Abstract
Rhodoquinone (RQ) is a required cofactor for anaerobic respiration in Rhodospirillum rubrum, and it is also found in several helminth parasites that utilize a fumarate reductase pathway. RQ is an aminoquinone that is structurally similar to ubiquinone (Q), a polyprenylated benzoquinone used in the aerobic respiratory chain. RQ is not found in humans or other mammals, and therefore, the inhibition of its biosynthesis may provide a novel antiparasitic drug target. To identify a gene specifically required for RQ biosynthesis, we determined the complete genome sequence of a mutant strain of R. rubrum (F11), which cannot grow anaerobically and does not synthesize RQ, and compared it with that of a spontaneous revertant (RF111). RF111 can grow anaerobically and has recovered the ability to synthesize RQ. The two strains differ by a single base pair, which causes a nonsense mutation in the putative methyltransferase gene rquA. To test whether this mutation is important for the F11 phenotype, the wild-type rquA gene was cloned into the pRK404E1 vector and conjugated into F11. Complementation of the anaerobic growth defect in F11 was observed, and liquid chromatography-time of flight mass spectrometry (LC-TOF-MS) analysis of lipid extracts confirmed that plasmid-complemented F11 was able to synthesize RQ. To further validate the requirement of rquA for RQ biosynthesis, we generated a deletion mutant from wild-type R. rubrum by the targeted replacement of rquA with a gentamicin resistance cassette. The ΔrquA mutant exhibited the same phenotype as that of F11. These results are significant because rquA is the first gene to be discovered that is required for RQ biosynthesis.
INTRODUCTION
Rhodoquinone (RQ) (Fig. 1, compound 1) is found in the mitochondrial membrane of parasitic helminths (43, 46) and other eukaryotic species capable of fumarate reduction, such as Euglena gracilis (17) and Caenorhabditis elegans (41). These species can adapt their metabolism to both aerobic and anaerobic conditions throughout their life cycle. Adult parasitic species such as Ascaris suum, Fasciola hepatica, and Haemonchus contortus rely heavily on fumarate reduction for their energy generation while inside a host organism, where the oxygen tension is very low (20, 45, 48). Under these conditions, the biosynthesis of RQ is upregulated; however, during free-living stages of their life cycle, the helminth parasites use primarily aerobic respiration, which requires ubiquinone (Q) (Fig. 1, compound 2) (20, 44, 48). The anaerobic energy metabolism of the helminths was reviewed previously (43, 46). Humans and other mammalian hosts use Q for aerobic energy metabolism but do not produce or require RQ; therefore, the discovery of molecules that selectively inhibit RQ biosynthesis may lead to highly specific antihelminthic therapeutics that do not have a toxic effect on the host (24).
Fig 1.
Proposed biosynthetic pathway of RQ (compound 1). Known Coq (from yeast) and Ubi (from E. coli) gene products required for the biosynthesis of Q (compound 2) are labeled. The pathways for yeast and E. coli both utilize p-hydroxybenzoic acid (compound 6) as an early precursor and merge again at the common intermediate (compound 8). For R. rubrum, we have shown that Q is a precursor to RQ. The number of isoprene units (n) varies by species (S. cerevisiae [yeast], n = 6; E. coli, n = 8; C. elegans and E. gracilis, n = 9; helminth parasites, n = 9 or 10; R. rubrum, n = 10; humans, n = 10). RQ is not found in yeast, E. coli, or humans.
The pathway of RQ biosynthesis has not been completely elucidated, and enzymes specifically required for RQ synthesis still must be identified. RQ is structurally similar to Q, an important lipid component involved in the aerobic respiratory chain (5, 40); however, they differ considerably in their redox potentials (−63 mV for RQ and +100 mV for Q) (1). RQ has an amino group on the benzoquinone ring in place of a methoxy group in Q. Rhodospirillum rubrum is an excellent model organism for the investigation of RQ biosynthesis. R. rubrum produces both RQ and Q and can survive in the presence or absence of oxygen by using a variety of metabolic substrates (19, 47, 49). Under anaerobic conditions, R. rubrum can grow photoheterotrophically in the light or by fermentation and respiratory pathways in the dark (9, 37). R. rubrum has been shown to exhibit fumarate reductase activity, and RQ has been proposed to function in fumarate reduction to maintain NAD+/NADH redox balance (9, 16). Rhodoquinol-fumarate activity was also recently reported for Rhodoferax fermentans, a genetically similar phototrophic purple bacterium (28). The complete genome of R. rubrum (ATCC 11170) was sequenced in 2005 (34). The 16S rRNA sequence of R. rubrum shows a high level of sequence similarity to cognate eukaryotic mitochondrial sequences (29). Therefore, knowledge of the RQ biosynthetic pathway acquired from R. rubrum may be transferable to eukaryotic species.
We recently showed that Q is a biosynthetic precursor to RQ in R. rubrum (4). The proposed pathway for RQ biosynthesis, in conjunction with the known steps in Q biosynthesis, is outlined in Fig. 1 (4, 21, 23, 40). R. rubrum mutant strain F11 does not make RQ, but it still produces Q, suggesting that it is defective for an enzyme that is specifically required for RQ biosynthesis (8, 12–14). F11 was generated after the mutagenesis of wild-type (WT) R. rubrum with N-methyl-N′-nitro-N-nitrosoguanidine and was isolated for its inability to grow photosynthetically under anaerobic conditions (7). The identification of a causative mutation in F11 should shed light on the RQ biosynthetic defect, but finding such a mutation in a heavily mutagenized strain is challenging. However, F11 is known to revert spontaneously after extended periods of anaerobic exposure in the light (7). Revertants of F11 that have regained the ability to synthesize RQ and can grow photosynthetically under anaerobic conditions have been identified (32, 33; this work). The number of genetic differences between a revertant and F11 was predicted to be low. Therefore, to find the causative mutation in F11, we isolated a revertant, RF111, which had the restored ability to grow anaerobically and to synthesize RQ. We sequenced the whole genomes of both F11 and RF111 to identify their differences. In this work, we identify a single-base-pair difference between these strains located in the rquA gene, we test whether the mutation in rquA is important for the F11 phenotype, and we reproduce the mutant phenotype in wild-type strain ATCC 11170 by deleting rquA. Our results demonstrate that we have identified the first gene known to be specifically required for RQ biosynthesis.
MATERIALS AND METHODS
Bacterial strains and growth.
Wild-type (WT) R. rubrum (ATCC 11170) was obtained from the ATCC (Manassas, VA). F11 was obtained from Juan Ramírez (Centro de Investigaciones Biológicas, CSIC, Madrid, Spain). Revertant strain RF111 was isolated from a liquid culture of F11 incubated under anaerobic conditions in the light for 16 days. R. rubrum strains were grown at 32°C in yeast extract-supplemented malate-ammonium-rich (SMN) medium (11) supplemented with nalidixic acid (Nx) (20 μg/ml). Anaerobic cultures were grown under light in glass screw-top centrifuge tubes for 4 to 6 days, as previously described (4). Aerobic cultures were grown in the dark with shaking at 200 rpm for 2 to 3 days.
Whole-genome sequencing of F11 and RF111.
Genomic DNA was isolated from F11 and RF111 by using a Purelink genomic DNA minikit (Invitrogen, Carlsbad, CA). DNA sequencing was carried out at the High Throughput Genomics Center, Department of Genome Sciences, University of Washington, Seattle, WA. The sequencing was performed with an Illumina Genome Analyzer IIx, with a 36-bp read length and one lane per sample. F11 and RF111 were sequenced twice independently using separate lanes and compared with the wild-type reference genome (ATCC 11170). The mean read depth was 72×. The data were analyzed with CASAVA (GAPipeline-1.3.2), and single-nucleotide polymorphisms detected in 10 or more separate reads were considered to be of high quality. The finished F11 sequence was deposited in the NCBI database.
Cloning of the putative methyltransferase from R. rubrum.
In the R. rubrum genome, there is a 346-bp gap between rquA and the gene upstream, which is predicted to contain a promoter, based on its propensity to undergo stress-induced duplex destabilization (3), and a 278-bp gap downstream of the next gene, which is oriented in the same direction. To construct plasmid pJRQ1, a 1.3-kb fragment containing rquA, including 374 bp of upstream sequence and 146 bp of downstream sequence, was amplified by Phusion high-fidelity PCR (New England BioLabs, Ipswich, MA) from WT R. rubrum genomic DNA using oligonucleotide primers with 5′ tails that contained BamHI or EcoRI restriction sites (5′-GCG GAT CCC TGG GCC GGG ACC TTA GCG T-3′ and 5′-GCG AAT TCG GGC CCA TGC GCT TTA TCA GGG-3′ [restriction sites are underlined]). The PCR amplicon was digested with BamHI and EcoRI and ligated into the BamHI and EcoRI sites in plasmid pRK404E1, a derivative of the broad-host-range vector RK2, which confers tetracycline resistance (35, 38). One round of Sanger sequencing (Clemson University Genomics Institute, Clemson, SC) from each direction confirmed the integrity of the rquA clone.
Transconjugation of R. rubrum and complementation of F11.
Plasmids pRK404E1 and pJRQ1 were transformed into Escherichia coli S17-1, which contains the chromosomal genes necessary for conjugation with R. rubrum (39). These plasmids were then conjugated into WT and F11 R. rubrum cells, which were plated onto selective medium containing tetracycline and nalidixic acid, as described previously (25). The conjugation efficiencies were 103 to 104 transconjugants per 1 ml of culture. SMN medium containing tetracycline was prepared without the addition of MgSO4, which chelates and inactivates the antibiotic (36). Tetracycline-resistant (Tcr) colonies were inoculated into lighted anaerobic cultures without antibiotics to test for the pJRQ1-dependent anaerobic rescue of F11, as tetracycline degrades into a toxic product when exposed to light (27). Following anaerobic growth without antibiotics, rescued F11 (F11::pJRQ1) was streaked onto selective medium to confirm plasmid DNA retention. DNA was isolated from F11::pJRQ1, and rquA DNA was specifically amplified from genomic DNA by using oligonucleotides that bind further upstream and downstream of rquA than those used to construct pJRQ1 (5′-TGC CCC GCA GGC TGA ATG GA-3′ and 5′-ATC TCG GTG GCG TCG CAA GG-3′). The amplified DNA was used as a template for Sanger sequencing.
Construction of an R. rubrum rquA knockout mutant.
Two 0.9-kb fragments, immediately 5′ and 3′ of rquA, were separately amplified by high-fidelity PCR from wild-type R. rubrum genomic DNA using oligonucleotide primers with 5′ tails containing restriction sites (5′-AAA CCC GTC GAC CGG GCG AAA TCG GCT TTT GAG-3′ and 5′-AGT ACT CGA CTG CCC AAT TGG CAG CAA TCC CTT TTC CGA CGG-3′, and 5′-CAA TTG GGC AGT CGA GTA CTA GTC GAG GGA CAA ATC GCG CA-3′ and 5′-TTT CCC CAT ATG GGT CGA TCT GGT CAT GCC CGC-3′ [restriction sites are underlined]). The amplicons were combined by using fusion PCR, resulting in a 2.1-kb product with ScaI and MfeI restriction sites within the fusion overlap and NdeI and SalI restriction sites at the 5′ and 3′ ends, respectively. This fusion product was digested with NdeI and SalI, which produced a 1.8-kb fragment due to an interior SalI site 300 bp from the 3′ end. This fragment was ligated into the NdeI and SalI sites of pUX19, a kanamycin-resistant (Kmr) suicide vector (50), forming plasmid pJRQ2. A gentamicin resistance (Gmr) cassette was amplified from plasmid pUCGM (51) by using oligonucleotide primers with 5′ tails that contained ScaI and MfeI restriction sites (5′-ATG CTT CAA TTG ACG TTG TAA AAC GAC GGC CAG TG-3′ and 5′-AGA ACC AGT ACT TCA CTC ATT AGG CAC CCC AGG C-3′ [restriction sites are underlined]). This was digested and ligated into the ScaI and MfeI sites of the fusion product within pJRQ2, forming Kmr Gmr suicide vector pJRQ3. Plasmid pJRQ3 was transformed into E. coli S17-1 cells and conjugated into wild-type R. rubrum cells (25). Nxr Gmr colonies were replica plated to identify Km-sensitive colonies that underwent a double-crossover recombination event to form the ATCC 11170::ΔrquA mutant. The putative deletion mutant colonies were grown in liquid cultures under aerobic and anaerobic conditions to observe the knockout phenotype. Sanger sequencing of the flanking regions of the Gmr cassette in genomic DNA from the ATCC 11170::ΔrquA strain confirmed the absence of rquA in the mutant.
Lipid extraction of cells.
Lipid extractions were performed on 0.2- to 0.4-g cell pellets of WT (aerobic and anaerobic), F11 (aerobic), F11::pJRQ1 (aerobic and anaerobic), and ATCC 11170::ΔrquA (aerobic) strains of R. rubrum as previously described (4). Dried extracts were resuspended in 100 μl of an 80:20 dilution of ethanol-hexane and further diluted (1:50 to 1:100) with ethanol such that concentrations of all extracts were normalized to 40 mg original wet pellet weight/ml ethanol.
LC-TOF-MS analysis of quinones.
Quinone separation was accomplished with an ultraperformance liquid chromatography (UPLC) system (Waters Acquity; Waters Corporation, Milford, MA), which was maintained at 4°C. Chromatography was performed by using a Luna RP-C18 column (3 μm, 50 by 3 mm; Phenomenex, Torrance, CA). All UPLC runs used a flow rate of 0.25 ml/min and an injection volume of 5 μl. All injections were performed in duplicate. RQ10 and Q10 were eluted between 7 and 7.5 min by using a gradient system containing water with 0.1% formic acid (buffer A) and acetonitrile with 0.1% formic acid (buffer B). The water and acetonitrile used were liquid chromatography-mass spectrometry (LC-MS)-grade Optima (Fisher Scientific, Pittsburgh, PA), and the formic acid was >99% packaged in sealed 1-ml ampoules (Thermo-Scientific, Rockford, IL). The gradient (buffer A-buffer B) method used was as follows: 0 to 3 min (30:70 dilution), 3 to 3.25 min (30:70 to 2:98), 3.25 to 5 min (2:98), 5 to 6 min (2:98 to 1:99), 6 to 8.75 min (1:99), 8.75 to 9 min (1:99 to 30:70), and 9 to 10 min (30:70). Mass spectral analysis was accomplished by use of a time of flight (TOF) mass spectrometer (Waters Premier XE; Waters Corporation, Milford, MA) in W-positive mode with an extended dynamic range. Mass Lynx V. 4.1 software was used for data acquisition and processing. All samples were analyzed for the presence of RQ10 (exact mass of 848.6921 for [M + H]+) and Q10 (exact masses of 863.6917 for [M + H]+ and 885.6736 for [M + Na]+). The following global conditions were used for analyses of all samples: a capillary voltage of 3,000 V, a sample cone voltage of 50 V, a desolvation temperature of 350°C, a source temperature of 130°C, a desolvation gas flow rate of N2 of 800 liters/h, and a cone gas flow rate of N2 of 50 liters/h. A 200-pg/μl leucine enkephalin lock mass standard was infused during data acquisition.
Nucleotide sequence accession number.
The finished F11 sequence was deposited in the NCBI database under GenBank accession number CP003046.
RESULTS AND DISCUSSION
Genetic evidence from early work performed by Del Valle-Tascón and coworkers suggested that a mutation in F11 occurred in a gene required for RQ biosynthesis, since a revertant, RF110, had regained the ability to produce RQ and to grow phototrophically in the absence of oxygen (7). Since RF110 was not available, we isolated another revertant, RF111, which has the same phenotype, including the ability to synthesize RQ. We expected that the RF111 genome would contain few sequence differences from its parent strain F11, and these differences may identify the causative mutation in F11 that prevents RQ biosynthesis.
Identification and sequence analysis of rquA.
The genomes of F11 and RF111 were sequenced and compared with WT reference strain ATCC 11170. Each of the genomes differed from the WT at about 75 positions. However, F11 differed from RF111 by only a single base pair, creating a nonsense mutation in the F11 gene Rru_A3227. The mutation, a G-to-A transition at position 3721692, converts a tryptophan codon at position 70 that is present in both the WT and RF111 to a premature stop codon in F11. This mutation occurs in the first one-third of the open reading frame of Rru_A3227. We hypothesize that this mutation is a significant cause of the F11 phenotype and that Rru_A3227 is required for the biosynthesis of RQ. We have therefore named the gene rquA, for rhodoquinone biosynthesis gene A.
The RquA protein shares sequence and predicted secondary structure similarities with class I S-adenosylmethionine (SAM)-dependent methyltransferases. Proteins of this class are not well conserved in primary structure, but they share a three-dimensional structure that includes a sheet of four parallel β-strands, within which four short signature motifs have been identified (30). When RquA is compared with experimentally confirmed methyltransferases, sequence similarity can be detected with the C- and O-methyltransferases UbiE/Coq5 and UbiG/Coq3, which are required for Q biosynthesis (2, 31). An alignment between RquA and these proteins shows that RquA shares a predicted secondary structure with methyltransferases and features within the signature motifs (Fig. 2). BLAST searches, however, made it clear that RquA is distinct from these methyltransferases and from the R. rubrum orthologs of UbiE and UbiG. RquA belongs to the orthologous protein cluster CLSK588563 (NCBI), which currently consists of 10 members, including a protein in Rhodoferax ferrireducens, a bacterium also known to synthesize RQ (10). The sequences of RquA and its orthologs conform well to the consensus methyltransferase signature motifs, except for some unusual substitutions in motif I (Fig. 3). In this motif, all members of the RquA cluster contain a glutamine in place of aspartate or glutamate (position 6 in Fig. 3), and most, including RquA, contain a valine in place of glycine (position 10 in Fig. 3) (30). Since motif I is involved in the binding of SAM, this raises the possibility that RquA does not bind SAM or that it binds SAM in a different way than other SAM-dependent methyltransferases do. We conclude that RquA has the structural appearance of a SAM-dependent methyltransferase, except that part of its SAM-binding motif does not conform to the consensus sequences of known SAM-binding motifs.
Fig 2.
Alignment of RquA with the SAM-dependent methyltransferase domains of four methyltransferases, showing the locations of predicted secondary structures and the methyltransferase signature motifs. The amino acid sequences of RquA of R. rubrum (R.r.), UbiE and UbiG of Escherichia coli (E.c.), and Coq5 and Coq3 of Saccharomyces cerevisiae (S.c.) were aligned with ClustalW (42). The secondary structures were predicted by PSIPRED (22) at the Ali2D server (http://toolkit.tuebingen.mpg.de/ali2d) and are displayed using C for random coil, E for β-sheet, and H for α-helix. The four methyltransferase signature motifs (boxes) were identified by using the secondary structure predictions and the consensus motifs and secondary structures described previously (30). Asterisks indicate positions that contact SAM in methyltransferases with known three-dimensional structures (30). RquA is 22 to 26% identical to these methyltransferases across this domain of ∼115 amino acids.
Fig 3.
Motif I sequence conservation among RquA orthologs compared with UbiE and UbiG orthologs in the same set of species. Amino acid conservation is depicted by using WebLogo (6). Boxes indicate the locations of signature motif I in each alignment, and asterisks indicate the positions in RquA orthologs that differ from the consensus signature motif I (30). Orthologs of RquA were identified as members of NCBI protein cluster CLSK588563 and were located in the following species: Aromatoleum aromaticum EbN1, Azoarcus sp. strain BH72, “Candidatus Accumulibacter phosphatis” clade IIA strain UW-1, Chromobacterium violaceum ATCC 12472, Laribacter hongkongensis HLHK9, Leptothrix cholodnii SP-6, Magnetococcus sp. strain MC-1, Methylocella silvestris BL2, Rhodoferax ferrireducens T118, and Rhodospirillum rubrum ATCC 11170. Orthologs of the E. coli UbiE and UbiG genes were identified in this set of species as the highest-scoring hits using BLASTp. The sequences were aligned by using ClustalW (42) to identify motif I.
Complementation of the F11 mutant phenotype.
In F11, the nonsense mutation in rquA should truncate the protein 20 amino acids upstream of motif I, severely interfering with its structure and function. The mutation may also have a polar effect on the downstream gene if rquA and the downstream gene are transcribed together. The gene is located 278 bp downstream of rquA and is oriented in the same direction. If a loss of the function of rquA is the cause of the F11 mutant phenotype, then the expression of a wild-type copy of rquA in F11 should complement F11, restoring RQ biosynthesis and anaerobic respiration. To test this, rquA was cloned into plasmid pJRQ1 and was transformed into E. coli strain S17-1, which can undergo conjugation with R. rubrum. S17-1 strains carrying pJRQ1 or an empty vector were conjugated to F11 and WT R. rubrum, and transconjugants that were resistant to both tetracycline (which selects for the plasmid) and nalidixic acid (which selects for R. rubrum) were selected. To assess anaerobic growth capabilities, colonies of F11 and the WT (with and without each plasmid) were inoculated into SMN medium without antibiotics and grown anaerobically in the light for 7 days. Phenotypic rescue was observed for F11::pJRQ1, as evidenced by its restored ability for anaerobic growth, whereas F11 complemented with the empty vector did not grow. The WT grew in the presence of either plasmid. All anaerobic cultures containing bacterial growth were streaked onto selective plates to verify that plasmid DNA was still in the strains. The plasmid from F11::pJRQ1 was isolated and digested with diagnostic restriction enzymes to confirm that the rquA insert was still present. Additionally, chromosomal rquA DNA of F11::pJRQ1 was sequenced, and the original mutation was still present. We conclude that the phenotypic rescue of F11 by the rquA plasmid was due to complementation and not due to reversion or recombination with the plasmid.
Deletion of rquA from the WT.
To further establish the importance of rquA in RQ synthesis and anaerobic growth and to eliminate the possibility that the F11 phenotype results from a combination of background mutations, we created a de novo deletion of rquA in a strain with a different genetic background, WT strain ATCC 11170. A Kmr Gmr suicide plasmid, pJRQ3, was constructed to contain a Gmr cassette in between two 0.9-kb fragments of DNA that flank the rquA gene. E. coli S17-1 was transformed with pJRQ3 and then conjugated with WT R. rubrum. Colonies with resistance to Nx and Gm, but not Km, were chosen for growth experiments. Colonies that retained Km resistance were assumed to have undergone a single-crossover event, which is indicative of the genomic incorporation of the whole plasmid, rather than the desired double-crossover recombination event. Growth assays were performed with the ATCC 11170::ΔrquA strain under both anaerobic and aerobic conditions, and growth was compared with those of the WT and F11. The deletion mutant shares the same phenotype as F11 and is incapable of anaerobic photoheterotrophic growth. We conclude that rquA is required for such anaerobic growth.
Detection of RQ and Q in lipid extracts.
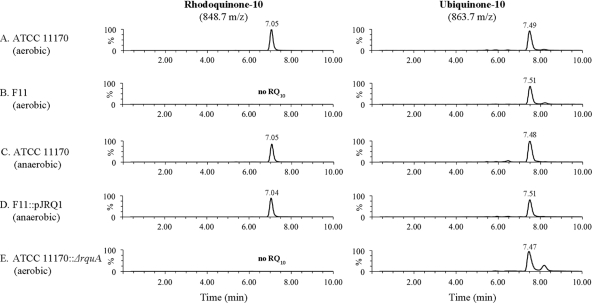
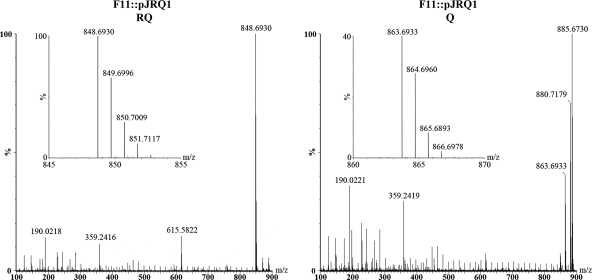
Lipid extracts from WT (ATCC 11170), F11, F11::pJRQ1, and ATCC 11170::ΔrquA cultures were analyzed by LC-TOF-MS to identify Q10 and RQ10 synthesized in vivo. The LC-MS chromatograms showed retention times of 7.5 min for Q10 and 7.0 min for RQ10. All samples contained 3 to 4 pmol Q10/mg wet pellet weight based on an external Q10 standard. The presence of Q10 in all samples functioned as a positive control by demonstrating that the lipid extraction successfully isolated polyprenylated quinones from the cells. RQ10 was found in the WT under both aerobic and anaerobic growth conditions (Fig. 4A and C) and in F11::pJRQ1 (Fig. 4D). The experimental mass of [RQ10 + H]+ in F11::pJRQ1 was found to be m/z 848.6930, which is within 1 ppm of the calculated exact mass (848.6921 atomic mass units [amu]) (Fig. 5). There was no RQ10 detected in F11 (Fig. 4B) or the ATCC 11170::ΔrquA mutant (Fig. 4E). A mass spectrum of Q10 from F11::pJRQ1 was also acquired for comparison. Both the [Q10 + H]+ and [Q10 + Na]+ parent ions were observed. The experimental mass for [Q10 + H]+ was determined to be m/z 863.6933, which is <2 ppm of the calculated exact mass (863.6917 amu) (Fig. 5), and that of [Q10 + Na]+ was m/z 885.6730, which is within 1 ppm of the exact mass (885.6736 amu) (Fig. 5). A small unidentified peak with a slightly longer retention time than that of Q10 appeared in all extracted ion chromatograms (m/z 863.7) from aerobic cultures; however, it was not present in anaerobic cultures and does not appear to be correlated to RQ levels. The LC-MS-TOF results confirm that RQ10 is synthesized in pJRQ1-complemented F11. The data definitively show that the rquA gene is required for RQ biosynthesis, as both F11 and the ΔrquA mutant were unable to synthesize RQ10.
Fig 4.
LC-MS chromatograms of R. rubrum lipid extracts. Extracted ion chromatograms are shown for [RQ10 + H]+ (m/z 848.7) (left column) and [Q10 + H]+ (m/z 863.7) (right column) in five R. rubrum samples (A to E). The retention times of RQ10 and Q10 were approximately 7 and 7.5 min, respectively, under LC conditions outlined in Materials and Methods. (A) ATCC 11170 (WT R. rubrum, grown aerobically); (B) F11 (RQ-deficient R. rubrum mutant, grown aerobically); (C) ATCC 11170 (WT R. rubrum, grown anaerobically); (D) F11::pJRQ1 (pJRQ1-complemented F11, grown anaerobically); (E) ATCC 11170::ΔrquA mutant (knockout mutant, grown aerobically). No RQ10 was found in either F11 or the ATCC 11170::ΔrquA strain.
Fig 5.
Mass spectra of RQ10 and Q10 in F11::pJRQ1. The left spectrum shows [RQ10 + H]+ at m/z 848.6930 (exact mass, 848.6921 amu). The right spectrum shows [Q10 + H]+ at m/z 863.6933 (exact mass, 863.6917 amu) and [Q10 + Na]+ at m/z 885.6730 (exact mass, 885.6736 amu).
Potential roles of RquA in RQ biosynthesis.
We have shown that rquA is required for RQ biosynthesis; however, its specific role is not yet known. RquA shares sequence similarity with other known methyltransferases. The region downstream of motif II is known to be involved in substrate binding (30), and this region is rich in hydrophobic residues in the methyltransferases involved in Q biosynthesis (UbiE and UbiG) as well as in RquA and its orthologs. Since UbiE and UbiG bind prenylated quinones (21, 23), it is likely that RquA also binds similar substrates. However, the SAM-binding motif (motif I) in RquA has unusual substitutions, and it does not resemble other known SAM-binding motifs. This observation, as well as the unlikely requirement of a methyltransferase for the conversion of Q to RQ, leaves the function of RquA uncertain.
One potential role for RquA as a methyltransferase is to produce an alternative pool of Q from demethyl ubiquinone (DMeQ) (11) (Fig. 1) that is used strictly for RQ biosynthesis. RquA may function in conjunction with an amino- or amidotransferase and other enzymes as part of a multicomponent complex for RQ biosynthesis that cannot function without an intact RquA. The inability of F11 to produce an alternate pool of Q may account for the defect in RQ biosynthesis if RquA is a required component of an RQ biosynthetic complex. A similar complex has been identified in Saccharomyces cerevisiae, where it was observed that the Coq3, Coq4, Coq5, Coq6, Coq7, and Coq9 polypeptides are required to interact as part of a Q biosynthetic complex (2, 15, 18, 26). A second possibility is that RquA evolved from a methyltransferase and is now used for an alternate function, such as demethylation or amino transfer, or it is required for the function or expression of RQ biosynthetic enzymes but is not involved directly in the enzymatic synthesis of RQ.
We plan to investigate whether RquA is associated with any other membrane proteins in order to test these hypotheses and identify other putative RQ biosynthetic enzymes. The heterologous expression of RquA in E. coli and characterization of its methyltransferase activity are under way in our laboratories. The identification of other enzymes involved in RQ biosynthesis in R. rubrum is also being pursued, using methods that include the bioinformatic screening of amino- and amidotransferase candidates and the preparation of deletion mutants. An RquA homolog has not been found in any of the sequenced helminth parasites; however, there are homologs in other RQ-producing bacterial species (e.g., R. ferrireducens and R. fermentans). While the RQ biosynthetic pathways may not be identical between R. rubrum and the helminth parasites, it is likely that there are some common proteins shared between these organisms. This work has laid the foundation for the further discovery of genes required for RQ biosynthesis in R. rubrum, which may ultimately be applicable to the helminth parasites.
ACKNOWLEDGMENTS
This research was supported in part by grants from the National Institutes of Health (award no. 1R15GM096398-01), the Howard Hughes Medical Institute through the Undergraduate Science Education Program (award to Gonzaga University), and the National Science Foundation CRIF-MU program (award no. CHE-0741868).
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation or National Institutes of Health.
We also thank Juan Ramírez (Centro de Investigaciones Biológicas, CSIC, Madrid, Spain) for the donation of the R. rubrum F11 mutant; Gary Roberts and Yaoping Zhang (University of Wisconsin, Madison) for assistance with conjugation protocols and the donation of pRK404E1, pUCGM, and S17-1; Christy Watson for advice on plasmid construction and molecular biology protocols; Steve Clarke (UCLA) for assistance in identifying methyltransferase motifs in RquA; and Catherine Clarke (UCLA) for consultation and proofreading of the manuscript.
Footnotes
Published ahead of print 22 December 2011
REFERENCES
- 1. Ackrell BAC, Johnson MK, Gunsalus RP, Cecchini G. 1992. Structure and function of succinate dehydrogenase and fumarate reductase, p 229–297 In Muller F. (ed), Chemistry and biochemistry of flavoenzymes, vol 3 CRC Press, Boca Raton, FL [Google Scholar]
- 2. Baba SW, et al. 2004. Yeast Coq5 C-methyltransferase is required for stability of other polypeptides involved in coenzyme Q biosynthesis. J. Biol. Chem. 279:10052–10059 [DOI] [PubMed] [Google Scholar]
- 3. Bi C-P, Benham CJ. 2004. WebSIDD: server for predicting stress-induced duplex destabilized (SIDD) sites in superhelical DNA. Bioinformatics 20:1477–1479 [DOI] [PubMed] [Google Scholar]
- 4. Brajcich BC, et al. 2010. Evidence that ubiquinone is a required intermediate for rhodoquinone biosynthesis in Rhodospirillum rubrum. J. Bacteriol. 192:436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Collins MD, Jones D. 1981. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implications. Microbiol. Rev. 45:316–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crooks GE, Hon G, Chandonia JM, Brenner SE. 2001. WebLogo: a sequence logo generator. Genome Res. 14:1188–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Del Valle-Tascón S, Gimenéz-Gallego G, Ramírez JM. 1975. Light-dependent ATP formation in a non-phototrophic mutant of Rhodospirillum rubrum deficient in oxygen photo reduction. Biochem. Biophys. Res. Commun. 66:514–519 [DOI] [PubMed] [Google Scholar]
- 8. Del Valle-Tascón S, Gimenéz-Gallego G, Ramírez JM. 1977. Photooxidase system of Rhodospirillum rubrum. I. Photooxidations catalyzed by chromatophores isolated from a mutant deficient in photooxidase activity. Biochim. Biophys. Acta 459:76–87 [DOI] [PubMed] [Google Scholar]
- 9. Ferguson SJ, Jackson JB, McEwan AG. 1987. Anaerobic respiration in the Rhodospirillaceae: characterisation of pathways and evaluation of roles in redox balancing during photosynthesis. FEMS Microbiol. Rev. 46:117–143 [Google Scholar]
- 10. Finneran KT, Johnsen CV, Lovley DR. 2003. Rhodoferax ferrireducens sp. nov., a psychrotolerant, facultatively anaerobic bacterium that oxidizes acetate with the reduction of Fe(III). Int. J. Syst. Evol. Microbiol. 53:669–673 [DOI] [PubMed] [Google Scholar]
- 11. Fitzmaurice WP, Saari LL, Lowery RG, Ludden PW, Roberts GP. 1989. Genes coding for the reversible ADP-ribosylation system of dinitrogenase reductase from Rhodospirillum rubrum. Mol. Gen. Genet. 218:340–347 [DOI] [PubMed] [Google Scholar]
- 12. Gimenéz-Gallego G, Del Valle-Tascón S, Ramírez JM. 1976. A possible physiological function of the oxygen-photoreducing system of Rhodospirillum rubrum. Arch. Microbiol. 109:119–125 [DOI] [PubMed] [Google Scholar]
- 13. Gimenéz-Gallego G, Del Valle-Tascón S, Ramírez JM. 1978. Photooxidase system of Rhodospirillum rubrum. II. Its role in the regulation of cyclic-photophosphorylation. Z. Pflanzenphysiol. 87:25–36 [Google Scholar]
- 14. Gimenéz-Gallego G, Ramírez-Ponce MP, Lauzurica P, Ramírez JM. 1982. Photooxidase system of Rhodospirillum rubrum. III. The role of rhodoquinone and ubiquinone in the activity preparations of chromatophores and reaction centers. Eur. J. Biochem. 121:343–347 [DOI] [PubMed] [Google Scholar]
- 15. Gin P, Clarke CF. 2005. Genetic evidence for a multi-subunit complex in coenzyme Q biosynthesis in yeast and the role of the Coq1 hexaprenyl diphosphate synthase. J. Biol. Chem. 280:2676–2681 [DOI] [PubMed] [Google Scholar]
- 16. Hiraishi A. 1988. Fumarate reduction systems in members of the family Rhodospirillaceae with different quinone types. Arch. Microbiol. 150:56–60 [Google Scholar]
- 17. Hoffmeister M, et al. 2004. Euglena gracilis rhodoquinone:ubiquinone ratio and mitochondrial proteome differ under aerobic and anaerobic conditions. J. Biol. Chem. 279:22422–22429 [DOI] [PubMed] [Google Scholar]
- 18. Hsieh EJ, et al. 2007. Saccharomyces cerevisiae Coq9 polypeptide is a subunit of the mitochondrial coenzyme Q biosynthetic complex. Arch. Biochem. Biophys. 463:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Imhoff JF. 1988. Anoxygenic phototrophic bacteria, p 207–240 In Austin B. (ed), Methods in aquatic bacteriology. Wiley & Sons, Ltd., Chichester, United Kingdom [Google Scholar]
- 20. Iwata F, et al. 2008. Change of subunit composition of mitochondrial complex II (succinate-ubiquinone reductase/quinol-fumarate reductase) in Ascaris suum during migration in the experimental host. Parasitol. Int. 57:54–61 [DOI] [PubMed] [Google Scholar]
- 21. Jonassen T, Clarke CF. 2000. Genetic analysis of coenzyme Q biosynthesis, p 185–208 In Kagan VE, Quinn PJ. (ed), Coenzyme Q: from molecular mechanisms to nutrition and health. CRC Press, Boca Raton, FL [Google Scholar]
- 22. Jones DT. 1999. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 292:195–202 [DOI] [PubMed] [Google Scholar]
- 23. Kawamukai M. 2009. Biosynthesis and bioproduction of coenzyme Q10 by yeasts and other organisms. Biotechnol. Appl. Biochem. 53:217–226 [DOI] [PubMed] [Google Scholar]
- 24. Kita K, Miyadera H, Saruta F, Miyoshi H. 2001. Parasite mitochondria as a target for chemotherapy. J. Health Sci. 47:219–239 [Google Scholar]
- 25. Liang JH, et al. 1991. Mutations in the draT and draG genes of Rhodospirillum rubrum result in loss of regulation of nitrogenase by reversible ADP-ribosylation. J. Bacteriol. 173:6903–6909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marbois B, et al. 2005. Coq3 and Coq4 define a polypeptide complex in yeast mitochondria for the biosynthesis of coenzyme Q. J. Biol. Chem. 280:20231–20238 [DOI] [PubMed] [Google Scholar]
- 27. Mather MW, McReynolds LM, Yu C-A. 1995. An enhanced broad-host-range vector for Gram-negative bacteria: avoiding tetracycline phototoxicity during the growth of photosynthetic bacteria. Gene 156:85–88 [DOI] [PubMed] [Google Scholar]
- 28. Miyadera H, et al. 2003. Complex II from phototrophic purple bacterium Rhodoferax fermentans displays rhodoquinol-fumarate reductase activity. Eur. J. Biochem. 270:1863–1874 [DOI] [PubMed] [Google Scholar]
- 29. Ochman H, Wilson AC. 1987. Evolutionary history of enteric bacteria, p 1649–1654 In Neidhardt FC, et al. (ed), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington, DC [Google Scholar]
- 30. Petrossian TC, Clarke SG. 2009. Multiple motif scanning to identify methyltransferases from the yeast proteome. Mol. Cell. Proteomics 8:1516–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poon WP, et al. 1999. Yeast and rat Coq3 and Escherichia coli UbiG polypeptides catalyze both O-methyltransferase steps in coenzyme Q biosynthesis. J. Biol. Chem. 274:21665–21672 [DOI] [PubMed] [Google Scholar]
- 32. Ramírez-Ponce MP, Gimenéz-Gallego G, Ramírez JM. 1980. A specific role for rhodoquinone in the photosynthetic electron-transfer system of Rhodospirillum rubrum. FEBS Lett. 114:319–322 [Google Scholar]
- 33. Ramírez-Ponce MP, Ramírez JM, Gimenéz-Gallego G. 1980. Rhodoquinone as a constituent of the dark electron-transfer system of Rhodospirillum rubrum. FEBS Lett. 119:137–140 [Google Scholar]
- 34. Reslewic S, et al. 2005. Whole-genome shotgun optical mapping of Rhodospirillum rubrum. Appl. Environ. Microbiol. 71:5511–5522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saegesser R, Ghosh R, Bachofen R. 1992. Stability of broad host cloning vectors in the phototrophic bacterium Rhodospirillum rubrum. FEMS Microbiol. Lett. 95:7–11 [Google Scholar]
- 36. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed, p A2.6 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 37. Schultz JE, Weaver PF. 1982. Fermentation and anaerobic respiration by Rhodospirillum rubrum and Rhodopseudomonas capsulata. J. Bacteriol. 149:181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scott HN, Laible PD, Hanson DK. 2003. Sequences of versatile broad-host-range vectors of the RK2 family. Plasmid 50:74–79 [DOI] [PubMed] [Google Scholar]
- 39. Simon R, Priefer UB, Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Biotechnology 1:784–791 [Google Scholar]
- 40. Soballe B, Poole PK. 1999. Microbial ubiquinones: multiple roles in respiration, gene regulation and oxidative stress management. Microbiology 145:1817–1830 [DOI] [PubMed] [Google Scholar]
- 41. Takamiya S, et al. 1999. Free-living nematodes Caenorhabditis elegans possess in their mitochondria an additional rhodoquinone, an essential component of the eukaryotic fumarate reductase system. Arch. Biochem. Biophys. 371:284–289 [DOI] [PubMed] [Google Scholar]
- 42. Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tielens AGM. 1994. Energy generation in parasitic helminths. Parasitol. Today 10:346–352 [DOI] [PubMed] [Google Scholar]
- 44. Van Hellemond JJ, Klockiewicz M, Gaasenbeek CPH, Roos MH, Tielens AGM. 1995. Rhodoquinone and complex II of the electron transport chain in anaerobically functioning eukaryotes. J. Biol. Chem. 270:31065–31070 [DOI] [PubMed] [Google Scholar]
- 45. Van Hellemond J, Luijten M, Flesch F, Gaasenbeek C, Tielens A. 1996. Rhodoquinone is synthesized de novo by Fasciola hepatica. Mol. Biochem. Parasitol. 82:217–226 [DOI] [PubMed] [Google Scholar]
- 46. Van Hellemond JJ, Van der Klei A, Van Weelden SWH, Tielens AGM. 2003. Biochemical and evolutionary aspects of anaerobically functioning mitochondria. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358:205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Verméglio A. 1995. Organization of electron transfer components and supercomplexes, p 279–295 In Blankenship RE, Madigan MT, Bauer CE. (ed), Anoxygenic photosynthetic bacteria. Kluwer Academic Publishers, Dordrecht, Netherlands [Google Scholar]
- 48. Yamashita T, et al. 2004. Rhodoquinone reaction site of the mitochondrial complex I, in parasitic helminth, Ascaris suum. Biochim. Biophys. Acta 1608:97–103 [DOI] [PubMed] [Google Scholar]
- 49. Zannoni D. 1995. Aerobic and anaerobic electron transport in anoxygenic phototrophic bacteria, p 949–971 In Blankenship RE, Madigan MT, Bauer CE. (ed), Anoxygenic photosynthetic bacteria. Kluwer Academic Publishers, Dordrecht, Netherlands [Google Scholar]
- 50. Zhang Y, Pohlman EL, Ludden PW, Roberts GP. 2000. Mutagenesis and functional characterization of the glnB, glnA, and nifA genes from the photosynthetic bacterium Rhodospirillum rubrum. J. Bacteriol. 182:983–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang Y, Pohlmann EL, Roberts GP. 2005. GlnD is essential for NifA activation, NtrB/NtrC-regulated gene expression, and posttranslational regulation of nitrogenase activity in the photosynthetic, nitrogen-fixing bacterium Rhodospirillum rubrum. J. Bacteriol. 187:1254–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]