Abstract
The glutamate dehydrogenase RocG of Bacillus subtilis is a bifunctional protein with both enzymatic and regulatory functions. Here we show that the rocG null mutant is sensitive to β-lactams, including cefuroxime (CEF), and to fosfomycin but that resistant mutants arise due to gain-of-function mutations in gudB, which encodes an otherwise inactive glutamate dehydrogenase. In the presence of CEF, ΔrocG ΔgudB mutant cells exhibit growth arrest when they reach mid-exponential phase. Using microarray-based transcriptional profiling, we found that the σW regulon was downregulated in the ΔrocG ΔgudB null mutant. A survey of σW-controlled genes for effects on CEF resistance identified both the NfeD protein YuaF and the flotillin homologue YuaG (FloT). Notably, overexpression of yuaFG in the rocG null mutant prevents the growth arrest induced by CEF. The YuaG flotillin has been shown previously to localize to defined lipid microdomains, and we show here that the yuaFGI operon contributes to a σW-dependent decrease in membrane fluidity. We conclude that glutamate dehydrogenase activity affects the expression of the σW regulon, by pathways that are yet unclear, and thereby influences resistance to CEF and other antibiotics.
INTRODUCTION
In Bacillus subtilis, a model system for the Gram-positive bacteria (36), the synthesis of glutamate is catalyzed uniquely by the heterodimeric product of the gltAB operon. Glutamate acts as a central metabolite providing the link between carbon and nitrogen metabolism (11, 40). The degradation of glutamate is catalyzed by the strictly catabolic glutamate dehydrogenase RocG (2). In addition to rocG, B. subtilis has a second glutamate dehydrogenase gene, gudB, whose product is cryptic due to an insertion of three amino acids close to the active site of this enzyme. However, null mutants of rocG rapidly accumulate spontaneous gain-of-function suppressor mutations in gudB that remove the repeat sequence encoding the three-amino-acid insertion, thereby resulting in the synthesis of active GudB (3, 12).
Recent studies have shown that RocG has a second activity as a regulatory protein. RocG, if glutamate is available, directly interacts with GltC, the transcription activator of the gltAB operon, thus inhibiting its activity (10, 15). However, whether it has additional functions remains largely unknown. In addition to RocG, several other bacterial enzymes are now known to regulate gene expression. Some act as transcription factors by direct binding to either DNA or RNA, and others modulate the activity of transcription factors either by covalent modification or by protein-protein interactions (9).
Cefuroxime (CEF) belongs to the group of broad-spectrum β-lactam cephalosporin antibiotics, with antimicrobial activity against both Gram-positive and Gram-negative bacteria (31). The mode of action of CEF is conventional: by binding to specific penicillin-binding proteins (PBPs), it inhibits the third and final stage of bacterial cell wall synthesis. In Gram-negative bacteria such as Escherichia coli, CEF shows high affinity for PBP3 (35). β-Lactams such as CEF are also known to exert their toxicity, at least in part, by generating reactive oxygen species (14, 22).
Three major mechanisms have been proposed for bacterial resistance to β-lactam antibiotics. The most common mechanism is the production of β-lactam-degrading enzymes (β-lactamases), which are widely disseminated among bacteria. The second mechanism, well studied in Gram-positive staphylococcal and streptococcal species, is alterations in PBPs, resulting in low affinities for β-lactams. The third mechanism is mediated by efflux pumps which prevent access of the β-lactams to the PBP targets (32, 43). B. subtilis also exhibits intrinsic resistance to a wide variety of β-lactams, including CEF. Currently, however, none of these mechanisms have been found to be applicable to B. subtilis. The extracytoplasmic function (ECF) sigma (σ) factors of B. subtilis regulate genes activated by cell wall antibiotics and are known, in several cases, to confer antibiotic resistance (18). The mechanism of activation by antibiotics is not well understood, but in the case of σW, stress activates a proteolytic cascade resulting in release of free σ factor from a transmembrane anti-σ, RsiW (17). A multiply mutant B. subtilis strain lacking all seven ECF sigma factors (σM, σX, σW, σV, σY, σZ, and σYlaC) has an increased sensitivity to β-lactams, including CEF. A similar sensitivity was noted for a triple mutant strain lacking σM, σW, and σX (28).
Here we address the influence of glutamate dehydrogenase activity on CEF resistance in B. subtilis. We were motivated by the serendipitous observation that rocG null mutant strains displayed an enhanced sensitivity to CEF. Our results demonstrate that glutamate dehydrogenase affects the activity of the ECF σ factor σW. Of the ∼60 genes in the σW regulon, we identify the yuaFGI operon as playing a pivotal role in CEF resistance. Our results reveal an unexpected link between central metabolism and antibiotic resistance.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. Deletion mutants were constructed by replacing genes with antibiotic resistance cassettes using long-flanking-homology (LFH) PCR as described previously (30, 39) in B. subtilis W168 (BGSC 1A1). Cells were routinely cultured in Luria-Bertani (LB) broth at 37°C with vigorous shaking or on solid LB medium containing 1.5% Bacto agar (Difco). Minimal medium contained 40 mM potassium morpholinepropanesulfonate (MOPS) (adjusted to pH 7.4 with KOH), 2 mM potassium phosphate buffer (pH 7.0), glucose (2%, wt/vol), (NH4)2SO4 (2 g/liter), MgSO4 · 7H2O (0.2 g/liter), trisodium citrate · 2H2O (1 g/liter), potassium glutamate (1 g/liter), tryptophan (10 mg/liter), 3 nM (NH4)6Mo7O24, 400 nM H3BO3, 100 μM FeCl3, 30 nM CoCl2, 10 nM CuSO4, 10 nM ZnSO4, and 80 nM MnCl2. Difco sporulation medium (DSM) agar was used for spore formation and maintenance of B. subtilis strains. The following antibiotics were used when appropriate: spectinomycin (Spec) (100 μg/ml), kanamycin (Kan) (15 μg/ml), chloramphenicol (Cat) (10 μg/ml), or macrolide-lincosamide-streptogramin B (MLS) (contains 1 μg/ml erythromycin and 25 μg/ml lincomycin) for B. subtilis strains and ampicillin (100 μg/ml) for E. coli DH5α.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Source or referencea |
|---|---|---|
| Strains | ||
| W168 | trpC2 | BGSC 1A1 |
| HB13528 | W168 rocG::spc | LFH PCR with W168 |
| HB13541 | W168 rocG::spc gudB::kan | HB13543 chromosomal DNA with HB13528 |
| HB13542 | W168 rocG::spc gudB+ | Gain-of-function mutation in gudB |
| HB13545 | W168 rocG::spc gudB::kan amyE::Pspac(hy)-rocG (cat) | pYH001 with HB13541 |
| HB13543 | W168 gudB::kan | LFH PCR with W168 |
| HB6156 | CU1065 yuaFGI::kan | 6 |
| HB13159 | W168 yuaFGI::kan | HB6156 chromosomal DNA with W168 |
| HB13547 | W168 yuaFG::mls | LFH PCR with W168 |
| HB13548 | W168 yuaGI::mls | LFH PCR with W168 |
| HB13568 | W168 yuaI::mls | LFH PCR with W168 |
| HB5331 | CU1065 yqeZ-yqfAB::kan | 6 |
| HB13566 | W168 yqeZ-yqfAB::kan | HB5331 chromosomal DNA with W168 |
| HB13567 | W168 yqeZ-yqfAB::kan yuaFG::mls | HB5331 chromosomal DNA with HB13547 |
| HB10102 | 168 sigW::mls | 27 |
| HB13549 | W168 sigW::mls | HB10102 chromosomal DNA with W168 |
| HB6208 | W168 sigW::spc | 6 |
| HB13558 | W168 sigW::spc yuaFG::mls | HB6208 chromosomal DNA with HB13547 |
| HB13557 | W168 sigW::mls rocG::spc gudB::kan | HB13549 chromosomal DNA with HB13541 |
| HB13571 | W168 yuaFG::mls amyE::Pspac(hy)-yuaFG (cat) | pYH002 with HB13547 |
| HB13572 | W168 rocG::spc gudB::kan amyE::Pspac(hy)-yuaFG | pYH002 with HB13541 |
| HB13574 | W168 sigW::mls amyE::Pspac(hy)-yuaFG (cat) | pYH002 with HB13549 |
| HB13042 | W168 amyE::Pxyl-sigW (cat) | 21 |
| HB13122 | W168 PfabHAFfabHA(P5*)-fabF amyE::Pxyl-sigW (cat) | 21 |
| HB13160 | W168 yuaFGI::kan amyE::Pxyl-sigW (cat) | HB6156 chromosomal DNA with HB13042 |
| HB13226 | W168 PfabHAFfabHA(P5*)fabF yuaFGI::kan amyE::Pxyl-sigW (cat) | HB6156 chromosomal DNA with HB13122 |
| HB13236 | W168 yqeZ-yqfAB::kan amyE::Pxyl-sigW (cat) | HB13566 chromosomal DNA with HB13042 |
| Plasmids | ||
| pPL82 | IPTG-inducible expression vector (amyE integration) | 33 |
| pYH001 | Pspac(hy)-rocG in pPL82 | This study |
| pYH002 | Pspac(hy)-yuaFG in pPL82 | This study |
Plasmid construction.
PCR and cloning for plasmid construction were performed by using standard techniques (34). The primers used in the present study are listed in Table S1 in the supplemental material. Ectopic expression of rocG and yuaFG at amyE was placed under the control of isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter Pspac(hy) using plasmid backbone pPL82 (33). For the construction of pYH001 (pPL82-rocG), the promoterless rocG gene was amplified from B. subtilis chromosomal DNA by PCR using the primers 5395 (rocG Pspac-F) and 5396 (rocG Pspac-R). pYH002 (pPL82-yuaFG) was constructed in a similar manner using the pair of primers 5561 (yuaFG Pspac-F) and 5563 (yuaFG Pspac-R). Construct integrity was verified by DNA sequencing. Plasmids were amplified in E. coli DH5α before transformation of B. subtilis strains.
Disk diffusion assays.
Disk diffusion assays were performed as described previously (29). Briefly, strains were grown in LB medium to an optical density at 600 nm (OD600) of 0.4. A 100-μl aliquot of these cultures was mixed with 4 ml of 0.7% LB soft agar (kept at 50°C) and directly poured onto LB agar plates (containing 15 ml of 1.5% LB agar). After 30 min at room temperature (to allow the soft agar to solidify), the plates were dried for 20 min in a laminar airflow hood. Filter paper disks containing the antibiotics to be tested were placed on the top of the agar, and the plates were incubated at 37°C overnight. The diameters of the inhibition zones were measured after subtraction of the diameter of the filter paper disk (6.5 mm). The following antibiotics and quantities were used in the disk diffusion assays: penicillin G, 100 μg; ampicillin, 100 μg; fosfomycin, 500 μg; vancomycin, 100 μg; and CEF, 1 μg or 3 μg.
RNA preparation and microarray analyses.
Total RNA was isolated from three biological replicates of W168 and HB13541 (rocG gudB double null mutant) grown in LB to mid-log phase (OD600 of 0.4), using the RNeasy minikit (Qiagen), followed by DNase treatment with Turbo DNA free (Ambion). The quantity and purity of RNA were determined using a NanoDrop spectrophotometer (NanoDrop Technology Inc., Wilmington, DE). cDNA labeling and microarray analysis were performed as described previously (16). Two microarrays were used in biological triplicates. The GenePix Pro 6.0 software package was used for image processing and analysis. Each expression value is representative of four separate measurements (duplicate spots on each of two arrays). Mean values and standard deviations (SDs) for the normalized microarray data sets were calculated with MS Excel. The normalized microarray data sets were filtered to remove those genes that were not expressed at levels significantly above background in either condition (sum of mean fluorescence intensity, <20). In addition, the mean and standard deviation of the fluorescence intensities were computed for each gene, and those for which the standard deviation was greater than the mean value were ignored. The fold change was calculated by using the average signal intensities for HB13541 divided by those for W168.
Quantitative real-time RT-PCR.
Measurement of transcript abundance was performed by quantitative real-time RT-PCR (qRT-PCR). cDNA was synthesized by using random hexamer primers and a TaqMan reverse transcription kit (Roche). qRT-PCR was carried out by using SYBR green (Bio-Rad) and gene-specific primer pairs 5403 (yqeZ qRT-F)/5404 (yqeZ qRT-R) and 5411 (yuaF qRT-F)/5412 (yuaF qRT-R) according to the manufacturer's instructions. Expression of yuaF and yqeZ was calculated as the fold change based on the threshold cycle (CT) values for each gene, as described previously (38). The level of 23S rRNA was used as a normalization control.
Fluorescence anisotropy.
Fluorescence anisotropy analysis of B. subtilis strains treated with 1,6-diphenyl-1,3,5-hexatriene (DPH) was performed as described previously (37) with slight modifications. Strains were grown to mid-log phase (OD600 of 0.4 ± 0.01) in LB supplemented with 2% xylose. A 0.5-ml sample of each culture was then washed once and suspended in 2 ml of phosphate buffer (100 mM, pH 7.0) containing 5 μM DPH. After a 30-min incubation at room temperature, fluorescence anisotropy measurements (λex = 358 nm, slit width = 10 nm; λem = 428 nm, slit width = 15 nm) were taken with a Perkin-Elmer LS55 luminescence spectrometer. The correction for the fluorescence intensity of nonlabeled cells was calculated as described by Kuhry et al. (23).
Microarray data accession number.
The microarray data set is available in the NCBI GEO database under accession number GSE34383.
RESULTS AND DISCUSSION
A rocG null mutant shows increased susceptibility to CEF.
We grew B. subtilis cells by repeated subculturing with selection for increasing resistance to both vancomycin and cephalosporin. In studies to be presented in detail elsewhere, we found that the evolved strains were significantly altered in gene expression as judged by global transcriptome analyses using cDNA microarrays. Of relevance for the present study, the genes upregulated in the evolved strains included rocG, encoding the sole catabolic glutamate dehydrogenase in B. subtilis. However, mutational inactivation of rocG did not affect antibiotic resistance in these resistant strains, and the relevant genetic determinants were ultimately determined using whole-genome resequencing (data not shown). Although it is not an important determinant of cephalosporin resistance in these strains, we made the serendipitous observation that rocG mutant strains are more sensitive to some cell wall antibiotics in an otherwise wild-type background (Fig. 1A). Here, we define this unexpected link between glutamate dehydrogenase and cephalosporin sensitivity and identify the relevant genetic determinants.
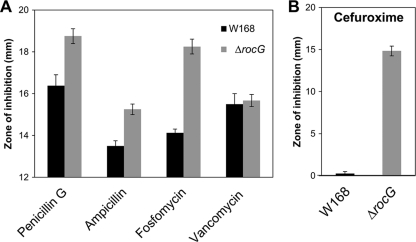
Fig 1.
Disk diffusion susceptibility testing of the rocG mutant with several antibiotics affecting cell wall biosynthesis. (A) The rocG mutant is sensitive to fosfomycin and slightly sensitive to penicillin G and ampicillin. (B) Disruption of rocG markedly increases sensitivity to CEF (1 μg). Each bar represents the average zone of inhibition, expressed as total diameter minus diameter of the filter paper disk (6.5 mm). At least three assays were performed with three independent clones of each strain. Error bars indicate the standard deviations.
To assess the potential role of rocG in conferring antibiotic resistance, we constructed an isogenic deletion mutant by homologous recombination in B. subtilis W168 (BGSC 1A1). Disk diffusion assays showed that disruption of rocG leads to susceptibility to β-lactams and fosfomycin but not to vancomycin (Fig. 1A). Although the rocG mutant is only slightly sensitive to penicillin G and ampicillin, it is notably sensitive to cefuroxime (CEF), a broad-spectrum cephalosporin (Fig. 1B). The rocG mutant showed a clear inhibition zone, but the wild type was only slightly affected by CEF, suggesting that RocG plays a crucial role in CEF resistance in B. subtilis. Since sensitivity to fosfomycin is less distinct than that to CEF, here we focused on identification of genetic factors that confer CEF resistance.
The lack of glutamate dehydrogenase activity influences CEF resistance.
The rocG mutant colonies grown on LB agar plates lyse more easily than wild-type colonies at room temperature. After 2 weeks, however, many new colonies arise and exhibit no lysis phenotype, eventually sporulating on LB agar plates (see Fig. S1 [left] in the supplemental material). Since it is known that rocG mutant strains rapidly accrue spontaneous gain-of-function mutations (previously designated gudB1) in gudB, which encodes an inactive glutamate dehydrogenase in B. subtilis 168 strains (3), we expected that this unusual phenotype would be due to mutations in gudB. Using DNA sequencing of gudB, we confirmed that these colonies are gudB gain-of-function mutants, as previously reported (3) (Fig. S1 [right] in the supplemental material). All of the sequenced colonies had a deletion of one copy of the 9-nucleotide direct repeat in the 5′ coding region of gudB.
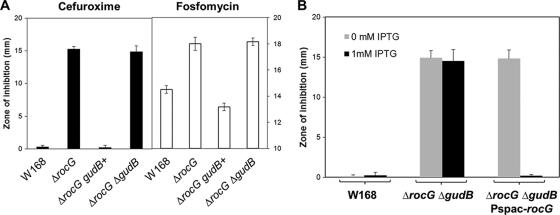
To further examine whether the spontaneous gain-of-function mutations in gudB, here denoted gudB+, suppresses the CEF-sensitive phenotype of a rocG mutant, we performed disk diffusion assays. These mutations restore normal resistance to CEF, and similar results were also obtained for fosfomycin resistance (Fig. 2A). Indeed, the rocG gudB+ strain is slightly more resistant to these antibiotics than the wild type, possibly due to the constitutive expression of gudB (3). To avoid complications due to these suppressor mutations, we constructed a rocG gudB double mutant (HB13541) and used this as a glutamate dehydrogenase-negative strain. Moreover, when rocG expression is placed under the control of the Pspac(hy) promoter, it complements the CEF-sensitive phenotype of the double mutant (Fig. 2B). Control experiments show that IPTG itself does not reduce CEF sensitivity.
Fig 2.
CEF resistance is distinctly influenced by the glutamate dehydrogenase activity. (A) Sensitivity to CEF and fosfomycin was determined by using disk diffusion assays. The spontaneous gain-of-function mutations in gudB restore normal resistance to these two antibiotics. (B) Induction of the rocG gene by IPTG (1 mM) complements the CEF-sensitive phenotype. Three independent experiments were performed for each strain, and the standard deviation is indicated by error bars.
The glutamate dehydrogenase RocG functions not only as a central metabolic enzyme but also as a regulatory protein by interaction with GltC (10, 15). GudB shares with RocG both a common enzymatic activity and an ability to regulate GltC (3). Thus, the effects of glutamate dehydrogenase on antibiotic resistance could, in principle, be due to the enzymatic activity of the protein or the regulatory function.
CEF arrests the growth of the rocG mutant cells at mid-exponential phase.
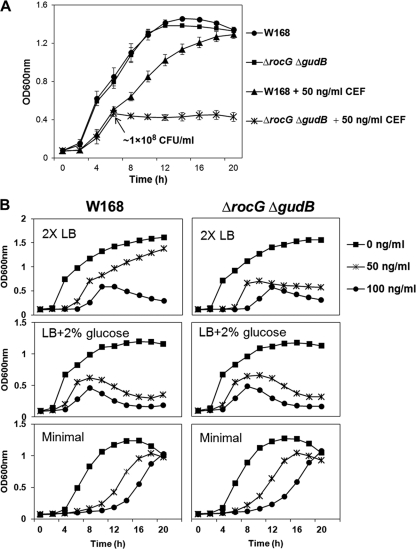
We next compared the growth behaviors of B. subtilis wild-type and rocG gudB double null mutant (ΔrocG ΔgudB) strains in the presence of CEF. CEF was added at the beginning of the culture, and growth was measured spectrophotometrically (by optical density at 600 nm) using a Bioscreen C microbial growth analyzer (Growth Curves USA, Piscataway, NJ) at 37°C with vigorous shaking. After reaching mid-exponential phase (OD600 of ∼0.5, which corresponds to about 1 × 108 CFU/ml), the rocG gudB mutant cells exhibit growth arrest in the presence of very low levels of CEF (50 ng/ml) (Fig. 3A).
Fig 3.
Mid-exponential-phase rocG mutant cells exhibit growth arrest in the presence of CEF. (A) Effect of CEF on the growth of the rocG gudB mutant. CEF (50 ng/ml) was added at the beginning of the culture. Liquid growth assays were performed in LB medium using a Bioscreen C growth analyzer. To determine CFU/ml, viable cell counts were estimated by plating diluted cultures on LB agar plates. Results from three independent experiments were averaged, and the standard deviation is indicated by error bars. (B) Effects of different culture media on the CEF-induced growth arrest of the rocG gudB mutant. Strains were grown in 2× LB, LB supplemented with 2% glucose, and glucose (2%) minimal medium in the presence or absence of CEF. Data are representative of at least three independent experiments.
Glutamate dehydrogenase is required for the catabolism of glutamate, arginine, ornithine, and proline, and transcription of rocG is strongly repressed by glucose and other easily metabolized carbon sources (3, 4, 5). Previously, in wild-type cells grown in nutrient broth, the total cellular activity of glutamate dehydrogenase was observed to be low in early exponential phase, with higher levels in the later stages of exponential growth (3). Thus, the CEF-induced growth arrest of the rocG gudB mutant appears to occur during the same growth phase when rocG would normally be upregulated. We therefore hypothesized that the CEF-induced growth arrest might be correlated with an inability of the rocG gudB mutant to metabolize alternative carbon sources and, as a corollary, that CEF somehow affects carbon source preferences.
We reasoned that if the growth arrest observed in the presence of CEF is due to a block in catabolism, then cells provided with a more abundant carbon supply should be delayed in growth arrest. Indeed, in 2× LB medium, growth impairment (for both the wild type and the rocG gudB double mutant) occurred at a somewhat higher OD600 value (approximately 0.7) than in LB (Fig. 3B versus A). However, wild-type cells actually had slightly increased sensitivity to CEF. We next tested the effects of providing cells with an abundant and easily metabolized carbon source (2% glucose). In LB supplemented with 2% glucose, both the wild-type and rocG gudB mutant strains exhibited growth arrest, and eventually lysis, in mid-logarithmic phase in the presence of low levels of CEF. Finally, in minimal medium containing 2% glucose, the wild-type and mutant strains showed similar responses to CEF: both strains showed a CEF-dependent growth lag but no longer displayed growth arrest in mid-logarithmic phase.
These results suggest that glucose-dependent repression of rocG leads even wild-type cells to behave phenotypically as glutamate dehydrogenase mutants. Moreover, simply providing cells with an easily metabolized carbon source is insufficient to bypass the growth arrest. Although the reasons for these medium-dependent differences are not entirely clear, these data suggest that CEF-induced growth arrest of the rocG gudB mutant in LB medium is correlated with a need for rocG activation.
Effect of alkaline growth pH on the susceptibility of B. subtilis to CEF.
In addition to its role in carbon catabolism, RocG may play a role in pH homeostasis (43). The arginine catabolism (roc) operon has been shown to be upregulated at high pH, presumably because arginine breakdown can lead to acidic products that counteract base stress (43). However, in some species arginine catabolism is upregulated at acidic pH. Ammonia (NH3), generated by glutamate dehydrogenase, can bind a proton (H+), leading to an increase in intracellular pH (pHi). It has been shown that during fermentation of amino acids by B. subtilis natto there is substantial ammonia production, much of which is due to glutamate dehydrogenase (20). We therefore reasoned that production of NH3 by RocG might affect cellular pH and, since cell membranes are permeable to NH3, also affect extracellular pH (pHe). However, no significant differences in pHe were observed between the B. subtilis wild-type and rocG gudB double mutant strains when measured at several different growth points in LB medium (data not shown).
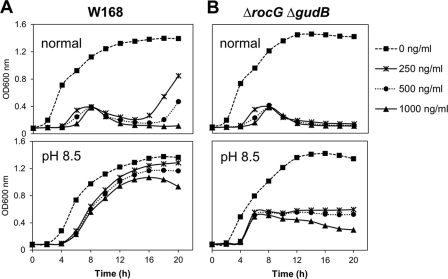
Next, we examined the effect of alkaline growth pH (conditions known to induce rocG expression and the σW regulon [42, 44]) on the susceptibility of B. subtilis to CEF. The wild-type strain showed a remarkable reduction in susceptibility to CEF under alkaline growth conditions (Fig. 4A). However, as shown in Fig. 4B, the rocG gudB double mutant still shows high susceptibility to CEF even in MOPS-buffered LB medium (pH 8.5). Thus, simply raising the pHe, in this case with buffer, is not sufficient to prevent growth arrest. We conclude that the role of RocG as being important for growth in the presence of low concentrations of CEF is not obviously linked to carbon catabolism or to a major role in pH homeostasis. It remains possible that RocG affects intracellular pH or by other means alters cell physiology to help cells resist the deleterious effects of CEF, but determining the mechanism of this connection requires further study.
Fig 4.
Effect of external alkaline pH on the susceptibility of wild-type (A) and rocG gudB mutant (B) strains of B. subtilis to CEF. Liquid growth assays were performed in LB medium (normal) or LB medium buffered with MOPS (pH 8.5) using a Bioscreen C growth analyzer. CEF was added at the beginning of the culture. A representative data set is shown.
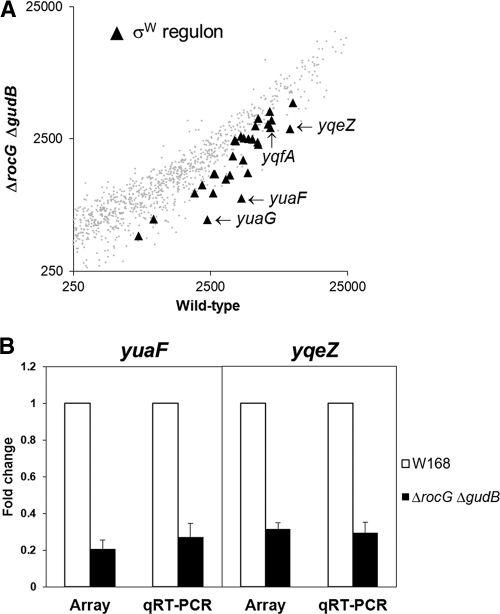
RocG is positively involved in controlling the expression of the σW regulon.
In order to better understand the precise molecular mechanism(s) by which RocG exerts its effects on CEF resistance, the gene expression profile (transcriptome) of the rocG gudB mutant was assessed using DNA microarrays. The rocG gudB double null mutant showed significant changes in gene expression relative to a wild-type strain. Significantly, a regulon-based expression analysis revealed that most of the σW regulon (8, 19), which is known to be related to resistance to cell wall antibiotics (18), is downregulated in the rocG gudB mutant (Fig. 5A). This is consistent with the observed susceptibility of the rocG mutant to fosfomycin (Fig. 1A), since σW is required for expression of FosB, the major fosfomycin resistance determinant in B. subtilis (7).
Fig 5.
A rocG null mutant displays decreased expression of the σW regulon. (A) Gene expression variation as measured by cDNA microarray analysis under nonstress conditions. RNA was extracted from cells grown in LB medium to an OD600 of 0.4. Black triangles indicate the σW regulon genes. (B) Quantitative real-time RT-PCR analysis of yuaF and yqeZ expression. Data were expressed as fold change relative to wild-type cells. The level of 23S rRNA was used as a normalization control. Three independent experiments were performed for each gene, and the standard deviation is indicated by error bars.
Within the σW regulon, expression of genes in the yuaFGI and yqeZ-yqfAB operons was strongly downregulated. In B. subtilis, YuaF is a member of the NfeD family with a potential role in maintaining membrane integrity (1), and YuaG (recently renamed FloT) is a putative flotillin-like protein (24, 45). The yqeZ gene encodes a second NfeD family protein, while yqfA encodes another flotillin homologue that has functions partially redundant with those of YuaG (26). The observed changes in transcript abundance for yuaF (fold change ± standard deviation [SD] = 0.21 ± 0.049) and yqeZ (0.32 ± 0.035) were further confirmed by qRT-PCR analysis (0.27 ± 0.078 and 0.29 ± 0.058, respectively), as shown in Fig. 5B. The qRT-PCR results were in direct agreement with cDNA microarray data.
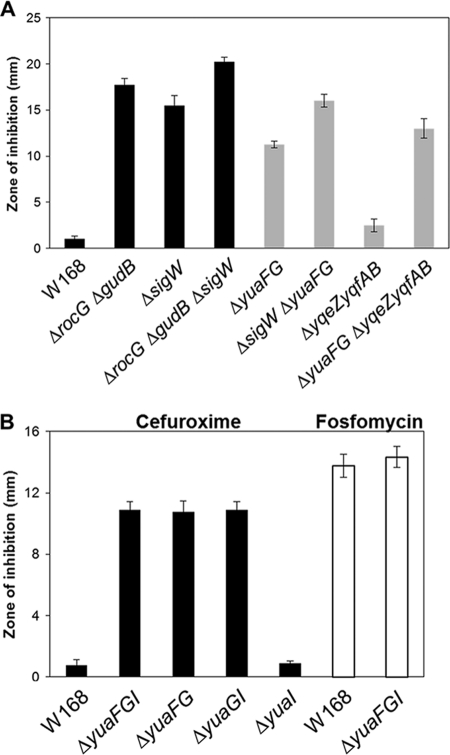
The yuaFGI operon is a major contributor to σW-dependent CEF resistance.
To determine the contribution of the σW regulon to CEF resistance, disk diffusion assays were performed on LB agar plates (Fig. 6). As predicted, the sigW mutant exhibited increased sensitivity to CEF (Fig. 6A). A survey of σW-controlled genes for effects on CEF resistance revealed that yuaFG plays a major role in CEF resistance, with yqeZ-yqfAB playing an accessory role (Fig. 6A). We also found that the first two genes in the yuaFGI operon were enough for it to exert its full effect on CEF resistance (Fig. 6B). These results are also consistent with studies suggesting a functional interaction between the NfeD protein YuaF and the flotillin YuaG (41). However, the yuaFGI operon does not confer fosfomycin resistance (Fig. 6B), consistent with the known involvement of another σW target gene, fosB (7). Together, these findings suggest that RocG affects CEF resistance by enhancing transcription of the σW regulon.
Fig 6.
A survey of the σW regulon identifies yuaFGI as a major determinant of CEF resistance. The CEF sensitivity was determined by disk diffusion assay, which was performed on LB agar plates with a filter paper disk containing 3 μg CEF. (A) Detailed identification of genes conferring CEF resistance in the yuaFGI operon and determination of fosfomycin sensitivity. (B) Involvement of the σW regulon in RocG-mediated CEF resistance. Three independent experiments were performed for each strain, and the standard deviation is indicated by error bars.
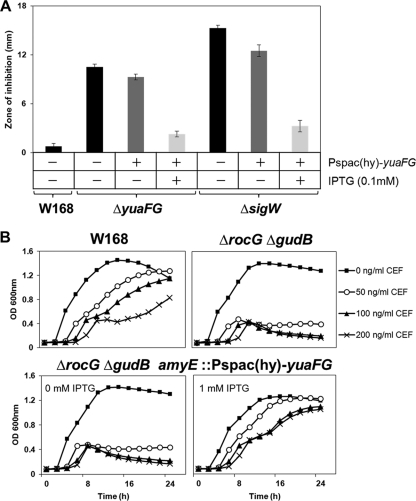
Overexpression of yuaFG in the rocG mutant prevents the growth arrest induced by CEF.
To confirm the involvement of the yuaFG genes in CEF resistance, these two genes were placed under the control of an IPTG-inducible promoter and the fusion was integrated ectopically at the amyE locus [amyE::Pspac(hy)-yuaFG] of the yuaFG mutant, the sigW mutant, and the rocG gudB double mutant. We performed disk diffusion assays in the yuaFG and the sigW mutant backgrounds bearing the Pspac(hy)-yuaFG fusion (Fig. 7A). In both strains, induction of yuaFG expression by IPTG (0.1 mM) restored CEF resistance (Fig. 7). These strains also show slightly lower sensitivity to CEF under noninducing conditions, possibly because the Pspac(hy) promoter has low expression in the absence of IPTG.
Fig 7.
yuaFG overexpression by IPTG induction rescues the CEF-sensitive phenotype. (A) Induction of yuaFG expression by IPTG (0.1 mM) restores CEF resistance in the yuaFG mutant and the sigW mutant. Three independent experiments were performed for each strain, and the standard deviation is indicated by error bars. (B) IPTG-dependent induction of yuaFG prevents the growth arrest of rocG mutant cells. The maximal effect was observed at a final concentration of 1 mM IPTG. Liquid growth assays were performed in LB medium using a Bioscreen C growth analyzer. A representative data set is shown.
We next determined whether the yuaFG genes can rescue the growth arrest induced by CEF in the rocG gudB mutant. Indeed, the rocG gudB mutant carrying the Pspac(hy)-yuaFG fusion showed growth arrest without IPTG induction but was rescued by induction of YuaFG synthesis (Fig. 7B). These effects occurred in an IPTG concentration-dependent manner, with a maximal effect at 1 mM (data not shown). These results suggest a pivotal role of the yuaFG genes in CEF resistance mediated by σW (and influenced by RocG) in B. subtilis.
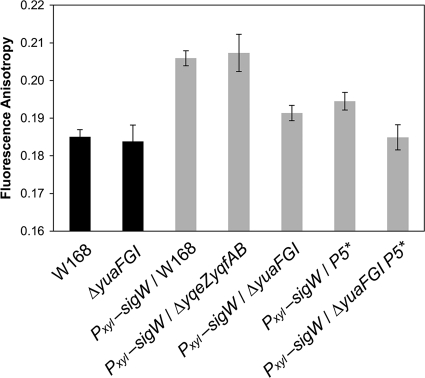
The yuaFGI operon reduces membrane fluidity under σW-inducing conditions.
To better understand how yuaFGI and yqeZ-yqfAB contribute to intrinsic CEF resistance, we investigated the influence of these genes on membrane fluidity. Both yuaG and yqfA encode putative flotillin-like proteins that are believed to organize the cell membrane into functional microdomains (1, 26). In addition, σW overexpression has previously been shown to reduce membrane fluidity by altering expression of fatty acid biosynthesis genes (21). The σW-dependent activation of a promoter (P5) within the fabHAF operon leads to an increase in the proportion of straight-chain fatty acids and an increase in overall chain length. Since activation of P5 accounts for some, but not all, of the σW-dependent decrease in membrane fluidity (21), we reasoned that upregulation of yuaFGI and/or yqeZ-yqfAB might alter membrane fluidity.
Membrane fluidity was assessed by measuring the fluorescence anisotropy of B. subtilis cells labeled with DPH (Fig. 8). Under normal growth conditions, both wild-type and yuaFGI knockout cells exhibited similar anisotropy levels. However, when sigW was overexpressed with a xylose-inducible promoter (Pxyl-sigW), the resulting increase in anisotropy was significantly lower in the yuaFGI knockout strain than in control cells. Since a higher anisotropy is indicative of a less-fluid membrane, these results indicate that expression of the yuaFGI operon reduces membrane fluidity when activated by σW. In contrast, deleting yqeZ-yqfAB had no effect on anisotropy levels, even under sigW overexpression conditions. The effect of yuaFGI on membrane fluidity is comparable to that of the σW-dependent promoter (P5) within the fabHAF operon (21). In a σW overexpression strain both lacking yuaFGI and containing a mutation (P5*) that abolishes P5 activity (Pxyl-sigW yuaFGI P5*), anisotropy levels were the same as in wild-type cells. This demonstrates that both P5 and yuaFGI function to reduce membrane fluidity and that they are the primary components of the σW regulon to do so.
Fig 8.
Inactivation of yuaFGI prevents the decrease in membrane fluidity induced by overexpression of σW. Cells were grown in LB medium with xylose (2%) to an OD600 of 0.4 and then incubated in phosphate buffer (100 mM, pH 7.0) with DPH (5 μM) at 25°C for 30 min. In strains containing the Pxyl-sigW construct, σW was expressed under the control of a xylose-inducible promoter. The membrane fluidity of each strain was determined via fluorescence anisotropy measurements. Data are presented as the average of at least three trials, and the standard error is indicated by error bars.
The effect of yuaFGI on membrane fluidity might explain how this operon contributes to CEF resistance. Adjustments in membrane fluidity can influence numerous properties of the lipid bilayer, such as permeability, protein mobility, and protein-protein interactions (25). However, not all changes in membrane fluidity result in CEF resistance, since the P5-inactive strain was not any more susceptible to CEF than the wild-type strain (data not shown). YuaG (FloT) has also been linked to the formation of lipid domains, which have been shown to regulate sporulation, biofilm formation, and other signal transduction pathways (13, 26).
Concluding remarks.
Our data demonstrate a previously unidentified regulatory effect of RocG on antibiotic resistance. Although glutamate dehydrogenase is relatively well studied in B. subtilis, the effects of glutamate dehydrogenase activity on the cell envelope stress response have thus far remained unknown. The present study indicates that the σW-dependent stress response is the link between RocG activity and CEF resistance. Glutamate dehydrogenase affects expression of the σW regulon, by mechanisms not yet resolved, and thereby contributes to CEF resistance. We specifically demonstrate that overexpression of the σW-regulated yuaFG operon prevents growth arrest of the rocG mutant in the presence of CEF. We also show that expression of yuaFGI operon reduces membrane fluidity under σW-inducing conditions and that this protein-based mechanism is additive with a previously described lipid-based pathway (21). These findings suggest that YuaFG influences CEF resistance by altering the physical properties of the membrane, but the origins of this effect are presently unclear. YuaFG are thought to help organize membrane microdomains (13, 26), and this could affect the assembly or activity of cell wall biosynthetic complexes known to be targeted by CEF. A future challenge will be to identify how glutamate dehydrogenase affects activity of the σW regulon (and thereby CEF and fosfomycin resistance) and how the activity of flotillin-like proteins affects cell wall biosynthesis pathways.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institutes of Health (GM047446) and was also supported by a Korea Research Foundation grant funded by the Korean government (KRF-2009-352-C00118) to Y. H. Lee.
Footnotes
Published ahead of print 16 December 2011
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Bateman A, et al. 2004. The Pfam protein families database. Nucleic Acids Res. 32:D138–D141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Belitsky BR. 2002. Biosynthesis of amino acids of the glutamate and aspartate families, alanine and polyamines, p 203–231 In Sonenshein AL, Hoch JA, Losick R. (ed), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC [Google Scholar]
- 3. Belitsky BR, Sonenshein AL. 1998. Role and regulation of Bacillus subtilis glutamate dehydrogenase genes. J. Bacteriol. 180:6298–6305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belitsky BR, Sonenshein AL. 1999. An enhancer element located downstream of the major glutamate dehydrogenase gene of Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 96:10290–10295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belitsky BR, Kim H-J, Sonenshein AL. 2004. CcpA-dependent regulation of Bacillus subtilis glutamate dehydrogenase gene expression. J. Bacteriol. 186:3392–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Butcher BG, Helmann JD. 2006. Identification of Bacillus subtilis σW-dependent genes that provide intrinsic resistance to antimicrobial compounds produced by bacilli. Mol. Microbiol. 60:765–782 [DOI] [PubMed] [Google Scholar]
- 7. Cao M, Bernat BA, Wang Z, Armstrong RN, Helmann JD. 2001. FosB, a cysteine-dependent fosfomycin resistance protein under the control of sigma (W), an extracytoplasmic-function sigma factor in Bacillus subtilis. J. Bacteriol. 183:2380–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cao M, et al. 2002. Defining the Bacillus subtilis sigma (W) regulon: a comparative analysis of promoter consensus search, runoff transcription/macroarray analysis (ROMA), and transcriptional profiling approaches. J. Mol. Biol. 316:443–457 [DOI] [PubMed] [Google Scholar]
- 9. Commichau FM, Stülke J. 2008. Trigger enzymes: bifunctional proteins active in metabolism and in controlling gene expression. Mol. Microbiol. 67:692–702 [DOI] [PubMed] [Google Scholar]
- 10. Commichau FM, Herzberg C, Tripal P, Valerius O, Stülke J. 2007. A regulatory protein-protein interaction governs glutamate biosynthesis in Bacillus subtilis: the glutamate dehydrogenase RocG moonlights in controlling the transcription factor GltC. Mol. Microbiol. 65:642–654 [DOI] [PubMed] [Google Scholar]
- 11. Commichau FM, Forchhammer K, Stülke J. 2006. Regulatory links between carbon and nitrogen metabolism. Curr. Opin. Microbiol. 9:167–172 [DOI] [PubMed] [Google Scholar]
- 12. Commichau FM, Gunka K, Landmann JJ, Stülke J. 2008. Glutamate metabolism in Bacillus subtilis: gene expression and enzyme activities evolved to avoid futile cycles and to allow rapid responses to perturbations in the system. J. Bacteriol. 190:3557–3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donovan C, Bramkamp M. 2009. Characterization and subcellular localization of a bacterial flotillin homologue. Microbiology 155:1786–1799 [DOI] [PubMed] [Google Scholar]
- 14. Dwyer DJ, Kohanski MA, Collins JJ. 2009. Role of reactive oxygen species in antibiotic action and resistance. Curr. Opin. Microbiol. 12:482–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gunka K, et al. 2010. Functional dissection of a trigger enzyme: mutations of the Bacillus subtilis glutamate dehydrogenase RocG that affect differentially its catalytic activity and regulatory properties. J. Mol. Biol. 400:815–827 [DOI] [PubMed] [Google Scholar]
- 16. Hachmann AB, Angert ER, Helmann JD. 2009. Genetic analysis of factors affecting susceptibility of Bacillus subtilis to daptomycin. Antimicrob. Agents Chemother. 53:1598–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heinrich J, Hein K, Wiegert TT. 2009. Two proteolytic modules are involved in regulated intramembrane proteolysis of Bacillus subtilis RsiW. Mol. Microbiol. 74:1412–1426 [DOI] [PubMed] [Google Scholar]
- 18. Helmann JD. 2002. The extracytoplasmic function (ECF) sigma factors. Adv. Microb. Physiol. 46:47–110 [DOI] [PubMed] [Google Scholar]
- 19. Huang XJ, Gaballa A, Cao M, Helmann JD. 1999. Identification of target promoters for the Bacillus subtilis extracytoplasmic function sigma factor, σW. Mol. Microbiol. 31:361–371 [DOI] [PubMed] [Google Scholar]
- 20. Kada S, Yabusaki M, Kaga T, Ashida H, Yoshida K. 2008. Identification of two major ammonia-releasing reactions involved in secondary natto fermentation. Biosci. Biotechnol. Biochem. 72:1869–1876 [DOI] [PubMed] [Google Scholar]
- 21. Kingston AW, Subramanian C, Rock CO, Helmann JD. 2011. A σW-dependent stress response in Bacillus subtilis that reduces membrane fluidity. Mol. Microbiol. 81:69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797–810 [DOI] [PubMed] [Google Scholar]
- 23. Kuhry J-G, Duportail G, Bronner C, Laustriat G. 1985. Plasma membrane fluidity measurements on whole living cells by fluorescence anisotropy of trimethylammoniumdiphenylhexatriene. Biochim. Biophys. Acta 845:60–67 [DOI] [PubMed] [Google Scholar]
- 24. Langhorst MF, Reuter A, Stuermer CA. 2005. Scaffolding microdomains and beyond: the function of reggie/flotillin proteins. Cell. Mol. Life Sci. 62:2228–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Los DA, Murata N. 2004. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta 1666:142–157 [DOI] [PubMed] [Google Scholar]
- 26. López D, Kolter R. 2010. Functional microdomains in bacterial membranes. Genes Dev. 24:1893–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luo Y, Helmann JD. 2009. Extracytoplasmic function sigma factors with overlapping promoter specificity regulate sublancin production in Bacillus subtilis. J. Bacteriol. 191:4951–4958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luo Y, Asai K, Sadaie Y, Helmann JD. 2010. Transcriptomic and phenotypic characterization of a Bacillus subtilis strain without extracytoplasmic function σ factors. J. Bacteriol. 192:5736–5745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mascher T, Hachmann AB, Helmann JD. 2007. Regulatory overlap and functional redundancy among Bacillus subtilis extracytoplasmic function sigma factors. J. Bacteriol. 189:6919–6927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mascher T, Margulis NG, Wang T, Ye RW, Helmann JD. 2003. Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon. Mol. Microbiol. 50:1591–1604 [DOI] [PubMed] [Google Scholar]
- 31. Neu HC, Fu KP. 1978. Cefuroxime, a beta-lactamase-resistant cephalosporin with a broad spectrum of gram-positive and -negative activity. Antimicrob. Agents Chemother. 13:657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poole K. 2004. Resistance to β-lactam antibiotics. Cell. Mol. Life Sci. 61:2200–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Quisel JD, Burkholder WF, Grossman AD. 2001. In vivo effects of sporulation kinases on mutant Spo0A proteins in Bacillus subtilis. J. Bacteriol. 183:6573–6578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd edCold Spring Harbor Laboratory Press, New York, NY [Google Scholar]
- 35. Scott LJ, Ormrod D, Goa KL. 2001. Cefuroxime axetil: an updated review of its use in the management of bacterial infections. Drugs 61:1455–1500 [DOI] [PubMed] [Google Scholar]
- 36. Sonenshein AL, Hoch JA, Losick R. (ed). 2002. Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC [Google Scholar]
- 37. Svobodová J, Svoboda P. 1988. Cytoplasmic membrane fluidity measurements on intact living cells of Bacillus subtilis by fluorescence anisotropy of 1,6-diphenyl 1,3,5-hexatriene. Folia Microbiol. 33:1–9 [DOI] [PubMed] [Google Scholar]
- 38. Talaat AM, et al. 2002. Genomic DNA standards for gene expression profiling in Mycobacterium tuberculosis. Nucleic Acids Res. 30:e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wach A. 1996. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast 12:259–265 [DOI] [PubMed] [Google Scholar]
- 40. Wacker I, et al. 2003. The regulatory link between carbon and nitrogen metabolism in Bacillus subtilis: regulation of the gltAB operon by the catabolite control protein CcpA. Microbiology 149:3001–3009 [DOI] [PubMed] [Google Scholar]
- 41. Walker CA, Hinderhofer M, Witte DJ, Boos W, Moller HM. 2008. Solution structure of the soluble domain of the NfeD protein YuaF from Bacillus subtilis. J. Biomol. NMR 42:69–76 [DOI] [PubMed] [Google Scholar]
- 42. Wiegert T, Homuth G, Versteeg S, Schumann W. 2001. Alkaline shock induces the Bacillus subtilis sigma(W) regulon. Mol. Microbiol. 41:59–71 [DOI] [PubMed] [Google Scholar]
- 43. Wilke MS, Lovering AL, Strynadka NC. 2005. β-Lactam antibiotic resistance: a current structural perspective. Curr. Opin. Microbiol. 8:525–533 [DOI] [PubMed] [Google Scholar]
- 44. Wilks JC, et al. 2009. Acid and base stress and transcriptomic responses in Bacillus subtilis. Appl. Environ. Microbiol. 75:981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang HM, et al. 2005. An alkali-inducible flotillin-like protein from Bacillus halodurans C-125. Protein J. 24:125–131 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.