 and C9 was evident following opsonization with the C3-deficient serum (Fig. 2E, lane 2), and such deposition was further enhanced by addition of purified C3. C3 binding, in particular that of the opsonic C3b fragment, in 10% pooled human sera was maximal by 5 min (Fig. 2E, lane 6), whereas C9 deposition on G. bethesdensis increased for up to 30 min. Inhibition of the classical and lectin complement activation pathways by Mg2+-EGTA decreased binding of both C3 and C9 to bacteria (Fig. 2E, compare lane 9 to lane 8), as did mild heat treatment (50°C for 20 min to inhibit the alternative pathway) (Fig. 2E, lane 10), suggesting that complement activation may depend on both pathways or that inhibition was incomplete. Consistent with its effect of decreasing complement activation on bacteria, Mg2+-EGTA also inhibited PMN phagocytosis of pHrodo-labeled G. bethesdensis by ∼50% (data not shown). Binding of C9 to bacteria did not occur in C3- or C6-deficient sera (data not shown), indicating that binding was due to complement activation rather than nonspecific adsorption. Thus, G. bethesdensis appears to be resistant to the membrane attack complex (MAC), possibly due to the presence of a capsule (15), smooth lipopolysaccharide (LPS), or some other mechanism.
Thus, G. bethesdensis is highly serum resistant and is internalized through a serum-dependent pathway, likely involving C3 activation through the classical or lectin pathway.
Inhibition of PMN apoptosis by G. bethesdensis.
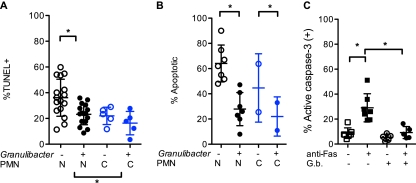
Regulation of the PMN life span by pathogens is thought to play an important role in microbial pathogenesis (28). To test whether G. bethesdensis regulated this aspect of PMN biology, we assessed apoptosis by enumerating apoptotic nuclei and, by using two FACS-based assays, measuring either the extent of DNA fragmentation correlating with late apoptosis or the degree of caspase-3 activation as a marker of early apoptosis. As shown in Fig. 3A and B and as previously reported (18), normal and CGD PMN undergo spontaneous apoptosis over 24 h in culture, with CGD PMN exhibiting significantly diminished apoptosis when assessed by TUNEL staining and a trend toward decreased apoptosis, as judged by morphology. Notably, G. bethesdensis significantly decreased apoptosis of normal PMN and, to some extent, of CGD PMN (Fig. 3). Antibody to Fas induces apoptosis of PMN and was shown in Fig. 3C to significantly increase apoptosis in the absence but not the presence of G. bethesdensis. Thus, unlike many pathogens such as Streptococcus, E. coli, and Staphylococcus aureus that have been shown to increase PMN apoptosis (19), G. bethesdensis, like Anaplasma phagocytophilum (30), Neisseria gonorrhoeae (25), and others (3), belongs to the relatively small list of pathogens that inhibit human neutrophil apoptosis. Whether such increases in the PMN life span play a role in vivo during Granulibacter infection remains to be determined.
Fig 3.
Inhibition of constitutive and Fas-induced PMN apoptosis by G. bethesdensis. (A) Constitutive apoptosis of PMN was assessed by a FACS-based TUNEL assay of normal (n = 16) and CGD (n = 5) PMN incubated for 24 h in the absence or presence of 10 G. bethesdensis CFU per PMN in 10% autologous serum in RPMI 1640, as described in Materials and Methods. (B) Apoptotic neutrophil morphology was assessed by microscopy after incubation as described for panel A of PMN from healthy subjects (N; n = 7) or subjects with CGD (C; n = 2). (C) Flow cytometry was used to measure activated caspase-3 in PMN treated for 6 h in the absence or presence of 1 μg/ml anti-Fas antibody in the absence or presence of G. bethesdensis (MOI, 10:1). Significance in all three studies was determined with a 2-way analysis of variance (ANOVA) and is indicated with an asterisk when P is ≤0.05.
Activation of the NADPH oxidase by G. bethesdensis.
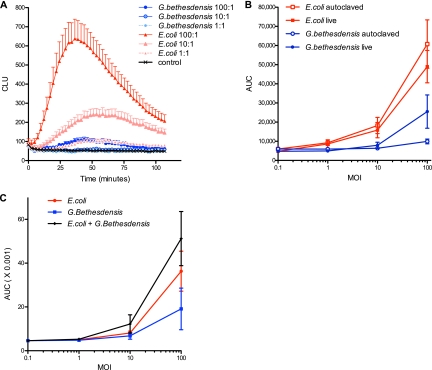
Since G. bethesdensis is internalized by normal and CGD PMN in a serum-dependent manner, we next assessed PMN activation using luminol-enhanced chemiluminescence to detect superoxide production by the phagocyte NADPH oxidase, the enzyme defective in patients with CGD. As previously shown (4), the “respiratory burst” of leukocytes occurs rapidly upon exposure to bacteria. Chemiluminescence was detected after stimulation by as few as 1 autoclaved E. coli organism per PMN (Fig. 4A). In contrast, 10 to 100 times more G. bethesdensis was required to achieve the same level of activation induced by a single E. coli CFU (Fig. 4A and B). Both live and heat-killed bacteria were tested (Fig. 4B), and while both forms of E. coli seemed equivalently potent, live G. bethesdensis induced somewhat greater chemiluminescence than heat-treated bacteria, possibly indicating the presence of a heat-labile stimulator of cell activation. Several microbes are known to directly inhibit activation of the NADPH oxidase (e.g., Anaplasma phagocytophilum [21] and Francisella tularensis [3]). To test whether G. bethesdensis inhibited NADPH oxidase activation in response to other stimuli, we incubated PMN with this organism alone or with E. coli. As shown in Fig. 4C, G. bethesdensis did not inhibit PMN activation induced by E. coli but rather caused an additive increase in chemiluminescence. Independent studies combining G. bethesdensis with different doses of phorbol ester (a potent receptor-independent activator of the NADPH oxidase) confirmed that G. bethesdensis did not inhibit production or detection of superoxide (data not shown). Together, these results suggest that rather than blocking activation of PMN, G. bethesdensis has a reduced ability to stimulate the PMN respiratory burst compared to E. coli.
Fig 4.
G. bethesdensis is 10 to 100 times less stimulatory of leukocyte chemiluminescence than E. coli. (A) Luminol-enhanced leukocyte chemiluminescence was measured in the presence of 10% autologous sera in response to autoclaved G. bethesdensis or E. coli at MOIs of 100, 10, 1, and 0 bacteria per cell (means ± SEM; n = 27 to 39 donors). CLU, chemiluminescence units. (B) The AUC of kinetic chemiluminescence data was measured to compare relative potencies of autoclaved and live bacteria (n = 6 donors). (C) Chemiluminescence of PMN exposed to E. coli alone, G. bethesdensis alone, or both together (mean ± SEM; n = 6 donors).
Given that G. bethesdensis is a weak activator of the respiratory burst and is a pathogen in patients with CGD, who are unable to generate antimicrobial reactive oxygen species (ROS), including superoxide and H2O2, we next tested the sensitivity of this organism to H2O2 as a model oxidant. Ten independent experiments were performed, with G. bethesdensis and E. coli exposed to different concentrations of H2O2 for 1 h. Nonlinear regression analysis of normalized results indicates that the sensitivity of G. bethesdensis and E. coli are not significantly different, with 50% inhibitory concentrations (IC50s) of 29.09 μM (95% confidence interval [CI] = 14.93 to 56.69 μM) and 52.67 (31.56 to 88.17 μM), respectively.
PMN-mediated killing of G. bethesdensis.
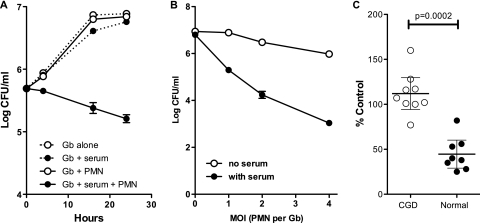
We next tested whether growth of G. bethesdensis could be inhibited by PMN in the absence or presence of 10% autologous serum. As shown in Fig. 5A, bacteria alone, in serum, or in the presence of PMN without serum grew during 24 h of incubation. However, in the presence of serum and normal PMN, G. bethesdensis was killed in a time-dependent fashion. Killing of G. bethesdensis was more effective with greater numbers of PMN (Fig. 5B). PMN from patients with CGD were unable to kill G. bethesdensis (Fig. 5C), although a bacteriostatic effect was evident. Importantly, while normal PMN kill about 50% of G. bethesdensis within 24 h in our bactericidal activity assays, they killed approximately 99% of another CGD pathogen, Burkholderia multivorans, when tested under identical conditions in our laboratory (14). It is possible that the poor activation of the NADPH oxidase by G. bethesdensis in normal PMN contributes to its relative resistance to killing by these cells. However, the addition of phorbol myristate acetate (PMA) at doses of between 5 and 100 ng/ml did not significantly alter PMN killing of this organism (data not shown). Several microbes either fail to activate the NADPH oxidase, actively interfere with activation or targeting of this enzyme (e.g., Neisseria gonorrhoeae [8], Francisella tularensis, and Helicobacter pylori [3]), or utilize catalase or superoxide dismutase to inactivate ROS. Our finding that G. bethesdensis does not inhibit PMN responses to other simultaneous stimuli (e.g., E. coli or PMA) suggests that it is a poor activator rather than an inhibitor of the NADPH oxidase. Compared to E. coli, G. bethesdensis is also 10 to 100 times less potent at stimulating production of cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) in whole blood ex vivo (K. A. Zarember and J. I. Gallin, unpublished observations). Studies are under way to isolate and characterize the LPS of G. bethesdensis as well as other potentially host-stimulatory compounds from this organism.
Fig 5.
G. bethesdensis is killed by normal PMN but not CGD PMN. (A) G. bethesdensis was cultured in the absence or presence of 10% serum and in the absence or presence of PMN at an MOI of 1:1. At the indicated time points, cultures were lysed and diluted as described in Materials and Methods, and the CFU were enumerated and plotted as means ± SEM (n = 6 healthy donors). (B) The number of CFU of G. bethesdensis after 24 h of culture in the presence of PMN at the indicated ratios in the absence or presence of serum (mean ± SEM; n = 6 healthy donors). (C) PMN from patients with CGD were bacteriostatic toward G. bethesdensis under conditions in which normal PMN reduced the number of viable CFU by >50% (individual data points are shown as percent control of initial counts, and the means ± SEM are depicted (n = 8 healthy donors and 9 donors with CGD). An unpaired t test was used to determine significance as shown.
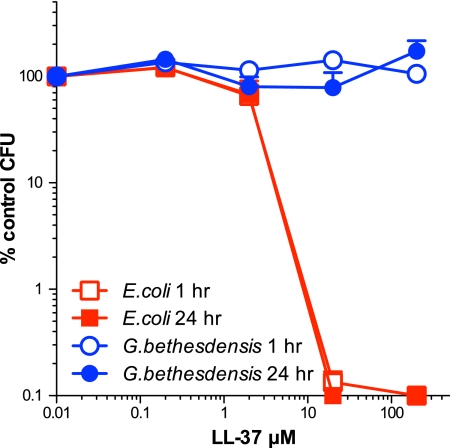
Given that CGD PMN failed to reduce the number of G. bethesdensis CFU, we conclude that killing of G. bethesdensis by normal human PMN is ROS dependent and that this organism is resistant to the nonoxidative antimicrobial factors of PMN. To test the latter prediction directly, we incubated G. bethesdensis and E. coli TOP10 with LL-37, a cationic antimicrobial cathelicidin peptide. While viability of E. coli was reduced by at least 3 logs within 1 h by 20 μM peptide, G. bethesdensis survived for at least 24 h in the presence of up to 200 μM LL-37 (Fig. 6). Resistance to LL-37 has been described for several pathogens and can be due to surface properties of microbes or the production of microbial peptidases that degrade LL-37 (24). It is currently unknown which G. bethesdensis genes confer resistance to LL-37 or the entire spectrum of nonoxidative microbicidal factors of the CGD PMN. Studies are under way to determine the transcriptional responses of this organism during phagocytic interaction with normal and CGD PMN.
Fig 6.
G. bethesdensis is resistant to cathelicidin peptide. Bacteria were cultured with the indicated dose of peptide in RPMI 1640 plus 10 mM HEPES (pH 7.4) for 1 and 24 h, and the remaining CFU/ml enumerated. Data shown represent the mean percentages of control ± SEM for 5 or 6 independent experiments.
Whether healthy humans can be infected by G. bethesdensis is currently unknown; however, this microbe appears to be resistant to several key innate defenses (e.g., serum and cathelicidin peptide) and is a poor stimulator of the leukocyte NADPH oxidase, the main source of superoxide required for the oxygen-dependent microbicidal armaments of the PMN. Aside from direct antimicrobial effects, ROS play important roles in the production of neutrophil extracellular traps (NETs) (10) and other antimicrobial host defenses. Further studies are required to determine which cells and mechanisms are responsible for the clearance of this organism in vivo and whether G. bethesdensis can persist in a latent form in healthy hosts or those with CGD. It remains to be tested whether the observed interactions between G. bethesdensis and components of the innate immune system are unique or whether these attributes are shared by other members of this growing family of opportunistic pathogens.
ACKNOWLEDGMENTS
This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases as well as the NIH Clinical Center. K.N. is supported through contract HHSN26120080001E.
The views expressed here are those of the authors and do not necessarily reflect those of the U.S. Government.
We thank the patients with CGD who have contributed to these studies.
Footnotes
Published ahead of print 19 December 2011
REFERENCES
- 1. Abdel-Haq N, et al. 2009. Asaia lannaensis bloodstream infection in a child with cancer and bone marrow transplantation. J. Med. Microbiol. 58:974–976 [DOI] [PubMed] [Google Scholar]
- 2. Alauzet C, et al. 2010. Gluconobacter as well as Asaia species, newly emerging opportunistic human pathogens among acetic acid bacteria. J. Clin. Microbiol. 48:3935–3942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allen LA, McCaffrey RL. 2007. To activate or not to activate: distinct strategies used by Helicobacter pylori and Francisella tularensis to modulate the NADPH oxidase and survive in human neutrophils. Immunol. Rev. 219:103–117 [DOI] [PubMed] [Google Scholar]
- 4. Baldridge CW, Gerard RW. 1932. The extra respiration of phagocytosis. Am. J. Physiol. 103:235–236 [Google Scholar]
- 5. Bibashi E, Sofianou D, Kontopoulou K, Mitsopoulos E, Kokolina E. 2000. Peritonitis due to Roseomonas fauriae in a patient undergoing continuous ambulatory peritoneal dialysis. J. Clin. Microbiol. 38:456–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bittar F, et al. 2008. Acetobacter indonesiensis pneumonia after lung transplant. Emerg. Infect. Dis. 14:997–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bortolussi R, Ferrieri P, Quie PG. 1983. Influence of growth temperature of Escherichia coli on K1 capsular antigen production and resistance to opsonization. Infect. Immun. 39:1136–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Criss AK, Seifert HS. 2008. Neisseria gonorrhoeae suppresses the oxidative burst of human polymorphonuclear leukocytes. Cell. Microbiol. 10:2257–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fanella S, Schantz D, Karlowsky J, Rubinstein E. 2009. Septic arthritis due to Roseomonas gilardii in an immunocompetent adolescent. J. Med. Microbiol. 58:1514–1516 [DOI] [PubMed] [Google Scholar]
- 10. Fuchs TA, et al. 2007. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176:231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gemski P, Cross AR, Sadoff JC. 1980. K1 Antigen-associated resistance to the bactericidal activity of serum. FEMS Microbiol. Lett. 9:193–197 [Google Scholar]
- 12. Gouby A, et al. 2007. Acetobacter cibinongensis bacteremia in human. Emerg. Infect. Dis. 13:784–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greenberg DE, et al. 2006. A novel bacterium associated with lymphadenitis in a patient with chronic granulomatous disease. PLoS Pathog. 2:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greenberg DE, et al. 2010. Antisense phosphorodiamidate morpholino oligomers targeted to an essential gene inhibit Burkholderia cepacia complex. J. Infect. Dis. 201:1822–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greenberg DE, et al. 2007. Genome sequence analysis of the emerging human pathogenic acetic acid bacterium Granulibacter bethesdensis. J. Bacteriol. 189:8727–8736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greenberg DE, et al. 2010. Recurrent Granulibacter bethesdensis infections and chronic granulomatous disease. Emerg. Infect. Dis. 16:1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Juretschko S, Beavers-May TK, Stovall SH. 2010. Nosocomial infection with Asaia lannensis in two paediatric patients with idiopathic dilated cardiomyopathy. J. Med. Microbiol. 59:848–852 [DOI] [PubMed] [Google Scholar]
- 18. Kasahara Y, et al. 1997. Involvement of reactive oxygen intermediates in spontaneous and CD95 (Fas/APO-1)-mediated apoptosis of neutrophils. Blood 89:1748–1753 [PubMed] [Google Scholar]
- 19. Kobayashi SD, et al. 2003. Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc. Natl. Acad. Sci. U. S. A. 100:10948–10953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kobayashi SD, Voyich JM, Buhl CL, Stahl RM, DeLeo FR. 2002. Global changes in gene expression by human polymorphonuclear leukocytes during receptor-mediated phagocytosis: cell fate is regulated at the level of gene expression. Proc. Natl. Acad. Sci. U. S. A. 99:6901–6906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mott J, Rikihisa Y. 2000. Human granulocytic ehrlichiosis agent inhibits superoxide anion generation by human neutrophils. Infect. Immun. 68:6697–6703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rihs JD, et al. 1993. Roseomonas, a new genus associated with bacteremia and other human infections. J. Clin. Microbiol. 31:3275–3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodriguez Lopez FC, et al. 2008. Granulibacter bethesdensis isolated in a child patient with chronic granulomatous disease. J. Infect. 57:275–277 [DOI] [PubMed] [Google Scholar]
- 24. Schmidtchen A, Frick IM, Andersson E, Tapper H, Bjorck L. 2002. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol. Microbiol. 46:157–168 [DOI] [PubMed] [Google Scholar]
- 25. Simons MP, Nauseef WM, Griffith TS, Apicella MA. 2006. Neisseria gonorrhoeae delays the onset of apoptosis in polymorphonuclear leukocytes. Cell. Microbiol. 8:1780–1790 [DOI] [PubMed] [Google Scholar]
- 26. Snyder RW, et al. 2004. Asaia bogorensis peritonitis identified by 16S ribosomal RNA sequence analysis in a patient receiving peritoneal dialysis. Am. J. Kidney Dis. 44:e15–17 [DOI] [PubMed] [Google Scholar]
- 27. Tuuminen T, Heinasmaki T, Kerttula T. 2006. First report of bacteremia by Asaia bogorensis, in a patient with a history of intravenous-drug abuse. J. Clin. Microbiol. 44:3048–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weinrauch Y, Zychlinsky A. 1999. The induction of apoptosis by bacterial pathogens. Annu. Rev. Microbiol. 53:155–187 [DOI] [PubMed] [Google Scholar]
- 29. Wright AE, Douglas SR. 1903. An experimental investigation of the role of the blood fluids in connection with phagocytosis. Proc. R. Soc. Lond. 72:357–370 [Google Scholar]
- 30. Yoshiie K, Kim HY, Mott J, Rikihisa Y. 2000. Intracellular infection by the human granulocytic ehrlichiosis agent inhibits human neutrophil apoptosis. Infect. Immun. 68:1125–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zarember KA, Sugui JA, Chang YC, Kwon-Chung KJ, Gallin JI. 2007. Human polymorphonuclear leukocytes inhibit Aspergillus fumigatus conidial growth by lactoferrin-mediated iron depletion. J. Immunol. 178:6367–6373 [DOI] [PubMed] [Google Scholar]
- 32. Zipfel PF, Wurzner R, Skerka C. 2007. Complement evasion of pathogens: common strategies are shared by diverse organisms. Mol. Immunol. 44:3850–3857 [DOI] [PubMed] [Google Scholar]