Abstract
Ignicoccus hospitalis, a hyperthermophilic, chemolithoautotrophic crenarchaeon was found to possess a new CO2 fixation pathway, the dicarboxylate/4-hydroxybutyrate cycle. The primary acceptor molecule for this pathway is acetyl coenzyme A (acetyl-CoA), which is regenerated in the cycle via the characteristic intermediate 4-hydroxybutyrate. In the presence of acetate, acetyl-CoA can alternatively be formed in a one-step mechanism via an AMP-forming acetyl-CoA synthetase (ACS). This enzyme was identified after membrane preparation by two-dimensional native PAGE/SDS-PAGE, followed by matrix-assisted laser desorption ionization–time of flight tandem mass spectrometry and N-terminal sequencing. The ACS of I. hospitalis exhibits a molecular mass of ∼690 kDa with a monomeric molecular mass of 77 kDa. Activity tests on isolated membranes and bioinformatic analyses indicated that the ACS is a constitutive membrane-associated (but not an integral) protein complex. Unexpectedly, immunolabeling on cells of I. hospitalis and other described Ignicoccus species revealed that the ACS is localized at the outermost membrane. This perfectly coincides with recent results that the ATP synthase and the H2:sulfur oxidoreductase complexes are also located in the outermost membrane of I. hospitalis. These results imply that the intermembrane compartment of I. hospitalis is not only the site of ATP synthesis but may also be involved in the primary steps of CO2 fixation.
INTRODUCTION
All members of the genus Ignicoccus are anaerobic hyperthermophiles that grow strictly chemolithoautotrophically by reduction of elemental sulfur using molecular hydrogen as an electron donor (22, 45). In contrast to all other known Archaea, they possess a highly unusual cell envelope. It lacks an S-layer but consists of two membranes, an “inner” (cytoplasmic) and an “outer” membrane (43, 48), forming a “periplasmic space” of variable width from 20 up to 1,000 nm (34, 45). In contrast to the “inner” membrane, the “outer” membrane of I. hospitalis lacks caldarchaeol core lipids (28) and contains multiple copies of a pore-forming complex, Ihomp1, a homo-oligomer of 6-kDa α-helical monomers (12). Furthermore, several proteins necessary for transport and metabolism were identified (13). These proteins may be necessary for the specific interaction of I. hospitalis with Nanoarchaeum equitans. These two organisms form an “intimate association,” the only known cultivable coculture of two Archaea (23, 30). Recently, an important and highly unexpected feature of I. hospitalis was described: its outermost membrane inhabits the primary (H2:sulfur oxidoreductase) and secondary (A1AO ATP synthase) proton pumps necessary for energy conservation and therefore must be energized (38). Simultaneously, it was shown that in I. hospitalis DNA and ribosomes are exclusively located inside its inner membrane, leading to a spatial separation of energy conservation and information processing. As a consequence, the compartment between the two membranes was renamed the “intermembrane compartment” (IMC) instead of the “periplasmic space” (38). It is the site of energy conservation and may contribute significantly to further metabolic activities of the cell.
I. hospitalis, as an obligate autotroph, is dependent on a high and constant supply with ATP for the formation of organic molecules from CO2. Remarkably, it uses a novel CO2 fixation pathway, the dicarboxylate/4-hydroxybutyrate cycle (24, 29). Acetyl coenzyme A (acetyl-CoA) acts as the primary acceptor molecule, while pyruvate synthetase and phosphoenolpyruvate (PEP) carboxylase are the CO2-fixing enzymes. Acetyl-CoA is regenerated via the characteristic intermediate 4-hydroxybutyrate. In these studies, I. hospitalis was shown to take up and incorporate low amounts of organic acids, such as acetate, pyruvate, or succinate (29). Up to 12% of its cell carbon was derived from these compounds. The assimilation of acetate requires an acetate-activating enzyme (in contrast to succinate or pyruvate). Two types of such reactions are known: a one-step mechanism catalyzed by the acetyl-CoA synthetase and a mechanism, usually present at acetate concentrations ≥30 mM in the environment, where two enzymes are involved, acetate kinase and phosphotransacetylase (62). This two-step mechanism has been found in several Bacteria (e.g., Escherichia coli, Corynebacterium glutamicum, and Azotobacter vinelandii [11, 49, 67, 70]) and a number of eukaryotic microbes (e.g., Chlamydomonas reinhardtii and Phytophthora sp. [2, 26]). However, in Archaea it was thus far only identified in Methanosarcina sp. (1). Although genes for the enzymes of the latter reaction were not detected in the I. hospitalis genome, two genes encoding for an acetyl-CoA synthetase (igni0256 and igni0257) were annotated (47). Two types of acetyl-CoA synthetases are known: an AMP-forming enzyme (ACS) and an ADP-forming enzyme (ACD) (17, 31). ACS activates acetate in a bi-uni-uni-bi ping pong mechanism to acetyl-CoA by consumption of ATP (62) and can be found in all three domains of life. The enzyme (EC 6.2.1.1) catalyzes the reaction:
| (1) |
Within the Archaea, an ACS has thus far been described for Methanosaeta consilii, Methanothermobacter thermoautotrophicus, Pyrobaculum aerophilum, Archaeoglobus fulgidus, and some halophiles, e.g., Haloarcula marismortui (8, 9, 25, 32). Some of the ACSs have been purified or enriched and characterized in their subunit composition. As an example, the ACS of P. aerophilum (PAE2867) is a homo-octameric protein complex with a total apparent molecular mass of 670 kDa, consisting of subunits with molecular masses of 75 kDa (9).
In contrast, the ADP-forming ACD converts acetyl-CoA into acetate. In vitro, this reaction is reversible, but for halophilic archaea it has been shown that in vivo only the acetate-forming reaction takes place (5, 6, 10). The ACD (EC 6.2.1.13) catalyzes the reaction:
| (2) |
This enzyme was first detected in eukaryotic protists (Entamoeba histolytica and Giardia lamblia [50, 54]). Meanwhile, it was also found in hyperthermophilic archaea, such as members of the genera Thermococcus and Desulfurococcus, as well as in Pyrococcus furiosus and A. fulgidus (strain 7324) (39, 55, 56) and in the genome of the bacterium Syntrophus aciditrophicus (41).
We present here the identification and the subcellular localization of the ACS of I. hospitalis, revealing further insights into the subcellular localization of basic metabolic pathways in this organism.
MATERIALS AND METHODS
Strain, medium, and growth conditions.
I. hospitalis KIN4/IT (DSM 18386) was obtained from our culture collection at the Institute of Microbiology and Archaeal Centre, University of Regensburg. It was grown in 1/2 SME-Ignicoccus medium at 90°C as described previously (45). The medium was covered with a gas phase of H2-CO2 (80/20 [vol/vol], 250 kPa), thereby using hydrogen as an electron donor and elemental sulfur as an electron acceptor. Mass cultivation was carried out in a 300-liter enamel-protected fermentor at 90°C at pH 5.5 to 6.0. To reach a final cell density of ∼108 I. hospitalis cells ml−1 in the fermentor, a flow rate of 60 liters of gas H2-N2-CO2 (15:65:20 [vol/vol/vol]) min−1 was applied. The cells were harvested by centrifugation (Padberg, Lahr, Germany), shock-frozen in liquid nitrogen, and stored at −70°C until further use.
A possible dependence of the ACS synthesis from culture conditions was tested by growing the organism in culture bottles (1 liter, each containing 250 ml of medium) either under chemolithoautotrophic conditions (in the absence of any organic compounds) or, alternatively, under mixotrophic conditions by adding either 1 mM acetate or 0.05% yeast extract (yielding acetate in a final concentration in the medium of ∼1 mM).
Membrane preparation and protein solubilization.
Frozen cells of I. hospitalis were resuspended in buffer A (25 mM Tris-HCl, 5 mM MgCl2, and 0.1 mM phenylmethylsulfonyl fluoride [PMSF], pH 7.5) and disrupted by three passages through a French press cell (Aminco, 3.5 MPa). Cell debris was removed from the extract by three centrifugation steps (3,000 × g, 6,750 × g, and then 9,500 × g for 15 min and at 4°C in a Beckman Avanti-J-25, JLA 16.250 rotor). The resulting supernatant (fraction F1) was subjected to ultracentrifugation (175,000 × g for 2 h at 4°C on a Beckman Optima LE-80K 70 Ti rotor) to separate the membranes (fraction F2) from the soluble cytoplasmic proteins (fraction F3). The membranes were resuspended in buffer B (100 mM HEPES-NaOH, 5 mM MgCl2, 10% [vol/vol] glycerol, 0.1 mM PMSF [pH 7.5]) with a tissue homogenizer. Triton X-100 (Roche) was added to a protein/detergent ratio of 1:1.5 and a detergent concentration of 2% (wt/vol) (incubation at 37°C for 2 h and at room temperature for 12 h). Membrane proteins and membrane-associated proteins were recovered from the extract by ultracentrifugation (140,000 × g, 2 h, 4°C; Beckman Optima LE-80K, 70.1Ti rotor). The obtained supernatant was designated the “solubilizate” (fraction F4), and the resuspended sediment is referred to as “insoluble membrane components” (fraction F5). Protein concentrations were determined with the BCA protein assay kit (Pierce Biotechnology).
hrCNE, SDS-PAGE, and Western blot analyses.
The membrane protein complexes were separated for 16 h at 300 V, 10 mA, and 50 W by high-resolution clear native electrophoresis (hrCNE) (69) using 5 to 13% gradient gels (68). The obtained complexes were cut out of the native gel and incubated for 1 h in a denaturing buffer (2% [wt/vol] SDS, 66 mM Na2CO3, 0.67% [vol/vol] β-mercaptoethanol) at room temperature, followed by 30 min of incubation at 60°C. The samples were analyzed on their subunit composition by a second-dimension Tricine-SDS-PAGE (57, 58). The gels were stained either with Coomassie brilliant blue R250 or silver using standard protocols (4, 44).
For raising specific antibodies, the identified acetyl-CoA synthetase complex was excised from an hrCNE gel and used for immunization of rabbits (Davids Biotechnology, Burgweinting, Germany). Proteins were transferred by electroblotting onto a polyvinylidene difluoride (PVDF) membrane (1 h; 20 V, constant) using a semidry transfer cell (Bio-Rad, Hercules, CA) as described previously (57). The transfer efficiency was checked by Ponceau S staining (0.1% [wt/vol] Ponceau S, 5% [vol/vol] acetic acid).
After the transfer, the membrane was blocked for 2 h using blocking solution (5% [wt/vol] milk powder in TBS buffer composed of 50 mM Tris-HCl [pH 8.0] and 150 mM NaCl). The primary antibody was diluted (1:5,000) in the blocking solution and applied to the membrane for 1 h. The PVDF membrane was washed twice with TBS-TT buffer (20 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.05% [vol/vol] Tween 20, 0.5% [vol/vol] Triton X-100), followed by washing in TBS buffer. After incubation with the secondary antibodies in TBS buffer for 1 h in the dark, the membrane was washed three times with TBS-TT buffer and stored in TBS buffer until detection. As secondary antibodies, anti-rabbit IgG peroxidase (Sigma, St. Louis, MO) and fluorescence-labeled anti-rabbit IgG DyLight 649 were used. For immunodetection by chemiluminescence, the peroxidase antibodies were incubated with the Lumi Light Western blotting substrate (Roche Diagnostics GmbH, Mannheim, Germany) and documented using a LAS-3000 Imager (Fujifilm, Düsseldorf, Germany). Fluorescence-labeled antibodies were excited at 635 nm in an FLA-5000 scanner (Fujifilm, Düsseldorf, Germany).
MALDI-TOF MS/MS and N-terminal sequencing.
The proteins separated by hrCNE (Fig. 1A) were identified by matrix-assisted laser desorption ionization–time of flight tandem mass spectrometry (MALDI-TOF MS/MS). The bands of interest were cut out of the gel, sliced in pieces, and incubated in the following four solutions (each for 30 min): 50 mM NH4HCO3, 50 mM NH4HCO3 in 25% (vol/vol) acetonitrile, 25% (vol/vol) acetonitrile, and finally 50% (vol/vol) acetonitrile. After lyophilization of the gel fragments, the proteins were digested with 2 μg of trypsin 100 μl−1 gel volume (sequencing grade; Roche Diagnostics GmbH, Mannheim, Germany) at 37°C for 12 h. The digested protein was extracted twice with 100 mM NH4HCO3 and finally with 100 mM NH4HCO3 in 40% (vol/vol) acetonitrile. Extracts were collected and lyophilized, and the peptides were analyzed by MALDI-TOF MS/MS using a 4700 proteomics analyzer (ABI). Proteins were identified searching the nonredundant National Center for Biotechnology Information database. Total protein scores (obtained by Mascot [35, 46]) higher than 80 were defined as significant (P < 0.05). Furthermore, a search in the databases of the Joint Genome Institute (JGI; http://jgi.doe.gov/) was carried out for verification.
Fig 1.

Native membrane protein and subunit composition. (A) In the nondenaturing electrophoresis, hrCNE, of fraction F4 (solubilized membrane and membrane-associated protein complexes) the two high-molecular-mass complexes are obvious which lead to the further analyzed complexes 1 (thermosome) and 2 (ACS). (B) SDS-PAGE of the complexes 1 and 2 from Fig. 1A. Molecular masses are indicated at the left in kilodaltons.
For N-terminal sequencing by Edman degradation (ABI Procise 492 protein sequencer; Applied Biosystems), proteins were transferred onto a PVDF membrane using standard methods (Transblot Semidry; Bio-Rad, Munich, Germany) and visualized with Coomassie brilliant blue R250. Phenylthiohydantoin amino acids were identified by high-pressure liquid chromatography.
Electron microscopy and immunolabeling on ultrathin sections.
After cultivation in 1/2 SME Ignicoccus medium inside the lumen of cellulose capillaries (52), cells of I. hospitalis were immobilized by high-pressure freezing (EM PACT2; Leica) and dehydrated by freeze-substitution fixation (8 h at −90°C, 8 h at −60°C, 6 h at −30°C, 94.6% [vol/vol] ethanol, 0.5% [vol/vol] glutaraldehyde, 0.5% [wt/vol] uranyl acetate, and 5.4% H2O [33]). Samples were rinsed in acetone at 0°C and embedded in Epon which was hardened at 60°C for 48 h (12, 48). For immunogold labeling, ultrathin sections on Formvar-coated nickel grids were incubated with phosphate-buffered saline (PBS) with 0.1% (wt/vol) glycine (for 5 min; blocking of aldehydes) and blocked with PBS containing 1% (wt/vol) bovine serum albumin (BSA; 5 min). The grids were then incubated with the primary anti-ACS antibody (a 1:100 dilution in PBS with 0.1% [wt/vol] BSA for 60 min), washed five times for 2 min each time (PBS with 0.1% [wt/vol] BSA), and then incubated with the secondary antibody (conjugated with 6-nm gold, 1:50 dilution in PBS with 0.1% [wt/vol] BSA, for 60 min). After washing (PBS with 0.1% [wt/vol] BSA, five times; PBS, twice) and fixation (PBS with 2% [vol/vol] glutaraldehyde, 5 min), the grids were washed (PBS, twice; water, three times) and finally contrasted with uranyl acetate (2% [wt/vol], 15 min). Digital electron micrographs were recorded using a slow-scan charge-coupled device camera (Tietz, Gauting, Germany), mounted on a CM12 transmission electron microscope (FEI Co., Eindhoven, Netherlands), which was operated at 120 keV.
Epifluorescence microscopy and immunolabeling on whole Ignicoccus cells.
Phase contrast and fluorescence microscopy were carried out with an Olympus BX 60 fluorescence microscope. Micrographs were recorded digitally using a Canon 60D camera (high resolution mode). Digital images were edited with Photoshop (Adobe). Phase-contrast and fluorescence images were merged using ImageJ (http://imagej.nih.gov/ij).
DNA of I. hospitalis cells was stained with SYTO9 (Invitrogen, USA). For optimal staining, 1 μl of the stock solution (1.5 mM in PBS buffer) was added to 8 μl of an I. hospitalis culture, followed by incubation for 5 min in the dark (fluorescence filter U-MNB, narrow band; excitation, 470 to 490 nm). For immunofluorescence, fresh log-phase cells were fixed by the addition of formaldehyde (30% [vol/vol] in PBS), washed with PBSG (PBS with 50 mM glycine) and PBS. Subsequently, PBS was replaced by PBSBT (PBS with 2% [wt/vol] BSA and 0.02% [vol/vol] Tween 20), and primary antibody (dilution 1:250) was added. After incubation for 2 h, the cells were washed with PBS, incubated with the secondary antibody (Alexa Fluor 546 labeling, goat anti-rabbit IgG [Invitrogen], 1:500 in PBS) for 2 h, and finally washed twice in PBS. All procedures were carried out at room temperature. For visualization, an NG filter combination (Olympus) was used.
Enzymatic tests for determination of acetate concentration in the media and formation of acetyl-CoA.
The concentration of acetate in the culture media was measured using an acetate detection kit for food bioanalytics (r-Biopharm, Darmstadt, Germany) according to the manufacturer's instructions. Samples (2 ml) were taken from fermentor cultures and adjusted to pH 10 (5 M NaOH), and the cells were sedimented by centrifugation at 13,000 × g. The supernatant was applied to the test. For mixotrophic growth conditions, 0.1% [wt/vol] yeast extract was added to the normal culture medium corresponding to a concentration of 7.4 mg of acetate liter of medium−1.
Enzymatic activity was determined by monitoring acetyl-CoA formation from acetate, ATP, and CoA by the hydroxamate reaction (1). Acetyl-hydroxamate formation from acetyl-CoA and hydroxylamine was assayed photometrically at 540 nm. The reaction buffer contained 100 mM Tris, 1.4% NaCl, 30 mM MgCl2, 400 mM potassium acetate, and 700 mM hydroxyl-ammonium chloride (pH 7.5). Before use, 10 mM ATP and 1 mM coenzyme A were added. The enzyme test was performed at 90°C under anaerobic conditions and was started by the addition of 0.15 mg of protein. As controls, the tests were carried out either without protein or without ATP and coenzyme A.
Enzymatic tests for determination of pyrophosphate formation.
The coproduction of pyrophosphate during the formation of acetyl-CoA was enzymatically monitored by the use of an autologous, enriched pyrophosphatase (14). This was combined with an enriched ACS fraction (>600 kDa) that had been obtained from membrane fraction F4 after size exclusion chromatography using an ÄKTA FPLC system with a Sephacryl 16/60 column (GE Healthcare). Using a colorimetric test system (21), the formation of orthophosphate from pyrophosphate provided by the hydroxamate reaction (reaction 1) was determined photometrically at 355 nm. The reaction buffer contained 100 mM Tris, 100 mM MES, 10 mM MgCl2, 40 mM Na2S2O5, and 200 mM KCl (pH 8.0). In 1.2 ml of buffer the ACS and pyrophosphatase from I. hospitalis were suspended, 4 mg of CoA was added to each tube, and the test was started with 6 μmol of ATP. After 3 min, 6 μmol of potassium acetate was added. The tests were performed at 90°C. As a control, assays were carried out without potassium acetate.
Resequencing of the ACS genes (igni0256 and igni0257).
Using two primers, PCR_fwd (ACGTACCGGTGCTCATCGTAAAGGGC) and PCR_rev (CGGACATAGGCGGGGTCTTGGGTTGG), a part of the I. hospitalis genome from bases 221050 to 223250 corresponding to the areas of the annotated ACS genes igni0256 and igni0257 (47) was amplified and resequenced using the primers seq_1 (CGACGCTCAAGCTAAGGTCG), seq_2 (GCGAGAAGTGGGTGGAGAAG), and seq_3 (CGACATAAGGGACGACGAC) (Entelechon, Regensburg, Germany).
Bioinformatic analyses.
Sequence alignments of the ACS were performed using the program CLUSTAL W2 (http://www.ebi.ac.uk/Tools/clustalw2/index.html). The sequences of ACSs of P. aerophilum (accession no. NP_560315), Homo sapiens (NP_061147), Salmonella enterica (Q8ZKF6), and M. consilii (AAA73007) were compared to the ACS of I. hospitalis. Prediction of transmembrane helices was performed using the programs HMMTOP (http://www.enzim.hu/hmmtop/), Phobius (http://phobius.sbc.su.se/), SignalP 3.0 Server (http://www.cbs.dtu.dk/services/SignalP/), SOSUI (http://bp.nuap.nagoya-u.ac.jp/sosui/), TMHMM Server v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM/), and TMpred (http://www.ch.embnet.org/software/TMPRED_form.html). The prediction of beta-barrels, the transmembrane beta-strands of outer membrane proteins, was done with PRED-TMBB (http://bioinformatics2.biol.uoa.gr/PRED-TMBB/input.jsp). A structural model of the ACS was created using the program I-TASSER (53), which automatically generates full-length protein three-dimensional structure predictions. It includes a benchmarked scoring system consisting of the confidential score (C-score), which estimates the accuracy of the prediction, and the TM score assessing the structural similarity of two protein structures. Using a C-score cutoff > −1.5 for the models of correct topology, both false-positive and false-negative rates are <0.1. A TM score of >0.5 corresponds approximately to two structures of similar topology.
RESULTS
Native membrane protein and subunit composition.
Solubilized membrane protein complexes and membrane associated proteins of I. hospitalis were separated by hrCNE and analyzed for their molecular mass and their involvement in energy consuming metabolic processes. After electrophoretic separation of the solubilized membrane proteins (fraction F4), several prominent high-molecular-mass complexes were revealed (Fig. 1A). For analysis of their subunit compositions, complexes with an apparent molecular mass higher than 600 kDa were separated using SDS-PAGE. The prominent complex at 1,000 kDa turned out to be composed of two different subunits with apparent masses of approximately 68 and 62 kDa, while the 670-kDa complex dissociated into 77-kDa monomers (Fig. 1B).
Identification of proteins with MALDI-TOF MS/MS and N-terminal sequencing.
For the identification of the 1,000- and 670-kDa protein complexes, the tryptic peptides were analyzed by MALDI-TOF MS/MS. The 1,000-kDa protein complex was identified as the beta subunit of the I. hospitalis thermosome (corresponding to Igni0897 [mass, 59 kDa]), based on a Mascot score of 374, a sequence coverage of 50% from the detected peptides, and a fragment score of well above 50, up to 103 (Table 1). For the protein complex with the apparent molecular mass of 670 kDa, identification by MALDI-TOF MS/MS resulted in two high score hits (Table 1): a predicted AMP-dependent synthetase and ligase (Igni0256 [mass, 47.3 kDa]), with a Mascot score of 312, and a predicted acyl-CoA synthetase (Igni0257 [mass, 25.7 kDa]), with a Mascot score of 380. The sequence coverages were 46 and 41%, respectively, and the identified peptides had fragment scores from 50 up to 191. Surprisingly, the molecular masses of Igni0256 and Igni0257 add up to 73 kDa and, therefore, correspond to the apparent molecular mass 77 kDa of the single protein obtained after SDS-PAGE. This result suggested that the 670-kDa protein complex of I. hospitalis is a homo-oligomer of one protein subunit. To confirm this suggestion, we accomplished an N-terminal sequencing of the native 670-kDa complex. Only one N terminus was detectable, consisting of the amino acids TEEQKTVLPISKG, which is in perfect agreement with the first 13 amino acids of the predicted product of gene igni0256. Thus, the 670-kDa protein complex of I. hospitalis is encoded only by one gene, consisting of the former genes igni0256 and igni0257. We were able to confirm this by resequencing the corresponding part of the I. hospitalis genome (base 221,050 to 223,250). The obtained ORF (igni0256/0257) codes for only one protein (73 kDa; 645 amino acids). It is the line up of the amino acid sequences of the former proteins Igni0256 and Igni0257 with the exchange of one single amino acid: threonine 412 against isoleucine 412 (according to sequence of Igni0256/0257). In addition, the generated antibody against the ACS was used for a Western blot experiment (Fig. 2). Only one positive reaction (at a molecular mass of ∼670 kDa) was obtained in the blot after hrCNE, demonstrating its specificity for the ACS.
Table 1.
Identification of protein complexes by MALDI-TOF MS/MS
| Sample complex size (kDa) | Examples of fragmented peptide sequence (5′–3′) | Fragment score | Mascot score | Sequence coverage (%) | Locus tag | Predicted function | Predicted molecular mass (kDa) |
|---|---|---|---|---|---|---|---|
| 1,000 | IAVLDAPLELEKPELDAEIR | 103 | 374 | 50 | igni0897 | Thermosome (beta subunit) | 58.8 |
| IVGAGGAPEVELALALR | 77 | ||||||
| 670 | AVISGQPLGDLSTLEDEASVEEVKR | 191 | 380 | 46 | igni0257 | Acyl-CoA synthetase | 25.7 |
| DEDGYFWILGR | 83 | ||||||
| AAIIWEGEPGETR | 78 | 312 | 41 | igni0256 | AMP-dependent synthetase and ligase | 47.3 | |
| YGVTTFYTAPTAIR | 67 |
Fig 2.

Nondenaturing electrophoresis, hrCNE, of fraction F4 and Western blot. The antibody generated against complex 2 of Fig. 1A was used in this blot. Only one positive reaction at an apparent molecular mass of ∼670 kDa was obtained. St, molecular masses in kilodaltons, are indicated at the left. Lane 1, hrCNE stained with Coomassie blue; lane 2, Western blot.
Multiple sequence alignment.
To create a multiple sequence alignment with CLUSTAL W2, the sequence of Igni0256/0257 was compared to selected enzymes from the databases. The ACSs from P. aerophilum, Homo sapiens, S. enterica, and M. consilii represented acetyl-CoA synthetases from all three domains of life and showed 10 highly conserved regions, which are important for substrate binding. For the I. hospitalis enzyme, three of the conserved regions (A1, A2, and A3) are part of the previous protein Igni0256 and regions A4 to A10 of the protein Igni0257. We also checked the Ignicoccus ACS sequence for the presence of amino residues putatively involved in acetate binding. Four such residues, which were identified for the S. enterica enzyme to be important for substrate binding (27) are found in a similar arrangement see (Fig. S1 in the supplemental material, marked in blue). Furthermore, a highly conserved lysine residue in region A10, which is essential for regulation of the enzyme, and the two argenine residues (A515 and A526 of S. enterica) for phosphate binding were found in the amino acid sequence of I. hospitalis (51) (see Fig. S1 in the supplemental material). In support of the assumption that the acetyl-CoA synthetase of I. hospitalis is not an ADP-forming enzyme, the sequences of Igni0256 and Igni0257 do not show significant similarity with, for example, the ACD of P. furiosus (genes pf1540 and pf1787), except for eight positions with identical amino acids out of 645 (data not shown). These results suggest that the 670-kDa protein complex of I. hospitalis is an ACS and that the genes igni0256 and igni0257 had been incorrectly sequenced and annotated.
Consumption of acetate and formation of acetyl-CoA and pyrophosphate by the ACS.
The concentration of acetate in the culture medium was measured during autotrophic and mixotrophic growth of I. hospitalis. The results showed that acetate is consumed by mixotrophically cultivated cells in the exponential growth phase (Fig. 3). On the other hand, under autotrophic culture conditions, no acetate production was detectable (Fig. 3). Both results are in line with the fact that I. hospitalis possesses an acetate assimilation pathway, indicating the presence of an ACS.
Fig 3.
Acetate consumption during growth of I. hospitalis. The figure shows the decrease of the acetate concentration in the medium during mixotrophic growth with 0.05% yeast extract (■) in relation to the cell concentration (▲). On the other hand, no significant acetate production was detectable under autotrophic culture conditions (▼).
The activity of the ATP and CoA-dependent acetyl-CoA formation of the ACS of I. hospitalis was determined in the crude extract (fraction F1) and in isolated membranes (fraction F4). As shown in Fig. 4, acetyl-CoA was formed within 30 min from acetate in the presence of ATP and coenzyme A in the crude extract. The acetyl-CoA concentration reached 4,300 nmol/mg of protein after 5 min. This corresponds to a specific activity of 0.86 U/mg for the ACS in the crude extract. In the isolated membranes, 92% of the total ACS activity was detectable using this assay (for comparison, in halophilic archaea specific activities of 0.02 to 0.03 U/mg of protein in the crude extract had been determined [5]).
Fig 4.
Acetyl-CoA formation by different cell fractions of I. hospitalis. Nearly identical amounts of acetyl-CoA per mg of protein were formed in the crude extract (■) and in isolated membranes (▼), while no significant formation was observed in the cytoplasmic fraction (▲).
The AMP-forming character of the identified acetyl-CoA synthesizing enzyme (instead of an ADP-forming complex, cf. equations 1 and 2 above) was proven by the determination of the coproduction of pyrophosphate, enzymatically monitored after the addition of an autologous pyrophosphatase. As shown in Fig. S2 in the supplemental material, the addition of acetate significantly raises the amount of released phosphate, as expected for an AMP-forming ACS.
Cellular localization of the ACS in I. hospitalis and other Ignicoccus species.
The subcellular localization of the ACS was studied by electron microscopy of immunogold labeled ultrathin sections using antibodies raised against the I. hospitalis ACS. Surprisingly, and coincident with the localization of the ATP synthase and the H2:sulfur oxidoreductase, a highly predominant labeling of the outermost membrane of the I. hospitalis cells was observed (Fig. 5). Almost no signal was found in the inner membrane, in the cytoplasm, or in the IMC of the cells. This result was supported by fluorescence light microscopy experiments of whole I. hospitalis cells, using the fluorescence labeled antibody generated against the ACS and SYTO9 for staining of the DNA (Fig. 6A to D).
Fig 5.
Localization of the ACS in the outermost membrane of I. hospitalis by immunoelectron microscopy (two representative examples [A and B]). Sections of high-pressure frozen, freeze-substituted, and Epon-embedded cells were incubated with anti-ACS (primary antibody; dilution, 1:100) and a 6-nm gold-labeled secondary antibody (dilution 1:50) and then visualized by transmission electron microscopy. C, cytoplasm; IM, inner membrane; OM, outermost membrane; IMC, intermembrane compartment. Bars, 200 nm.
Fig 6.
Epifluorescence microphotographs of cells from different Ignicoccus species. Bars, 1.5 μm. (A, E, I, and M) Phase-contrast images. (B, F, J, and N) Merged phase-contrast images and SYTO9 fluorescence signals (DNA stain; green). (C, G, K, and O) The localization of the ACS was elucidated by homologous primary antibodies, which were detected by a secondary antibody coupled to Alexa Fluor 546 (red). (D, H, L, and P) Merge of SYTO9 and Alexa Fluor 546 signals.
To elucidate whether the localization of the ACS is restricted to I. hospitalis or a characteristic of all Ignicoccus species, similar staining experiments were carried out with cells of I. islandicus, I. pacificus, and the new isolate Ignicoccus sp. strain Mex13A. In all strains, a distribution pattern similar to that of I. hospitalis cells was observed (Fig. 6E to P), indicating that the subcellular location of the ACS in the outermost membrane is a unique feature of members of the genus Ignicoccus.
Structure prediction.
The results of the immunogold labeling on ultrathin sections of I. hospitalis cells showed that the ACS is located at the outermost membrane. Therefore, secondary structure predictions by computational analyses were carried out to gain further insight whether the ACS has a leader peptide and to determine whether it is membrane associated or even an integral membrane protein. All programs used (TMPred, TMHMM, HMMTOP, Phobius, SignalP, and SOSUI) predict that the ACS of I. hospitalis has no signal peptide and no transmembrane helices. The search for beta-barrels with PRED-TMBB was unsuccessful, too. A structural model of the ACS of I. hospitalis was created using I-Tasser. The predicted structure had a nearly maximal confidence score (C score) of 1.98, and the estimated accuracy of the model was 0.99 (as given by the TM score). Typically, the C scores obtained by this program range from −5 to 2, where a C score of >−1.5 indicates a correct fold (53) and a TM score of >0.5 indicates a model of correct topology. The ACS model of I. hospitalis showed a mushroom-like structure consisting of a foot-domain and a globular head-domain (see Fig. S3A in the supplemental material). The foot-domain, which corresponds to the C-terminal part of the ACS, might act as an anchor docking the enzyme to the outermost membrane of the cell. Furthermore, a deep pocket was identified in the globular head-domain, most probably representing a cave leading to the reactive center of the enzyme which contains four typical amino acid residues for substrate binding (I301, T302, V377, W405; numbering for ACS of I. hospitalis) in the same configuration as in the S. enterica ACS (PDB entry 1pg3) (see Fig. S3B and C in the supplemental material).
Is the ACS of I. hospitalis an inducible enzyme?
Due to fact that I. hospitalis grows chemolithoautotrophically but is able to incorporate acetate from the medium, we checked the possibility whether the ACS is (part of) an inducible genetic system in this organism. Solubilized membrane proteins of autotrophically and mixotrophically (either with 0.1% yeast extract or with 7.5 mg of acetate liter−1) cultivated cells were applied to SDS-PAGE and tested for the presence of ACS using the antibody raised against the enzyme of I. hospitalis. However, in all experiments the ACS complex could be detected in similar amounts (data not shown), indicating that this complex is not induced by acetate in this organism.
DISCUSSION
The formation of acetyl-CoA is an important metabolic reaction for I. hospitalis, since this molecule also represents the primary acceptor in the CO2 fixation pathway of this organism. The catalyzing enzyme, the acetyl-CoA synthetase, was separated from other solubilized protein complexes by high-resolution clear native electrophoresis. The prepared membranes used for the solubilization contained both the outermost and the inner membrane since their specific biophysical separation is not yet possible. The native complex exhibited an apparent molecular mass of ∼670 kDa, indicating that the complex may be formed from approximately eight (or nine) identical subunits of an apparent molecular mass of 77 kDa. This composition would correspond to the ACS of another hyperthermophilic crenarchaeon, P. aerophilum, whose native ACS exhibits a molecular mass of ∼610 kDa and is composed of eight identical subunits with molecular masses of 75 kDa (9). Both complexes are unique in their stoichiometry and significantly distinct in their molecular masses from ACS complexes of other organisms. In the eukaryotes Saccharomyces cerevisiae and Penicillium chrysogenum, the native ACSs are dimers of around 151 and 139 kDa, respectively, while the dimeric ACS from M. consilii exhibits a molecular mass of 148 kDa (15, 31, 33, 40). Furthermore, monomeric native ACSs exist, like the enzymes in E. coli or Spinacea oleracea, with apparent molecular masses of only 73 or 72 kDa (36, 71). Higher oligomerization states of enzyme complexes were already observed for the formyltransferase of Methanopyrus kandleri. This enzyme can form monomers, dimers, or tetramers depending on the salt concentration in the medium, in which only the tetrameric form is stable and active (60, 61). Higher oligomerization states of cytoplasmic enzymes were discussed to contribute to their heat stability in thermophilic prokaryotes (66).
The obtained 670-kDa complex of I. hospitalis was identified by MALDI-TOF MS/MS with high scores, revealing the former proteins Igni0256 and Igni0257 of the annotated genome (JGI). The functions of the identified 26- and 47-kDa proteins were predicted as “acyl-CoA synthetase” (Igni0257) and “AMP-dependent synthase and ligase” (Igni0256) (47). In contrast to the bioinformatic data, biochemical analysis of the 670-kDa complex by SDS-PAGE revealed only one single subunit with a molecular mass of ∼77 kDa. This was supported by N-terminal sequencing of the complex where only a single peptide was identified, demonstrating that the ACS of I. hospitalis is encoded by only one open reading frame and is a homo-oligomer. Simultaneously, these results indicate a wrong sequencing and annotation of genes igni0256 and igni0257. While the homo-oligomeric structure was thus far found in all investigated ACS complexes, some ADP-forming acetyl-CoA synthetases are known which are composed of two different subunits, e.g., in P. furiosus or P. aerophilum (7, 42).
The enzyme was shown to catalyze the conversion of acetate to acetyl-CoA by forming pyrophosphate from ATP. This reaction is typical for the AMP-forming ACS. Comparison of amino acid sequences of AMP- and ADP-forming acetyl-CoA synthetases from organisms of all three domains of life supported the result that the ACS of I. hospitalis is an AMP-forming enzyme. Ten highly conserved motifs (A1 to A10) typical for an AMP-forming ACS were found, while comparison with the sequence of the ADP-forming ACS (ACD) from P. furiosus (10) yielded no congruity. Structural analyses of the AMP-forming ACS of S. enterica showed (19) that several of the highly conserved sequences are responsible for binding the substrate to the enzyme. Also, a highly conserved lysine residue was found in motif A10 of all sequences (including K602 in I. hospitalis). This residue is essential for catalysis and posttranslational modifications such as acetylation and deacetylation (9, 19, 62). For many ACSs, e.g., from Bacillus subtilis and E. coli, only a regulation at the transcription level has been described thus far (18, 37). In B. subtilis it was shown that the acuABC operon encodes a protein acetyltransferase (AcuA) and a protein deacetylase (AcuC) that controls the activity of the AMP-forming acetyl-CoA synthetase (16). Although no AcuA proteins could be detected by analysis of the genome of I. hospitalis, two proteins with significant similarity to AcuC of B. subtilis were found. Nevertheless, the relevance of these proteins for the activity of the ACS in I. hospitalis needs to be evaluated. For the ACSs of S. enterica and S. cerevisiae it was shown that an acetylation of the highly conserved lysine residue inactivates the whole enzyme (64, 65). In S. enterica the acetyl-CoA-dependent protein acetyltransferase (Pat) is responsible for the acetylation (deactivation) of the ACS (63). In the genome of I. hospitalis a similar enzyme, acetyl-CoA acetyltransferase, can be found. Furthermore, sirtuin proteins, e.g., the NAD+-dependent CodB sirtuin in S. enterica, are essential for the activity of the ACS in vivo, because they activate the ACS via deacetylation of the acetylated ACS (64). The mammalian mouse acetyl-CoA synthetase or the human acetyl-CoA synthetase is also regulated by reversible site-specific acetylation on K661 or K642. In both cases the sirtuins are deacetylating the acetyl-CoA synthetases and therefore activating the acetyl-CoA synthetase again (20, 59). However, no such proteins are annotated in the genome of I. hospitalis.
Bioinformatic analyses predicted no transmembrane helices, but a putative membrane anchor was identified in the structure of the ACS (foot-domain). This suggests an association of the complex with one of the I. hospitalis membranes, although in other Archaea no ACS complex was observed to be membrane associated. Therefore, it was surprising that immunolabeling experiments on ultrathin sections of I. hospitalis cells not only confirmed the membrane association but even more showed an unexpected subcellular location of the ACS at the outermost membrane. As a consequence, the outermost membrane of I. hospitalis is now known not only to harbor the energy conserving machinery (ATP synthase and H2:sulfur oxidoreductase) (38) but also the acetate activating ACS complex which consumes ATP.
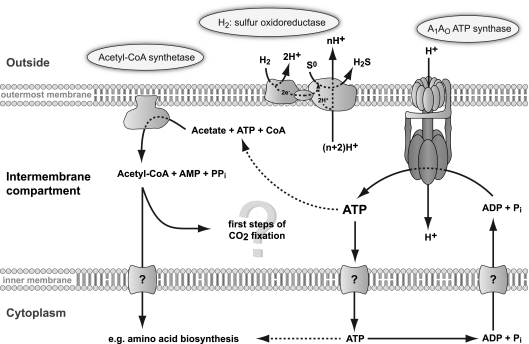
Figure 7 summarizes the identified reactions and the localization of the corresponding enzyme complexes in the IMC and in the outermost membrane of I. hospitalis. Protons are translocated by the H2:sulfur oxidoreductase, thereby creating a chemiosmotic ion gradient across the outermost membrane that fuels the ATP synthase. As a consequence, ATP is generated from ADP and Pi in the IMC. Since biosynthesis processes such as replication, transcription, and translation take place in the cytoplasm ATP has to be imported from the ICM (38). However, it is unknown at present, how the exchange of ATP and ADP across the inner (cytoplasmic) membrane is achieved.
Fig 7.
Scheme of ATP conservation and ATP consumption in the IMC of I. hospitalis.
The localization of the ACS at the outermost membrane and thus in the IMC avoids a transmembrane transport, as ATP is directly available to the complex. This is the first proven energy-consuming metabolic process in the IMC of I. hospitalis, leading to the suggestion that not only the formation of acetyl-CoA but also further steps of the CO2 fixation pathway may take place here. Candidates are especially the energy-consuming fixation steps by pyruvate synthetase and PEP carboxylase forming pyruvate and oxaloacetate, respectively (3, 24). Since the ACS shows an identical subcellular distribution in all other available Ignicoccus strains, this might be a general characteristic for all members of this genus.
Our results thus open up a field for further investigations of physiological reactions in the IMC supporting the hypothesis that members of the genus Ignicoccus and I. hospitalis in particular are highly organized prokaryotic cells.
Supplementary Material
ACKNOWLEDGMENTS
We thank Michael Thomm for ongoing support, Reinhard Wirth for stimulating discussions, Thomas Hader and Konrad Eichinger for technical support, Dietmar Birzer for visualizing the PDB data of the ACS, and Sara Wennige for the cultivation of cells. We thank Eduard Hochmuth and Rainer Deutzmann, Department of Biochemistry, University of Regensburg, for N-terminal sequencing and MALDI-TOF MS/MS analyses.
This study was supported by Deutsche Forschungsgemeinschaft grants HU703/2-1 (H.H. and R.R.) and SFB807 (V.M.).
Footnotes
Published ahead of print 13 January 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Aceti DJ, Ferry JG. 1988. Purification and characterization of acetate kinase from acetate-grown Methanosarcina thermophila. Evidence for regulation of synthesis. J. Biol. Chem. 263:15444–15448 [PubMed] [Google Scholar]
- 2. Atteia A, et al. 2006. Pyruvate formate-lyase and a novel route of eukaryotic ATP synthesis in Chlamydomonas mitochondria. J. Biol. Chem. 281:9909–9918 [DOI] [PubMed] [Google Scholar]
- 3. Berg IA, Ramos-Vera WH, Petri A, Huber H, Fuchs G. 2010. Study of the distribution of autotrophic carbon fixation cycles in Crenarchaeota. Microbiology 156:256–269 [DOI] [PubMed] [Google Scholar]
- 4. Blum H, Beier H, Gross HJ. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93–99 [Google Scholar]
- 5. Bräsen C, Schönheit P. 2001. Mechanisms of acetate formation and acetate activation in halophilic archaea. Arch. Microbiol. 175:360–368 [DOI] [PubMed] [Google Scholar]
- 6. Bräsen C, Schönheit P. 2004. Regulation of acetate and acetyl-CoA converting enzymes during growth on acetate and/or glucose in the halophilic archaeon Haloarcula marismortui. FEMS Microbiol. Lett. 241:21–26 [DOI] [PubMed] [Google Scholar]
- 7. Bräsen C, Schönheit P. 2004. Unusual ADP-forming acetyl-coenzyme A synthetase from the mesophilic halophilic euryarchaeon Haloarcula marismortui and from the hyperthermophilic crenarchaeon Pyrobaculum aerophilum. Arch. Microbiol. 182:277–287 [DOI] [PubMed] [Google Scholar]
- 8. Bräsen C, Schönheit P. 2005. AMP-forming acetyl-CoA synthetase from the extremely halophilic archaeon Haloarcula marismortui: purification, identification, and expression of the encoding gene, and phylogenetic affiliation. Extremophiles 9:355–365 [DOI] [PubMed] [Google Scholar]
- 9. Bräsen C, Urbanke C, Schönheit P. 2005. A novel octameric AMP-forming acetyl-CoA synthetase from the hyperthermophilic crenarchaeon Pyrobaculum aerophilum. FEBS Lett. 579:477–482 [DOI] [PubMed] [Google Scholar]
- 10. Bräsen C, Schmidt M, Grötzinger J, Schönheit P. 2008. Reaction mechanism and structural model of ADP-forming acetyl-CoA synthetase from the hyperthermophilic archaeon Pyrococcus furiosus: evidence for a second active site histidine residue. J. Biol. Chem. 283:15409–15418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown TDK, Jones-Mortimer MC, Kornberg HL. 1977. The enzymic interconversion of acetate and acetyl-coenzyme A in Escherichia coli. J. Gen. Microbiol. 102:327–336 [DOI] [PubMed] [Google Scholar]
- 12. Burghardt T, Näther DJ, Junglas B, Huber H, Rachel R. 2007. The dominating outer membrane protein of the hyperthermophilic archaeum Ignicoccus hospitalis: a novel pore-forming complex. Mol. Microbiol. 63:166–176 [DOI] [PubMed] [Google Scholar]
- 13. Burghardt T, et al. 2009. The interaction of Nanoarchaeum equitans with Ignicoccus hospitalis: proteins in the contact site between two cells. Biochem. Soc. Trans. 37:127–132 [DOI] [PubMed] [Google Scholar]
- 14. Daxer S. 2011. Master's thesis. University of Regensburg, Regensburg, Germany [Google Scholar]
- 15. Frenkel EP, Kitchens RL. 1977. Purification and properties of acetyl-coenzyme A synthetase from bakers' yeast. J. Biol. Chem. 252:504–507 [PubMed] [Google Scholar]
- 16. Gardner JG, Grundy FJ, Henkin TM, Escalante-Semerena JC. 2006. Control of acetyl-coenzyme A synthetase (AcsA) activity by acetylation/deacetylation without NAD+ involvement in Bacillus subtilis. J. Bacteriol. 188:5460–5468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glasemacher J, Bock AK, Schmid R, Schönheit P. 1997. Purification and properties of acetyl-CoA synthetase (ADP-forming), an archaeal enzyme of acetate formation and ATP synthesis, from the hyperthermophile Pyrococcus furiosus. Eur. J. Biochem. 244:561–567 [DOI] [PubMed] [Google Scholar]
- 18. Grundy FJ, Turinsky AJ, Henkin TM. 1994. Catabolite regulation of Bacillus subtilis acetate and acetoin utilization genes by CcpA. J. Bacteriol. 176:4527–4533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gulick AM, Starai VJ, Horswill AR, Homick KM, Escalante-Semerena JC. 2003. The 1.75 Å crystal structure of acetyl-CoA synthetase bound to adenosine-5′-propylphosphate and coenzyme A. Biochemistry 42:2866–2873 [DOI] [PubMed] [Google Scholar]
- 20. Hallows WC, Lee S, Denu JM. 2006. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc. Natl. Acad. Sci. U. S. A. 103:10230–10235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heinonen KJ, Lahti RJ. 1981. A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Anal. Biochem. 113:313–317 [DOI] [PubMed] [Google Scholar]
- 22. Huber H, et al. 2000. Ignicoccus gen. nov., a novel genus of hyperthermophilic, chemolithoautotrophic Archaea, represented by two new species, Ignicoccus islandicus sp. nov. and Ignicoccus pacificus sp. nov. Int. J. Syst. Evol. Microbiol. 50:2093–2100 [DOI] [PubMed] [Google Scholar]
- 23. Huber H, et al. 2002. A new phylum of Archaea represented by a nanosized hyperthermophilic symbiont. Nature 417:63–67 [DOI] [PubMed] [Google Scholar]
- 24. Huber H, et al. 2008. A dicarboxylate/4-hydroxybutyrate autotrophic carbon assimilation cycle in the hyperthermophilic archaeum Ignicoccus hospitalis. Proc. Natl. Acad. Sci. U. S. A. 105:7851–7856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ingram-Smith C, Smith KS. 2007. AMP-forming acetyl-CoA synthetases in Archaea show unexpected diversity in substrate utilization. Archaea 2:95–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ingram-Smith C, Martin SR, Smith KS. 2006. Acetate kinase: not just a bacterial enzyme. Trends Microbiol. 14:249–253 [DOI] [PubMed] [Google Scholar]
- 27. Ingram-Smith C, Woods BI, Smith KS. 2006. Characterization of the acyl substrate binding pocket of acetyl-CoA synthetase, Biochemistry 45:11482–11490 [DOI] [PubMed] [Google Scholar]
- 28. Jahn U, Summons R, Sturt H, Grosjean E, Huber H. 2004. Composition of the lipids of Nanoarchaeum equitans and their origin from its host Ignicoccus sp. strain KIN4/I. Arch. Microbiol. 182:404–413 [DOI] [PubMed] [Google Scholar]
- 29. Jahn U, Huber H, Eisenreich W, Hügler M, Fuchs G. 2007. Insights into the autotrophic CO2 fixation pathway of the archaeon Ignicoccus hospitalis: comprehensive analysis of the central carbon metabolism. J. Bacteriol. 189:4108–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jahn U, et al. 2008. Nanoarchaeum equitans and Ignicoccus hospitalis: new insights into a unique, intimate association of two Archaea. J. Bacteriol. 190:1743–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jetten MS, Stams AJ, Zehnder AJ. 1989. Isolation and characterization of acetyl-coenzyme A synthetase from Methanothrix soehngenii. J. Bacteriol. 171:5430–5435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jetten MS, Stams AJ, Zehnder AJ. 1992. Methanogenesis from acetate: a comparison of the acetate metabolism in Methanothrix soehngenii and Methanosarcina spp. FEMS Microbiol. Rev. 88:181–198 [Google Scholar]
- 33. Jogl G, Tong L. 2004. Crystal structure of yeast acetyl-coenzyme A synthetase in complex with AMP. Biochemistry 43:1425–1431 [DOI] [PubMed] [Google Scholar]
- 34. Junglas B, et al. 2008. Ignicoccus hospitalis and Nanoarchaeum equitans: ultrastructure, cell-cell interaction, and 3D reconstruction from serial sections of freeze-substituted cells and by electron cryotomography. Arch. Microbiol. 190:395–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koenig T, et al. 2008. Robust prediction of the MASCOT score for an improved quality assessment in mass spectrometric proteomics. J. Proteome Res. 7:3708–3717 [DOI] [PubMed] [Google Scholar]
- 36. Kumari S, Tishel R, Eisenbach M, Wolfe AJ. 1995. Cloning, characterization, and functional expression of acs, the gene which encodes acetyl coenzyme A synthetase in Escherichia coli. J. Bacteriol. 177:2878–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kumari S, et al. 2000. Regulation of acetyl coenzyme A synthetase in Escherichia coli. J. Bacteriol. 182:4173–4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Küper U, Meyer C, Müller V, Rachel R, Huber H. 2010. Energized outer membrane and spatial separation of metabolic processes in the hyperthermophilic archaeon Ignicoccus hospitalis. Proc. Natl. Acad. Sci. U. S. A. 107:3152–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Labes A, Schönheit P. 2001. Sugar utilization in the hyperthermophilic, sulfate-reducing archaeon Archaeoglobus fulgidus strain 7324: starch degradation to acetate and CO2 via a modified Embden-Meyerhof pathway and acetyl-CoA synthetase (ADP-forming). Arch. Microbiol. 176:329–338 [DOI] [PubMed] [Google Scholar]
- 40. Martinez-Blanco H, et al. 1992. Isolation and characterization of the acetyl-CoA synthetase from Penicillium chrysogenum: involvement of this enzyme in the biosynthesis of penicillins. J. Biol. Chem. 267:5474–5481 [PubMed] [Google Scholar]
- 41. McInerney MJ, et al. 2007. The genome of Syntrophus aciditrophicus: life at the thermodynamic limit of microbial growth. Proc. Natl. Acad. Sci. U. S. A. 104:7600–7605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Musfeldt M, Selig M, Schönheit P. 1999. Acetyl coenzyme A synthetase (ADP forming) from the hyperthermophilic archaeon Pyrococcus furiosus: identification, cloning, separate expression of the encoding genes, acdAI and acdBI, in Escherichia coli, and in vitro reconstitution of the active heterotetrameric enzyme from its recombinant subunits. J. Bacteriol. 181:5885–5888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Näther DJ, Rachel R. 2004. The outer membrane of the hyperthermophilic archaeon Ignicoccus: dynamics, ultrastructure, and composition. Biochem. Soc. Trans. 32:199–203 [DOI] [PubMed] [Google Scholar]
- 44. Neuhoff V, Arold N, Taube D, Ehrhardt W. 1988. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis 9:255–262 [DOI] [PubMed] [Google Scholar]
- 45. Paper W, et al. 2007. Ignicoccus hospitalis sp. nov., the host of ‘Nanoarchaeum equitans.’ Int. J. Syst. Evol. Microbiol. 57:803–808 [DOI] [PubMed] [Google Scholar]
- 46. Perkins DN, Pappin DJC, Creasy DM, Cottrell JS. 1999. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20:3551–3567 [DOI] [PubMed] [Google Scholar]
- 47. Podar M, et al. 2008. A genomic analysis of the archaeal system Ignicoccus hospitalis-Nanoarchaeum equitans. Genome Biol. 9:R158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rachel R, Wyschkony I, Riehl S, Huber H. 2002. The ultrastructure of Ignicoccus: evidence for a novel outer membrane and for intracellular vesicle budding in an archaeon. Archaea 1:9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reinscheid DJ, et al. 1999. Cloning, sequence analysis, expression and inactivation of the Corynebacterium glutamicum pta-ack operon encoding phosphotransacetylase and acetate kinase. Microbiology 145:503–513 [DOI] [PubMed] [Google Scholar]
- 50. Reeves RE, Warren LG, Susskind B, Lo HS. 1977. An energy-conserving pyruvate-to-acetate pathway in Entamoeba histolytica. Pyruvate synthase and a new acetate thiokinase. J. Biol. Chem. 252:726–731 [PubMed] [Google Scholar]
- 51. Reger AS, Carney JM, Gulick AM. 2007. Biochemical and crystallographic analysis of substrate binding and conformational changes in acetyl-CoA synthetase. Biochem. 46:6536–6546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rieger G, Müller K, Hermann R, Stetter KO, Rachel R. 1997. Cultivation of hyperthermophilic Archaea in capillary tubes resulting in improved preservation of fine structures. Arch. Microbiol. 168:373–379 [Google Scholar]
- 53. Roy A, Kucukural A, Zhang Y. 2010. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 5:725–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sanchez LB, Müller M. 1996. Purification and characterization of the acetate forming enzyme, acetyl-CoA synthetase (ADP-forming) from the amitochondriate protist, Giardia lamblia. FEBS Lett. 378:240–244 [DOI] [PubMed] [Google Scholar]
- 55. Schäfer T, Schönheit P. 1991. Pyruvate metabolism of the hyperthermophilic archaebacterium Pyrococcus furiosus: acetate formation from acetyl-CoA and ATP synthesis are catalyzed by an acetyl-CoA synthetase (ADP-forming). Arch. Microbiol. 155:366–377 [Google Scholar]
- 56. Schäfer T, Selig M, Schönheit P. 1993. Acetyl-CoA synthetase (ADP-forming) in Archaea, a novel enzyme involved in acetate and ATP synthesis. Arch. Microbiol. 159:72–83 [Google Scholar]
- 57. Schägger H. 2006. Tricine-SDS-PAGE. Nat. Protoc. 1:16–22 [DOI] [PubMed] [Google Scholar]
- 58. Schägger H, von Jagow G. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:223–231 [DOI] [PubMed] [Google Scholar]
- 59. Schwer B, Bunkenborg J, Verdin RO, Andersen JS, Verdin E. 2006. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc. Natl. Acad. Sci. U. S. A. 103:10224–10229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shima S, et al. 1998. Lyotropic-salt-induced changes in monomer/dimer/tetramer association equilibrium of formyltransferase from the hyperthermophilic Methanopyrus kandleri in relation to the activity and thermostability of the enzyme. Eur. J. Biochem. 258:85–92 [DOI] [PubMed] [Google Scholar]
- 61. Shima S, et al. 2000. A mutation affecting the association equilibrium of formyltransferase from the hyperthermophilic Methanopyrus kandleri and its influence on the enzyme's activity and thermostability. Eur. J. Biochem. 267:6619–6623 [DOI] [PubMed] [Google Scholar]
- 62. Starai VJ, Escalante-Semerena JC. 2004. Acetyl-coenzyme A synthetase (AMP forming). Cell. Mol. Life Sci. 61:2020–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Starai VJ, Escalante-Semerena JC. 2004. Identification of the protein acetyl-transferase (pat) enzyme that acetylates acetyl-CoA synthetases in Salmonella enterica. J. Mol. Biol. 340:1005–1012 [DOI] [PubMed] [Google Scholar]
- 64. Starai VJ, Celic I, Cole RN, Boeke JD, Escalante-Semerena JC. 2002. Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science 298:2390–2392 [DOI] [PubMed] [Google Scholar]
- 65. Starai VJ, Takahashi H, Boeke JD, Escalante-Semerena JC. 2003. Short-chain fatty acid activation by acyl-coenzyme A synthetases requires SIR2 protein function in Salmonella enterica and Saccharomyces cerevisiae. Genetics 163:545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sterner R, Liebl W. 2001. Thermophilic adaptation of proteins. Crit. Rev. Biochem. Mol. Biol. 36:39–106 [DOI] [PubMed] [Google Scholar]
- 67. Tauchert K, Jahn A, Oelze J. 1990. Control of diauxic growth of Azotobacter vinelandii on acetate and glucose. J. Bacteriol. 172:6447–6451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wittig I, Braun HP, Schägger H. 2006. Blue native PAGE. Nat. Protoc. 1:418–428 [DOI] [PubMed] [Google Scholar]
- 69. Wittig I, Karas M, Schägger H. 2007. High resolution clear native electrophoresis for in-gel functional assays and fluorescence studies of membrane protein complexes. Mol. Cell Proteomics 6:1215–1225 [DOI] [PubMed] [Google Scholar]
- 70. Wolfe AJ. 2005. The acetate switch. Microbiol. Mol. Biol. Rev. 69:12–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zeiher CA, Randall DD. 1991. Spinach leaf acetyl-coenzyme A synthetase: purification and characterization. Plant Physiol. 96:382–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.