Abstract
The specific aminoacylation of the phospholipid phosphatidylglycerol (PG) with alanine or with lysine catalyzed by aminoacyl-phosphatidylglycerol synthases (aaPGS) was shown to render various organisms less susceptible to antibacterial agents. This study makes use of Pseudomonas aeruginosa chimeric mutant strains producing lysyl-phosphatidylglycerol (L-PG) instead of the naturally occurring alanyl-phosphatidylglycerol (A-PG) to study the resulting impact on bacterial resistance. Consequences of such artificial phospholipid composition were studied in the presence of an overall of seven antimicrobials (β-lactams, a lipopeptide antibiotic, cationic antimicrobial peptides [CAMPs]) to quantitatively assess the effect of A-PG substitution (with L-PG, L-PG and A-PG, increased A-PG levels). For the employed Gram-negative P. aeruginosa model system, an exclusive charge repulsion mechanism does not explain the attenuated antimicrobial susceptibility due to PG modification. Additionally, the specificity of nine orthologous aaPGS enzymes was experimentally determined. The newly characterized protein sequences allowed for the establishment of a significant group of A-PG synthase sequences which were bioinformatically compared to the related group of L-PG synthesizing enzymes. The analysis revealed a diverse origin for the evolution of A-PG and L-PG synthases, as the specificity of an individual enzyme is not reflected in terms of a characteristic sequence motif. This finding is relevant for future development of potential aaPGS inhibitors.
INTRODUCTION
The Gram-negative bacterium Pseudomonas aeruginosa is the dominant pathogen infecting cystic fibrosis patients (44, 55, 90). It is well known for its successful adaptation to environmental niches, which also includes various pH conditions of the habitat (84). Due to a defect in the bicarbonate ion transport, the airway surface liquid of the lung from cystic fibrosis patients was found acidified to pH values of <6.5, which is relevant for cystic fibrosis pathogenesis (7). Under acidic conditions, P. aeruginosa lipid homeostasis results in the formation of up to 6% of an aminoacyl ester of phosphatidylglycerol (PG). This specific synthesis of alanyl-phosphatidylglycerol (A-PG) catalyzed by alanyl-phosphatidylglycerol synthase (A-PGS) also mediates the resistance against the antimicrobial compounds protamine sulfate, cefsulodin, and sodium lactate and against CrCl3 (43).
Such a modification of the polar lipid head group of PG is a widely used strategy enabling bacteria to cope with compounds that are potentially harmful for the integrity of the cell membrane. It has been shown that cationic antimicrobial peptides (CAMPs), but also various cationic antibiotics, have the ability to directly interact with the negatively charged membrane as an antibacterial target. One important bacterial response to such compounds is the aminoacylation of PG resulting in a reduction of the overall net negative charge of the membrane. The resulting aminoacyl-phosphatidylglycerol (aaPG) molecules can either be zwitter-ionic (A-PG) or alternatively they carry an overall positive net charge, as is the case for lysyl-phosphatidylglycerol (L-PG) (20, 34, 43, 56, 75, 82). The resulting charge characteristics of the bacterial envelope were also proposed to have a profound impact on biophysical properties like membrane fluidity and lipid head group interaction (72, 76, 89).
L-PG formation under low-pH conditions has been described for Staphylococcus aureus, Enterococcus faecalis (formerly known as Streptococcus faecalis) (35), and Rhizobium tropici (82). The enzyme responsible for the formation of L-PG in S. aureus was identified during studies of the bacterial immune escape mechanisms (65). S. aureus and Listeria monocytogenes multiple peptide resistance factor gene (mprF) mutant strains are found defective of L-PG synthesis and are thereby rendered sensitive to cationic defensins (65, 87). The comparison of deletion strains from S. aureus and P. aeruginosa with the corresponding wild-type strains did not indicate a growth phenotype (43, 65). In a related S. aureus analysis, the deleted strain showed identical amounts of the major membrane lipids other than L-PG (65), and the L-PG deficiency had no major impact on the membrane proteome (80).
Besides the well-described effect of L-PG formation, also the reduction of the cellular PG content was described as a mechanism lowering the overall negative surface charge, thereby rendering Bacillus subtilis and P. aeruginosa more resistant to daptomycin and polymyxin, respectively (8, 24, 25).
Most organisms encode only a single aminoacyl-phosphatidylglycerol synthase (aaPGS); however, for the Gram-positive Clostridium perfringens SM101, two homologous genes were identified, one coding for a lysyl-phosphatidylglycerol synthase (L-PGS) and an additional one coding for an A-PGS (75). The formation of A-PG and L-PG was shown to be tRNA dependent with Ala-tRNAAla and Lys-tRNALys as the substrates, respectively (30, 43, 75, 83). Standard aaPGS enzymes are capable of synthesizing a single aaPG derivative. However, for the Enterococcus faecium enzyme, a relaxed specificity for lysine, arginine, and alanine was shown. Besides this, the orthologous Bacillus subtilis aaPGS facilitates PG aminoacylation with lysine and alanine (73). It was hypothesized that the parallel synthesis of different aaPG molecules allows these organisms a more elaborate remodeling of membrane lipids, providing resistance to a broader spectrum of antibiotics or environmental stresses (73). Interestingly, to date there is no experimental data describing the cellular physiology and the related spectrum of antibiotic resistance as a result of differing aaPG molecules. According to this, it is not clear if the L-PG molecule has the ability to substitute cellular A-PG function to a certain extent. One would expect compatible roles of A-PG and L-PG under conditions where the electrostatic interaction of the phospholipid head group with a positively charged compound is the key step of antimicrobial action. The comparison of bacterial mutant strains providing an altered lipid composition might be an attractive tool to study the impact of the overall surface charge and/or lipid composition. Besides this, it will be interesting to see if such a charge repulsion is relevant in the presence of several antimicrobial compounds or if various mechanisms are responsible for the individual susceptibility to specific antimicrobials.
Almost all aaPGS enzymes share a two-domain architecture consisting of an N-terminal transmembrane domain that is highly variable in size followed by an additional C-terminal domain carrying all key amino acid residues responsible for A-PG or L-PG synthesis (30). A flippase activity for the S. aureus enzyme was attributed to the hydrophobic N-terminal domain (19). The variant L-PGS protein from C. perfringens and also the A-PGS protein from P. aeruginosa with a truncation of the complete hydrophobic domain still enabled the specific synthesis of L-PG and A-PG, respectively (30, 73). The water-soluble P. aeruginosa A-PGS domain with an N-terminal deletion of 542 amino acids was the basis for an extended mutagenesis study which initially allowed for the postulation of an enzymatic mechanism (30). The analysis of derivatives of PG indicated that the polar head group of the phospholipid is specifically recognized by the enzyme, whereas modification of an individual fatty acid or even the deletion of a single fatty acid did not abolish A-PG synthesis (30). A-PGS substrate recognition was further analyzed by using alanylated microhelices instead of the natural substrate Ala-tRNAAla. It was shown that the enzyme even tolerated mutated versions of such a minimal substrate. Out of the huge tRNA molecule, only the presence of the five terminal base pairings of the acceptor stem is essential for substrate recognition. According to these results, it was concluded that the alanyl moiety of the substrate is recognized with high specificity, whereas the tRNA molecule is responsible mainly for the activation of the amino acid (30).
The inhibition of aaPGS was proposed as a promising strategy to render pathogenic bacteria more susceptible to antibacterial molecules, including the wide range of naturally occurring antimicrobial agents of the human host (65). For the future development of such strategies, it could be beneficial to elucidate principles of A-PGS and L-PGS substrate recognition.
In the present study, we make use of different P. aeruginosa mutant strains containing an artificially altered lipid composition to investigate the impact on bacterial resistance in the presence of a total of seven antimicrobial compounds. According to these experiments, an exclusive charge repulsion mechanism does not explain the attenuated antimicrobial susceptibility due to A-PG formation in the Gram-negative P. aeruginosa, as it was proposed for several Gram-positive organisms. Our data indicate that an accurate A-PG content is essential for lipid homeostasis. This fine-tuning of cellular A-PG concentrations is also demonstrated on the transcriptional level, since an overall of five antimicrobials clearly affected the specific promoter activity of the A-PGS gene.
In the second part of this investigation, we elucidate the substrate specificity of an overall of nine as-yet-uncharacterized aaPGS enzymes (Bacillus licheniformis, Bacillus thuringiensis, Burkholderia phymatum, Kineococcus radiotolerans, Methanosarcina barkeri, Paenibacillus polymyxa, Pseudomonas aeruginosa PA14, Pseudomonas putida, and Streptococcus thermophilus). The newly identified sequences give us the opportunity to generate a significant group of A-PG-specific enzymes which are then compared bioinformatically to the group of L-PG-specific synthases to figure out the evolutionary origin of aaPGS substrate specificity.
MATERIALS AND METHODS
Construction and growth curves of complemented P. aeruginosa strains.
Genomic complementation variants were constructed on the basis of a markerless deletion mutant (the PAO1 ΔPA0920 strain) which is devoid of open reading frame (ORF) PA0920 responsible for A-PG synthesis (43). To ensure an identical transcriptional control for all complemented strains, the original P. aeruginosa promoter region (187 bp upstream of ORF PA0920) was implemented upstream of the newly introduced aaPGS genes (PA0920 from P. aeruginosa, Sa113 [also termed mprF] from S. aureus, and ORF BSU08425 [mprF] from B. subtilis) at the attB locus of the PAO1 ΔPA0920 genome (Fig. 1A).
Fig 1.
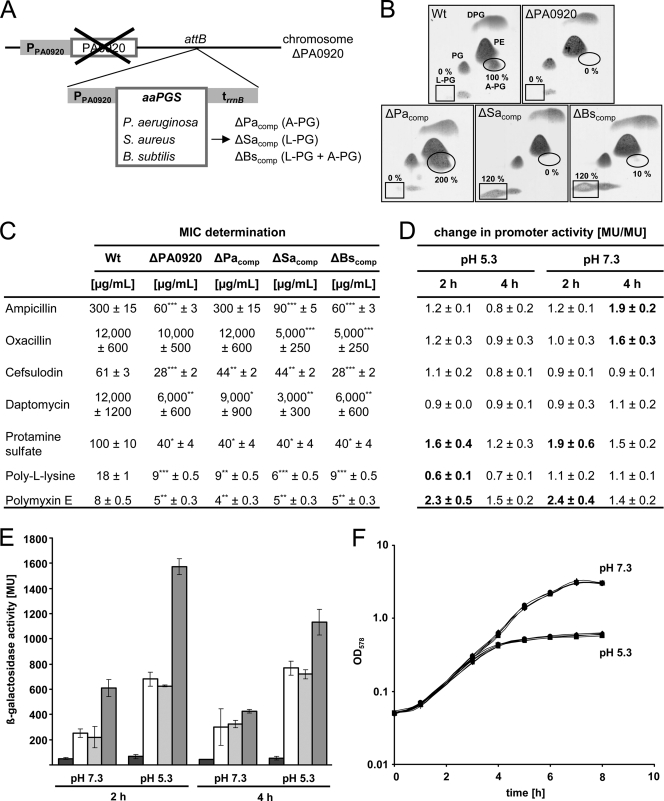
Construction and analysis of complemented P. aeruginosa mutant strains. (A) Chimeric P. aeruginosa mutant strains were constructed by the chromosomal complementation of the ΔPA0920 deletion mutant with orthologous aaPGS genes from S. aureus (ΔSacomp) and B. subtilis (ΔBscomp) strains at the attB locus, and analogously a homologous complemented strain (ΔPacomp strain) was generated. Resulting strains are devoid of any resistance marker, and the related specificity of the individual aaPGS gene for the synthesis of A-PG and/or L-PG is indicated. PPA0920, native promoter region of ORF PA0920 (187 bp); trrnB, rrnB terminator sequence (158 bp); attB, attachment site B locus. (B) Lipid composition of P. aeruginosa mutant strains. Cells were cultivated in AB medium (pH 5.3) and harvested, and polar lipids were extracted and separated by two-dimensional TLC. Lipids were visualized by molybdatophosphoric acid staining. The specific positions of A-PG and L-PG are indicated by an ellipse and a rectangle, respectively. Relative amounts of A-PG and L-PG were quantified using Gelscan 6.0 software. The A-PG level of the wild-type strain was defined as 100%, and all other values were related to this. (C) MICs were determined by treating the strains with gradually varying inhibitor concentrations in AB medium, pH 5.3. Significance analysis revealed P values of ≤0.1 (*), P values of ≤0.05 (**), and P values of ≤0.01 (***). (D) The promoter activity of the P. aeruginosa A-PGS gene was analyzed using a transcriptional PPA0920-lacZ fusion. Cells were grown in AB medium, pH 5.3 and 7.3, in the presence of subinhibitory concentrations of antimicrobials, and β-galactosidase activity was determined after 2 and 4 h of cultivation, respectively. To evaluate the individual effect of each compound, observed MU were expressed as an induction ratio (the ratio of Miller units in the presence and absence of the antimicrobial compound). A ratio of >1 indicates transcriptional activation due to the presence of a specific compound. (E) Selected promoter activities (from panel D) are indicated to demonstrate the influence of antibiotics, pH, and the cultivation time. MU values for untreated cultures are indicated with white bars and for the promoterless control strain KS11 with black bars. Promoter activities in the presence of daptomycin and polymyxin E are indicated in light gray and dark gray, respectively. (F) Growth curves for P. aeruginosa variants were determined by measuring the turbidity at 578 nm in AB medium, pH 5.3 and pH 7.3. Rhombus, wild type; quadrat, ΔPA0920 strain; triangle, ΔPacomp strain; dot, ΔSacomp strain; plus, ΔBscomp strain. For experimental details, see Materials and Methods. A-PG, alanyl-phosphatidylglycerol; L-PG, lysyl-phosphatidylglycerol; PE, phosphatidylethanolamine; PG, phosphatidylglycerol, DPG, diphosphatidylglycerol.
For this purpose, a DNA fragment consisting of the native A-PGS promoter PPA0920 (187 bp) and the appropriate cloning sites (SphI, BamHI) and the rrnB terminator sequence (158 bp; Invitrogen, Darmstadt, Germany) was synthesized by GENEART AG (Regensburg, Germany) (for the sequence, see Table S2 in the supplemental material). This synthetic fragment (promoter-terminator construct) was inserted into the mini-CTX2 vector (33) via the restriction sites SacI and HindIII. The resulting vector (mini-CTX2-PTC) was then used to amplify an appropriate SacI-SphI promoter fragment. This fragment, in combination with amplified genes PA0920 of P. aeruginosa PAO1, Sa113 of S. aureus NCTC 8325, and BSU08425 of B. subtilis subsp. subtilis strain 168, was then ligated into the SacI and BamHI sites of the mini-CTX2-PTC vector (primers for the amplification of the individual SphI-BamHI fragments are summarized in Table S2 in the supplemental material, primer C). Transfer of these vectors into ΔPA0920 PAO1 was obtained by diparental mating using Escherichia coli ST18 as the donor (88). The CTX integrase provided by the mini-CTX2 vector promoted integration of the respective vectors into the neutral attB phage attachment site of the parental strain chromosome. Finally, markerless complemented strains were generated by removal of the FRT-flanked vector sequences using the pFLP2-encoded FLP recombinase (32). This resulted in the homologously complemented P. aeruginosa strain (ΔPacomp strain) and the chimeric strains complemented with the aaPGS genes from S. aureus (ΔSacomp strain) and from B. subtilis (ΔBscomp strain). The accurate gene integration was verified by DNA sequence analysis. Strains and plasmids are summarized in Table S1 in the supplemental material.
For the determination of growth curves, strains were cultivated in 60 ml of AB medium (31) at 37°C and 200 rpm. The pH of the medium was adjusted using an appropriate phosphate buffer. Growth was monitored by measuring culture turbidity at 578 nm.
Lipid composition of complemented strains.
The ΔPacomp, ΔSacomp, and ΔBscomp complementation variants, the PAO1 wild type, and the ΔPA0920 strain were grown in AB medium at pH 7.3 and 5.3 to late stationary phase (24 h). Identical amounts of cells were harvested by centrifugation (according to the optical density at 578 nm [OD578]). Cell pellets were stored at −20°C and subsequently subjected to lipid extraction. Analysis of lipids was performed by two-dimensional thin-layer chromatography (TLC) as described elsewhere (43).
Quantification of polar lipids.
Overall amounts of polar lipids after molybdatophosphoric acid staining were quantified using the Gelscan 6.0 software (BioSciTec GmbH, Frankfurt, Germany). For the chimeric mutant strains of P. aeruginosa, the amount of aaPG molecules was related to the A-PG content of the wild-type strain (A-PG content set as 100%).
Phenotyping of complemented strains using Phenotype MicroArray.
The initial characterization of the complemented strains was performed by using microplates PM9 to PM20 and IF-10 medium of the Phenotype MicroArray system (Biolog Inc., Hayward, CA) according to the manufacturer's protocol. These plates provide tests for pH and salt stresses and measure sensitivities to chemical compounds like antimetabolites, antibiotics, and other inhibitors. Phenotypical changes were monitored by the OmniLog system and the accessory software (Biolog Inc.). For all compounds showing phenotypical alterations, a second independent experimental setup was performed. Therefore, bacteria were inoculated in AB medium at a pH of 5.3 and subjected to different compounds at various concentrations in 96-well plates. After being covered with a gas-permeable membrane (Breathe-Easy sealing membrane; Diversified Biotech, Dedham), plates were incubated at 37°C without shaking. Growth was monitored by turbidity measurement at 595 nm using a model 680 microplate reader (Bio-Rad, Munich, Germany) in the absence of the sealing membrane.
MIC determination.
For MIC determination in the presence of the antimicrobials ampicillin (Roth, Karlsruhe, Germany), daptomycin (Novartis, Nürnberg, Germany), oxacillin, cefsulodin, poly-l-lysine, protamine sulfate, and polymyxin E (Sigma-Aldrich, St. Louis, MO), a modified method according to Andrews (3) was used. Strains were cultivated in AB medium (pH 5.3) to late exponential phase (4 h) and diluted to an OD578 of 0.2. In 96-well plates (order no. 82.1581.001; Sarstedt AG Co., Nümbrecht, Germany), 50 μl of AB medium (pH 5.3) at a 3-fold concentration was supplemented with 100 μl of the respective antimicrobial compound (aqueous solution) and inoculated with 50 μl of the bacterial suspension. Each experiment was performed in quadruplicate. Plates were covered with a gas-permeable membrane (Breathe-Easy sealing membrane) and incubated for 18 h in a plate incubator (PHMP-4; Grant Bio, Cambridgeshire, United Kingdom) at 37°C and 350 rpm. Subsequently, bacterial growth was analyzed with a model 680 microplate reader (Bio-Rad), monitoring OD595 in the absence of the sealing membrane.
This experimental setup allows for the accurate determination of the MIC by gradually narrowing the range of the employed antimicrobial concentrations. The lowest concentration resulting in an OD595 of <0.05 (ampicillin, oxacillin, cefsulodin, poly-l-lysine, polymyxin E) or OD595 of <0.1 (daptomycin) was defined as the MIC of the respective compound. For protamine sulfate, the lowest concentration was determined as the MIC at which a constant minimum level of OD595 was reached. Concentration intervals were successively adjusted, ranging from 4 to 6%, according to the final MIC of the respective experiment. All values were reproduced on the basis of three independent sets of experiments. The relative standard deviations for MIC determinations were evaluated on the basis of the OD595-concentration plots. Values of approximately 5% (10% for protamine sulfate and daptomycin) were obtained. Significance of the presented data was calculated using a paired Student t test.
Promoter activity analysis.
Promoter activity studies made use of the PAO1-PPA0920-lacZ strain as described elsewhere (43). An identical P. aeruginosa strain which is devoid of the PPA0920 promoter sequence was employed as a control (PAO1 KS11) (79). The strains were cultivated in acidic (pH 5.3) and neutral (pH 7.3) AB medium for 2 h at 37°C and 200 rpm. Then the antimicrobial compounds were added and cells were further incubated for 4 h. Antimicrobials often have a tremendous influence on the cell morphology (21, 81), which might influence the validity of the related Miller units (MU). Such problems were circumvented by additionally using an experimental approach which relates bacterial transcript levels to the overall bacterial protein content obtained from Bradford determinations (37). The following subinhibitory compound concentrations were employed: one-fourth of the MIC obtained for the wild-type strain in the presence of ampicillin, oxacillin, cefsulodin, protamine sulfate, and poly-l-lysine; one-sixth of the MIC for polymyxin E; and one-eighth of the MIC for daptomycin. After 2 h and 4 h of compound application, the cell morphology was analyzed microscopically and samples were taken for the subsequent determination of β-galactosidase activity (70) and also to quantify the related protein content. In all cases, the appropriate pH value of 5.3 for the individual culture conditions was verified. All experiments were performed in triplicate. For Bradford determinations, cell pellets (culture volume of 1 ml) were incubated in 60 to 600 μl of 50 mM NaOH at 95°C and 1,400 rpm for 5 min. After sedimentation of cell debris, protein concentration was determined using Bradford reagent (Sigma-Aldrich) according to the manufacturer's instructions. Miller units were also calculated on the basis of the overall protein content. The results based on this alternative methodology did not show any differences when related to promoter activity values observed from optical density measurement.
For the evaluation of the individual effect of each antimicrobial compound at different growth phases and under different pH conditions, the observed promoter activity was expressed as an induction ratio (the ratio of Miller units in the presence and absence of the compound). Standard deviations were calculated according to reference 86.
Additionally, the lipid composition of the control strain was analyzed in the presence of antimicrobial compounds showing promoter induction. Therefore, lipid analyses were performed for the following conditions: ampicillin (one-fourth the MIC; pH 5.3 for 2 h and pH 7.3 for 4 h), oxacillin (one-fourth the MIC; pH 5.3 for 2 h and pH 7.3 for 4 h), protamine sulfate (one-fourth the MIC; pH 5.3 for 2 h and pH 7.3 for 2 h), and polymyxin E (one-sixth the MIC; pH 5.3 for 2 h and pH 7.3 for 4 h). Lipid extraction and subsequent two-dimensional TLC were performed as described above.
Bacterial strains and media for the investigation of aaPGS specificities.
The following strains were analyzed with respect to the synthesis of aaPG in vivo: Bacillus licheniformis ATCC 14580, Bacillus thuringiensis serovar Berliner ATCC 10792, Burkholderia phymatum STM 815, Kineococcus radiotolerans SRS 30216, Paenibacillus polymyxa ATCC 842, Pseudomonas aeruginosa UCBPP-PA14, Pseudomonas putida KT2440, and Streptococcus salivarius subsp. thermophilus ATCC 19258 (see Table S1 in the supplemental material).
B. licheniformis, B. thuringiensis, and P. polymyxa were cultured in Spizizen's minimal medium (SMM) according to reference 29, B. phymatum in R2A medium (67), P. aeruginosa PA14 and P. putida in AB medium (31), S. thermophilus in tryptic soy broth (TSB) medium (96) supplemented with 0.3% yeast extract, and K. radiotolerans in LB medium (78). B. phymatum was cultured at 30°C, and all other strains were cultured at 37°C. Cultures were inoculated using an overnight culture (in a ratio of 1:100) and agitated at 200 rpm (S. thermophilus was without agitation).
Induction of aaPG synthesis in response to acidic conditions.
To ensure acidic growth conditions, LB or R2A medium was acidified with 0.3 M HCl to pH 5, and then a phosphate buffer (pH 5.0) was added to a final concentration of 45 mM. SMM was acidified using 85% phosphoric acid, and AB medium was supplemented with phosphate buffer (pH 5.0) to a final concentration of 45 mM. In all cases, pH values were verified throughout cultivation. B. licheniformis, B. thuringiensis, P. polymyxa, and P. putida were cultivated for 8 h, P. aeruginosa PA14 for 24 h, and S. thermophilus for 72 h. For B. phymatum and K. radiotolerans, the pH of the culture was reequilibrated using 0.3 M HCl after 7 h and then further cultivated for 1 h. The overall lipid composition of each bacterial strain was analyzed (neutral and acidic conditions) by two-dimensional TLC (molybdatophosphoric acid staining), and the relative amount of aaPG molecules was related to the overall polar lipid content. To verify the identity of A-PG and L-PG, an additional TLC experiment was analyzed by ninhydrin staining (detection of free amino groups) (43).
Lipid analysis by mass spectroscopy.
Identification of L-PG and diglucosyl diacylglycerol from S. thermophilus and B. licheniformis was performed by mass spectrometry as described elsewhere (87).
Plasmid construction and recombinant analysis of aaPGS activity (full-length protein).
Gene sequences of Bphy_4019 (B. phymatum STM 815), Krad_4555 (K. radiotolerans SRS 30216), Bthur0008_13400 (B. thuringiensis ATCC 10792), and Mbar_A2435 (Methanosarcina barkeri strain Fusaro ATCC 29787) were amplified by PCR using primer A (see Table S2 in the supplemental material). The specific sequence from P. polymyxa ATCC 842 was amplified using random primers Paeni1SacIfw and Paeni1KpnIrv (see Table S2A). All PCR fragments were cloned into pBAD-His-A (Invitrogen, Karlsruhe, Germany) to obtain the following plasmids (see Table S1 in the supplemental material): pBAD-His-A/Bp4019 for Bphy_4019 (XhoI/HindIII), pBAD-His-A/Krad4555 for Krad_4555 (SacI/HindIII), pBAD-His-A/Bthur0008_13400 for Bthur0008_13400 (XhoI/KpnI), pBAD-His-A/Ppoly_aaPGS for aaPGS from P. polymyxa (SacI/KpnI), and pBAD-His-A/A2435 for Mbar_A2435 (XhoI/XhoI). Sequences of all inserts were verified (GATC Biotech AG, Konstanz, Germany).
For recombinant protein production in E. coli TOP10, cells were grown at 37°C and 200 rpm to an OD578 of 0.5, and protein synthesis was induced using 0.2% (wt/vol) l-(+)-arabinose (2.0% for pBAD-His-A/Krad4555 and pBAD-His-A/A2435). After 3 h of incubation, cells were harvested (20 min, 4°C, 3,000 × g) and washed using 50 mM HEPES-NaOH, pH 7.8. After an additional sedimentation (20 min, 4°C, 4,000 × g), cells were suspended according to their culture turbidity and disrupted by French press (Thermo Fisher Scientific, Waltham, MA) passage at 19,200 lb/in2. The crude cellular extracts were subjected to the in vitro activity assay which consisted of 40 μM [1-14C]l-alanine (51 mCi/mmol) or 7 μM [U-14C]l-lysine (288 mCi/mmol) (Moravek Biochemicals, Brea, CA), 2 mM ATP, an ATP regenerating system (18 mM creatine phosphate, 25 U creatine phosphokinase; Sigma-Aldrich), and the crude cellular extract in a final volume of 500 μl. Assays were incubated for 1 h at 37°C with agitation and subsequently stored at −20°C. Lipids were extracted and visualized by two-dimensional TLC and autoradiography. E. coli TOP10 harboring the empty pBAD-His-A vector was employed as a control.
Plasmid construction and recombinant analysis of aaPGS activity (C-terminal domain).
The C-terminal aaPGS domains from B. licheniformis ATCC 14580, S. thermophilus ATCC 19258, and Bacillus anthracis strain Sterne were identified on the basis of sequence alignments according to reference 30. Gene sequences encoding amino acid residues 519 to 850 (B. licheniformis), 504 to 851 (S. thermophilus), and 528 to 861 (B. anthracis) were amplified by PCR using chromosomal DNA (B. licheniformis, S. thermophilus). For the L-PGS domain from B. anthracis, a synthetic gene (GENEART AG, Regensburg, Germany) was employed which has been adaptated according to the codon usage of E. coli. The PCR fragment from B. anthracis was cloned into the NdeI/XhoI sites of pET22b(+) (Merck, Darmstadt, Germany), resulting in pET22b(+)/BAS1375Δaa1-527. Fragments from B. licheniformis and S. thermophilus were cloned into the BamHI/XhoI sites of vector pGEX-6P-1 (GE Healthcare, Freiburg, Germany), leading to pGEX-6P-1/YfiXΔaa1-518 and pGEX-6P-1/StMprFΔaa1-503 (see Table S1 in the supplemental material). Vectors were transferred into E. coli BL21 (λDE3).
Cells were cultivated to an optical density of 0.5, and protein production was induced using 50 μM [pGEX-6P-1/YfiXΔaa1-518, pET22b(+)/BAS1375Δaa1-527] or 300 μM (pGEX-6P-1/StMprFΔaa1-503) isopropyl-β-d-thiogalactopyranoside (GERBU Biotechnik GmbH, Wieblingen, Germany). After an additional cultivation for 4 h at 37°C (pGEX-6P-1/YfiXΔaa1-518) or overnight [pET22b(+)/BAS1375Δaa1-527, 25°C; pGEX-6P-1/StMprFΔaa1-503, 17°C], cells were harvested (15 min, 4°C, 4,000 × g) and subsequently stored at −20°C. Lipids were extracted and analyzed as described above.
Sequence alignment, WebLogo, and sequence identity analysis.
For the comparison of the C-terminal aaPGS amino acid sequences (Tables 1 and 2), the ClustalW2 software (50) was used. This alignment (or specific groups of this alignment) was employed to generate a WebLogo for all aaPGS enzymes (or alternatively for the A-PGS or L-PGS group) (11). Sequence identity values were calculated using the Lasergene software package (DNASTAR Inc., Madison, WI).
Table 1.
Substrate specificity of orthologous aaPGS enzymes, cellular aaPG content, and acidic induction
| Strain | Enzyme/ORF | Method(s) for specificity determination (reference)a | Specificity (reference) | % aaPG of overall lipid content (reference)b | Acidic inductionc |
|---|---|---|---|---|---|
| Bacillus anthracis strain Sterne | BAS1375 | ACTD, E (77) | L-PG (77) | 10 (77) | ND |
| Bacillus licheniformis ATCC 14580 | YfiX | ACTD, C, D, E | L-PG | 3 | – |
| Bacillus thuringiensis ATCC 10792 | Bthur0008_13400 | AFL, BFL, C, D | L-PG | 10 | – |
| Streptococcus thermophilus ATCC 19258 | stu1256 | ACTD, C, E | L-PG | 10 | ND |
| Pseudomonas aeruginosa UCBPP-PA14 | PA14_52350 | C | A-PG | 0.5/4 | + |
| Pseudomonas putida KT2440 | PP_1202 | C | A-PG | 0.5/5 | + |
| Burkholderia phymatum STM 815 | Bphy_4019 | AFL, BFL, D | A-PG | 0.5 | – |
| Kineococcus radiotolerans SRS 30216 | Krad_4555 | AFL, BFL, D | A-PG | 0.5 | – |
| Paenibacillus polymyxa ATCC 842 | A-PGS | AFL, BFL, C, D | A-PG | 4 | – |
| Methanosarcina barkeri ATCC 29787 | Mbar_A2435 | BFL | A-PG | ND | ND |
Methods for specificity determination using either recombinant full-length protein (FL) or C-terminal domain (CTD): A, lipid analysis by molybdatophosphoric acid staining of E. coli cells producing the recombinant protein; B, in vitro assay using 14C-amino acids. Specificity determination using native strains: C, lipid analysis by molybdatophosphoric acid staining; D, lipid analysis by ninhydrin staining; E, mass spectrometry of respective lipid spot.
Cellular aaPG amounts have been determined from two-dimensional TLC analyses. Values are related to the overall lipid content. The potential induction of aaPG synthesis under acidic growth conditions was analyzed as described in Materials and Methods, and values for neutral conditions (pH 7.3)/acidic conditions (pH 5.3) are indicated. ND, not determined.
ND, not determined; −, no increase of cellular aaPG concentrations; +, significantly elevated cellular aaPG concentrations under acidic growth conditions (see Fig. S2 and S3 in the supplemental material).
Table 2.
Substrate specificity of previously described aaPGS enzymes
| Strain | Enzyme/ORF | Specificity | Reference(s) |
|---|---|---|---|
| Agrobacterium tumefaciens | LpiA | L-PG | 68, 73 |
| Bacillus anthracis | BAS1375 | L-PG | 77 |
| Bacillus subtilis | MprF | L-PG, A-PG | 73, 74, 82 |
| Caulobacter crescentus | CC_2047 | L-PG | 10, 39 |
| Clostridium perfringens | CPR_1564 | A-PG | 73, 75 |
| CPR_1258 | L-PG | 75 | |
| Enterococcus faecium | ZP_000604896 | L-PG, A-PG, R-PG | 73 |
| Listeria monocytogenes | Lmo1695 | L-PG, L-DPG | 87 |
| Mycobacterium smegmatis | LysX | L-PG | 58 |
| Mycobacterium tuberculosis | LysX | L-PG | 59 |
| Pseudomonas aeruginosa PAO1 | A-PGS (PA0920) | A-PG | 30, 43 |
| Rhizobium tropici | LpiA | L-PG | 82 |
| Staphylococcus aureus | MprF | L-PG | 19, 65, 83 |
Phylogenetic tree construction.
The software iTOL was used to generate the phylogenetic tree of prokaryotes containing representative members of different classes of organisms (51). The presence of aaPGS enzymes was individually inspected by using the C-terminal domain of P. aeruginosa in BLAST analysis (1). Absence of aaPGS proteins was indicated by an E value of <e−40. For Mycobacteria containing fusion proteins of an aaPGS enzyme and a tRNA synthetase (59), an E value of <e−20 was used.
RESULTS
A-PGS-dependent resistance phenotypes of P. aeruginosa.
The modification of PG with alanine or lysine was shown to render various organisms less susceptible to antimicrobial agents. The well-established Gram-negative model organism P. aeruginosa PAO1 was employed to characterize sophisticated phenotypes which are dependent on the aminoacylation of the phospholipid PG. The wild-type strain and the A-PGS-deficient (ΔPA0920) strain were cultivated under 1,152 different culture conditions using the Phenotype MicroArray system (Biolog, Hayward, CA) at neutral pH conditions. This high-throughput test system inter alia contains a wide variety of antibiotics, antimetabolites, and inhibitors. It is based on the reduction of a tetrazolium dye as a result of the cell's metabolism. The resulting purple formazan can be monitored photometrically. The analysis revealed a set of 30 conditions, where the P. aeruginosa wild-type strain and the ΔPA0920 knockout mutant showed phenotypic differences (data not shown).
In a second set of experiments, the results of the commercial phenotype array were reevaluated under acidic growth conditions at pH 5.3 to ensure maximum cellular A-PG content (43). All components were individually mixed in 96-well plates, thereby employing concentrations of the individual inhibiting agents. The corresponding phenotypes of the wild-type and ΔPA0920 strains were determined by directly measuring bacterial growth at an OD595 in the absence of the tetrazolium dye. This type of experiment was also employed to analyze compounds showing aaPGS-mediated resistance phenotypes (24, 40, 47, 64) or a direct influence on membrane integrity in related studies with other microbes (28, 61, 71).
Under the employed growth condition, the most distinct resistance phenotypes for the wild-type strain in comparison to the ΔPA0920 strain were observed in the presence of ampicillin, oxacillin, cefsulodin, daptomycin, protamine sulfate, poly-l-lysine, and polymyxin E (compare structures denoted in Fig. S1 in the supplemental material; complementation experiments are summarized in Fig. 1C). For several β-lactams and daptomycin, related resistance phenotypes have also been described for the Gram-positive model organisms S. aureus and B. subtilis (24, 40, 47, 64). The cephalosporin cefsulodin and the cationic peptide protamine sulfate have been recently identified in our previous P. aeruginosa study (43).
Characterization of A-PGS-dependent resistance phenotypes using MIC determinations.
Subsequently, the individual phenotypes were more quantitatively evaluated by using a fine-tuned linear concentration range in the microarray test system. Each OD595 measurement was averaged from four parallel growth experiments. The resulting MIC was reproduced with three independent sets of experiments. In all cases, a significantly higher MIC was observed for the wild-type strain than for the ΔPA0920 mutant strain.
For the β-lactam antibiotics ampicillin and oxacillin, MIC reductions from 300 μg/ml to 60 μg/ml and from 12 mg/ml to 10 mg/ml were observed, respectively, whereas the cephalosporin cefsulodin resulted in a MIC reduction from 61 μg/ml to 28 μg/ml. Treatment with the lipopeptide daptomycin revealed a MIC of 12 mg/ml for the wild-type strain and of 6 mg/ml for the deletion mutant. For the CAMP protamine sulfate, the MIC was reduced from 100 μg/ml to 40 μg/ml, and for the cationic peptide poly-l-lysine, a significant MIC reduction from 18 μg/ml to 9 μg/ml was observed. For the third CAMP, polymyxin E, a MIC reduction from 8 μg/ml to 5 μg/ml was determined (Fig. 1C).
Obviously, the A-PGS-mediated resistance of P. aeruginosa results in alterations of the MIC in a range which is comparable to values observed for several multiresistant clinical isolates of P. aeruginosa (69, 85). Of note, the employed concentrations for experiments in the presence of oxacillin and daptomycin are three orders of magnitude higher than standard clinical application (17, 94). The established P. aeruginosa test system allows for the efficient determination of gradual resistance phenotypes. This is an important prerequisite for the subsequent analysis of chimeric aaPGS strains of P. aeruginosa.
Construction of chimeric aaPGS strains of P. aeruginosa by site-specific chromosomal integration.
Orthologous aaPGS enzymes showing different substrate specificities are key players for the lipid homeostasis in response to specific antibacterial compounds in different organisms. To date, it is not clear whether the formation of A-PG or L-PG or the concurrent production of both modified phospholipids results in comparable resistance mechanisms. In the present study, we compare a wild-type P. aeruginosa strain with a deletion mutant strain which is devoid of A-PG synthesis (ΔPA0920 strain). This mutant strain was specifically complemented by introducing the homologous ORF PA0920 encoding the A-PGS and the corresponding promoter region (187 bp upstream) from P. aeruginosa at the attB locus of the genome (ΔPacomp strain). Alternatively, the ΔPA0920 strain was cross-complemented by introducing the orthologous ORF Sa113 from S. aureus (ΔSacomp strain) or by inserting ORF BSU08425 from B. subtilis (ΔBscomp strain), both also known as mprF. For these two chimeric mutant strains, the original P. aeruginosa promoter region (187 bp) was implemented at the same position located upstream of the corresponding aaPGS genes. This mutant design enables a consistent transcriptional response for all complemented mutant strains in the absence of any resistance marker. The aaPGS gene from S. aureus is responsible for the formation of L-PG, whereas the gene from B. subtilis enables the parallel formation of L-PG and A-PG (65, 73). The construction of the complemented mutant strains is schematically represented in Fig. 1A.
Lipid composition of P. aeruginosa mutant strains.
In a recent study for P. aeruginosa PAO1, a maximum A-PG content of 6% (in relation to the total phospholipid content) was observed under growth conditions at pH 5.3 (43). To unambiguously verify that the complemented mutant strains still underlie an identical transcriptional control mechanism as described for the wild-type strain, the overall lipid content of all mutants was analyzed. The program Gelscan 6.0 was used to determine relative amounts of polar lipids after two-dimensional TLC separation. The A-PG content of the P. aeruginosa wild-type strain was set as 100%, and all other values of aaPG molecules for the complemented strains were related to this.
The complemented ΔPacomp strain.
For the complemented P. aeruginosa ΔPacomp strain, significant amounts of A-PG were observed under acidic growth conditions, as described for the wild-type strain (Fig. 1B). However, the employed lipid quantification for the ΔPacomp strain revealed an overall amount of 200% A-PG compared to that of the wild-type strain. This might be a result of the varying genomic context of the attB integration site, which was employed for the insertion of the A-PGS gene in combination with the natural P. aeruginosa promoter. Our experimental strategy resulted in a mutant showing an artificially increased A-PG content. This strain is a versatile tool to study resistance phenotypes under conditions of artificially elevated A-PG levels.
The chimera complemented with the S. aureus L-PGS gene (ΔSacomp strain).
The cross-complementation with the L-PGS gene (mprF) from S. aureus resulted in the formation of L-PG, which is in agreement with previous studies (19, 52, 65, 83) (Fig. 1B). In comparison to the P. aeruginosa wild-type strain, an overall L-PG content of 120% was determined. The positive net charge of the artificially synthesized L-PG molecule results in a partial reversal of the charge characteristics of the lipid bilayer of this mutant. Therefore, the ΔSacomp strain is an appropriate tool to verify the present repulsion model describing reduced susceptibility of Gram-positive organisms in the presence of cationic antimicrobials.
The chimera complemented with the B. subtilis aaPGS gene (ΔBscomp strain).
The cross-complementation with the aaPGS gene from B. subtilis indicated the dominant formation of two aaPGs under the employed growth conditions. The amount of the observed L-PG and A-PG was related to the corresponding A-PG content of the wild-type P. aeruginosa strain (compare Fig. 1B). The employed growth conditions resulted in the formation of 10% A-PG and 120% L-PG, which reflects the aaPG ratio (A-PG/L-PG) as observed for the B. subtilis aaPGS under in vitro conditions (73). This strain allows for the analysis of potential resistance phenotypes due to the concomitant production of A-PG and L-PG.
All chimeric mutant strains clearly facilitated aaPG formation in the presence of the natural aminoacyl-tRNA substrates from P. aeruginosa. These observations are in good agreement with our previous in vitro experiments, which indicated that the individual sequence of the tRNA moiety of the aaPGS substrate is not a determinant for substrate recognition (30). The overall cellular A-PG contents of P. aeruginosa were almost identical after 6.5 and 24 h. When all mutant strains were cultivated under standard conditions, no difference in the corresponding growth curves at pH 5.3 and pH 7.3 were observed, respectively (Fig. 1F). The employed quantitative two-dimensional TLC lipid analysis did not reveal any differences in the cellular concentrations of standard lipids diphosphatidylglycerol (DPG), phosphatidylethanolamine (PE), and PG of the respective mutant strains (Fig. 1B).
Phenotypes in the presence of β-lactam antibiotics.
The complemented ΔPacomp, ΔSacomp, and ΔBscomp strains were used to study the impact of increased or specifically altered aaPG contents in the bacterial membrane. This is of relevance, since the low intrinsic susceptibility of P. aeruginosa to β-lactam antibiotics is attributed to the low permeability of the cellular envelope (26) together with the presence of chromosomally encoded detoxification systems, such as multidrug efflux systems or antibiotic-inactivating enzymes (2). According to this, the employed oxacillin and daptomycin concentrations of this study are not capable of treating P. aeruginosa infections. However, the incorporation of A-PG into the inner and the outer bacterial membranes of P. aeruginosa (43) resulted in the lowering of the susceptibility to the β-lactam antibiotics ampicillin, oxacillin, and cefsulodin (Fig. 1C), which might be a result of a lower membrane permeability (27).
Ampicillin resistance phenotypes.
The complemented P. aeruginosa ΔPacomp strain showed a fully restored phenotype in the presence of ampicillin as indicated by a MIC of 300 μg/ml (Fig. 1C). Obviously, the significantly increased A-PG content of this mutant strain had no deleterious influence on the observed resistance phenotype. However, for the chimeric ΔBscomp mutant strain, an identical MIC value of 60 μg/ml was obtained, as observed for the deletion mutant. These results clearly indicate that an additional aaPG content of 10% A-PG together with 120% L-PG does not reduce the ampicillin susceptibility of the ΔPA0920 strain. In contrast to this, a MIC of 90 μg/ml was observed for the ΔSacomp chimeric strain, which facilitates the synthesis of L-PG at a level of 120%. This might indicate that the specific production of L-PG can partially restore the resistance phenotype of the wild-type strain. However, full resistance to ampicillin is dependent on the synthesis of A-PG in the context of the employed P. aeruginosa model.
Oxacillin resistance phenotypes.
Also for this β-lactam, the homologous ΔPacomp mutant strain showed a fully restored phenotype in the presence of a significantly elevated A-PG content, as indicated by a MIC of 12 mg/ml (Fig. 1C). Obviously, the highly increased A-PG content of 200% was not harmful for the bacterial cell in the presence of oxacillin. However, for the ΔBscomp and ΔSacomp chimeric mutant strains, an increased susceptibility with respect to this antibiotic was observed, even in comparison to the P. aeruginosa ΔPA0920 deletion strain. MIC determinations resulted in values of 5 mg/ml for ΔBscomp and ΔSacomp strains, which is considerably lower than the MIC of the ΔPA0920 strain (10 mg/ml). These results clearly indicate that the artificial lipid composition of ΔBscomp and ΔSacomp strains renders these strains highly susceptible to this antibiotic. Obviously, minor alterations in the chemical structure of the employed compound (see Fig. S1 in the supplemental material) have important consequences for the observed resistance phenotypes, as indicated by a 40-fold increase of the wild-type MIC in the presence of ampicillin (300 μg/ml) or oxacillin (12 mg/ml). Analyses for the outer membrane protein OmpF of E. coli indicated that this porin allows for an increased transfer of zwitter-ionic β-lactams, like ampicillin, whereas a significantly lower transfer rate was observed in the presence of mono- or dianionic β-lactams, like oxacillin (see Fig. S1) (63, 92, 95). According to the dramatic increase of the MIC in the presence of oxacillin, an analogous mechanism for P. aeruginosa seems reasonable. However, the observed effects in response to the different complementation strategies greatly differ in the presence of ampicillin versus oxacillin. This might indicate that differing mechanisms (e.g., of repulsion, of polarity, of fluidity) are responsible for the acquired resistance against a specific β-lactam.
Cefsulodin resistance phenotypes.
In experiments using the cephalosporin cefsulodin, the complemented P. aeruginosa ΔPacomp strain was able to only partially restore the resistance phenotype of the wild-type strain. In the presence of increased A-PG levels of this strain, a MIC of 44 μg/ml (Fig. 1C) was determined, whereas concentrations of 61 μg/ml and 28 μg/ml were observed for the wild-type strain and the ΔPA0920 strain, respectively. This might indicate that cefsulodin resistance is dependent on an accurate A-PG level. This hypothesis is also confirmed by the MICs obtained for ΔBscomp and ΔSacomp strains, which are 28 μg/ml and 44 μg/ml, respectively. Neither the presence of L-PG nor the presence of L-PG and A-PG restored the antibiotic resistance of the wild-type strain. However, the relative level of 120% L-PG (ΔSacompstrain) resulted in a comparable effect as observed for the homologously complemented strain. Under conditions of cefsulodin treatment, L-PG partially restored the phenotype of the wild type.
Phenotypes in the presence of daptomycin.
The new lipopeptide antibiotic daptomycin (see Fig. S1 in the supplemental material) shows activity against a wide range of Gram-positive bacteria (17). A direct interaction with PG of the cytoplasmic membrane via Ca2+ binding was proposed (42). Pore formation might be initiated by aggregated oligomers, which then results in membrane depolarisation and cell death (62). P. aeruginosa possesses a high intrinsic resistance to daptomycin, which might be attributed to an efficient outer membrane barrier.
Treatment of the ΔPacomp, ΔSacomp, and ΔBscomp complemented strains resulted in different MICs. For the ΔPacomp strain, a MIC of 9 mg/ml was detected, while wild-type and deletion strains showed MICs of 12 mg/ml and 6 mg/ml, respectively (Fig. 1C). Here, the increased amount of A-PG in the ΔPacomp strain might partially restore the wild-type phenotype. For the ΔSacomp strain, a MIC of 3 mg/ml indicated a decreased daptomycin resistance as a result of L-PG formation. In the presence of both A-PG and L-PG in the ΔBscomp strain, a MIC of 6 mg/ml was determined. The minor amounts of A-PG might be responsible for the slightly increased MIC in comparison to the ΔSacomp strain.
These results might indicate that only the specific formation of A-PG is responsible for an increased resistance to daptomycin, whereas the formation of L-PG significantly increases the susceptibility of the P. aeruginosa model system. In contrast to this, the formation of L-PG in S. aureus and B. subtilis is a key process leading to a reduced susceptibility in the presence of daptomycin (22, 24, 41, 60).
Phenotypes in the presence of cationic antimicrobial peptides.
Antimicrobial peptides are important for the host defense against several bacteria. These peptide molecules are composed of a hydrophobic and a highly cationic domain which allows them to interact with bacterial membranes (97). Microbes have evolved resistance mechanisms against such CAMP molecules, for example, by alteration of the charge characteristics of the membrane (49). Mainly on the basis of experiments with such CAMP molecules, the charge repulsion model was postulated as a mechanism describing the reduced susceptibility due to the formation of L-PG in Gram-positive organisms (65).
The CAMPs protamine sulfate, poly-l-lysine, and polymyxin E (see Fig. S1 in the supplemental material) were employed for the phenotyping of the ΔPacomp, ΔSacomp, and ΔBscomp complemented strains. For these types of molecules, interaction with the bacterial membrane was described as a mode of antibacterial action in Gram-positive organisms (4, 9, 18, 38, 48, 53). However, in the present investigation, no complementation of the Gram-negative model organism P. aeruginosa was observed in the presence of protamine sulfate, poly-l-lysine, and polymyxin E. These findings clearly differ from the results of related complementation experiments employing Gram-positive model organisms (59, 65).
In the presence of the CAMP protamine sulfate, all complemented strains (ΔPacomp, ΔSacomp, ΔBscomp) showed an identical MIC of 40 μg/ml as determined for the ΔPA0920 deletion strain (wild-type strain, 100 μg/ml). Using poly-l-lysine, MICs of 9 μg/ml for ΔPacomp and ΔBscomp complemented strains as well as for the ΔPA0920 strain were determined, whereas the wild-type strain showed a MIC of 18 μg/ml. The ΔSacomp chimeric mutant strain even showed a more increased susceptibility in the presence of poly-l-lysine, as indicated by a MIC of 6 μg/ml. Comparable results were also obtained in the presence of the cyclic CAMP polymyxin E. None of the complemented strains had the ability to partially restore the phenotype of the wild-type strain (8 μg/ml). MIC determinations resulted in values of 5 μg/ml for the ΔSacomp and ΔBscomp strains and for the deletion mutant. Neither the formation of L-PG (ΔSacompstrain) nor formation of L-PG and A-PG (ΔBscomp strain) has any influence on polymyxin E resistance. In the case of the ΔPacomp chimeric mutant, a further reduction of the MIC to 4 μg/ml indicated a detrimental effect due to A-PG formation (Fig. 1C). One might propose that the overall size of the employed CAMP molecules impedes the direct interaction with the inner membrane of the employed Gram-negative model organism.
Cellular response in the presence of antimicrobial agents.
Lipid homeostasis is a dynamic process which is highly relevant under very different environmental stresses. Analysis of the transcriptional regulation of the native A-PGS promoter PPA0920 is a versatile tool to study the influence of the antimicrobial compounds employed in the present study. It is well described that antibiotics often induce changes in the transcriptional and translational profiles as a cellular response to emerging stress conditions (6, 12, 93). To determine the effects of the employed antimicrobials to the native A-PGS promoter, we used the chromosomal transcriptional promoter-lacZ reporter gene fusion PAO1-PPA0920-lacZ (43). In a recent P. aeruginosa study, efficient A-PGS promoter induction was shown under acidic growth conditions (43). According to this, the influence of all compounds used in the present investigation was studied concomitantly at pH values of pH 5.3 and pH 7.3. For all types of experiments, a dedicated concentration of the corresponding antimicrobial (ranging from 0.08- to 0.25-fold MIC) was employed to allow for appropriate growth. At different time points, β-galactosidase activity was determined using OD578 measurements, and also Bradford protein determinations were employed to cope with morphological alterations as a result of the used compounds.
In all cases, a significant induction of the promoter activity in the presence or absence of antimicrobial compounds was observed under acidic conditions (pH 5.3) compared to values obtained at a pH of 7.3 (1.2- to 2.6-fold). This influence is exemplarily shown in Fig. 1E for daptomycin and polymyxin E.
The comparison of β-galactosidase activities revealed an increase from 253 to 681 MU and from 300 to 768 MU due to the pH alteration from 7.3 to 5.3 at 2 and 4 h, respectively. In the presence of the lipopeptide daptomycin, almost identical values of 218 and 625 MU (pH 7.3 and 5.3, 2 h) and of 325 and 720 MU (pH 7.3 and 5.3, 4 h) were observed. Obviously, the promoter is not modulated in the presence of daptomycin. However, treatment with polymyxin E resulted in Miller units of 609 and 1,571 under conditions of pH 7.3 and 5.3 (2 h), respectively. Besides the existing pH effect, presence of polymyxin E additionally elevates the promoter activity by factors of 2.4 (pH 7.3) and 2.3 (pH 5.3). After 4 h of treatment, a slightly diminished impact of polymyxin E was indicated by MU values of 425 and 1,132 (pH 7.3 and 5.3) according to a factor of 1.4 and 1.5, respectively.
A comparable dominant influence of acidic pH conditions was observed for all antimicrobial compounds analyzed in the present investigation. However, the overall extent of acidic induction was significantly modulated also in the presence of protamine sulfate and poly-l-lysine. Notably, the extent of this additional transcriptional regulation is growth phase dependent and varies over the time course of the experiment.
Under growth conditions at pH 7.3, polymyxin E, ampicillin, and oxacillin resulted in clearly elevated induction levels in comparison to untreated cells.
To evaluate the individual effect of each compound at different growth phases and under different pH conditions, the observed promoter activity was expressed as an induction ratio (the ratio of MU in the presence and absence of the antimicrobial compound) summarized in Fig. 1D. Upon β-lactam application, strongly induced promoter activities for ampicillin and oxacillin of 1.9-fold and 1.6-fold (pH 7.3, 4 h) were observed, respectively. In contrast to this, the induction ratio remained almost unaffected in the presence of the β-lactam cefsulodin. A clear decrease of the promoter activity to a level of 0.7 was observed for poly-l-lysine at a pH of 5.3 at 4 h. Cell treatment with the CAMP molecule protamine sulfate resulted in an increased induction under all employed experimental conditions. For protamine sulfate induction, values of 1.6 and 1.2 at pH 5.3 (2 h and 4 h) and of 1.9 and 1.5 at pH 7.3 (2 h and 4 h) were observed. These results might indicate that the induction of A-PG synthesis in P. aeruginosa is beneficial in the presence of the two CAMP molecules polymyxin E and protamine sulfate, which might indicate a related mode of action for these two peptides. Clearly differing results were obtained for the CAMP poly-l-lysine with a decreased induction ratio of 0.6 and 0.7 at a pH of 5.3 at 2 h and 4 h, respectively.
These results argue for a variable mode of A-PG function, even for the related class of CAMP molecules, which is reflected in the individual up- and downregulation (protamine sulfate, polymyxin E versus poly-l-lysine) of A-PGS gene transcription. However, under conditions of antimicrobial treatment, several growth phases are relevant, each showing different transcriptional levels (Fig. 1D). In light of these results, the lipid composition of the P. aeruginosa wild-type strain was evaluated in the presence of the respective compounds after 2 or 4 h. Only in the presence of oxacillin a slightly increased A-PG level was determined at a pH of 7.3 (data not shown). These results might indicate that cellular A-PG levels in P. aeruginosa underlie a temporal fine-tuning, as indicated in our transcriptional analysis. However, for our experimental setup, the hierarchical key signal for the overall cellular A-PG content is the environmental pH value. It will be of interest to decipher the different modes of signal recognition for the subsequent transcriptional control in P. aeruginosa in future analyses.
Substrate specificity of aaPGS enzymes.
For the intended development of aaPGS inhibitors, it is of importance to classify the related enzymes from different organisms according to their substrate specificity. However, to date the annotation of A-PG- versus L-PG-specific enzymes is not possible on the basis of bioinformatic analyses. This drawback might be attributed to the fact that only few biochemical analyses of A-PG synthesizing systems are described in the literature (30, 73, 75). Besides this, it was recently shown that the aaPGS family is highly diverse and also contains members showing a relaxed substrate specificity: for the B. subtilis enzyme, the dominant formation of L-PG and A-PG, and for the E. faecium protein, the dominant formation of L-PG and A-PG in the presence of minor amounts of arginyl-PG was demonstrated in in vitro experiments (73). Moreover, a recent study dealing with the enzymatic mechanism of aaPGS proteins revealed a set of 41 amino acid residues which were fully conserved among A-PG and L-PG synthesizing systems (30), which might indicate an identical enzymatic mechanism. With several studies, it was possible to clearly attribute the catalytic acyl-ester transferase activity to the C-terminal domain of A-PGS and L-PGS. From these results, it was concluded that this C-terminal part of the enzyme is responsible for the specific recognition of the aminoacyl-tRNA substrate (19, 30, 73).
In the present study, we intended to resolve underlying mechanisms for the development of different aaPGS substrate specificities. For this purpose, we employed well-established techniques to elucidate the substrate specificity of an overall of nine new enzymes, with the aim to identify additional proteins showing A-PG specificity. Different experimental strategies (analysis of the overall organism, of the recombinant enzyme, or of C-terminal domains; use of radioactively labeled amino acids) were employed, reflecting the overall cellular aaPG content or the presence of paralogous aaPGS sequences in the respective organism. Besides this, the overall induction of aaPG production under acidic conditions was analyzed.
The aaPGSs of B. anthracis, of B. licheniformis, of B. thuringiensis, and of S. thermophilus catalyze the formation of L-PG.
The spore-forming bacterium B. anthracis is the cause of the acute and often lethal disease anthrax (45). B. thuringiensis is well known as a source of insecticidal proteins and is therefore employed in agriculture (36). The nonpathogenic B. licheniformis is used for the industrial production of bacitracin (a polypeptide antibiotic) and exoenzymes like proteases and amylases (91). The dairy industry widely uses S. thermophilus because of its lactose fermentation ability (13).
For the analysis of the specificity of the paralogous aaPGS enzymes from B. anthracis, from B. licheniformis, and from S. thermophilus, the catalytic C-terminal domain was recombinantly overproduced in E. coli as described previously (30). The homologous enzyme from B. thuringiensis was analogously produced as a full-length protein. The employed E. coli host does not possess any aaPGS-related genes and therefore allows for the direct determination of additional phospholipids after lipid extraction, two-dimensional TLC, and molybdatophosphoric acid staining (negative control; see Fig. S2A in the supplemental material) (43). In all cases, the additional formation of L-PG (see Fig. S2A) in comparison to related control experiments was determined. L-PG synthesis by the B. thuringiensis enzyme was confirmed with experiments in the presence of radioactively labeled amino acids (see Fig. S2A). The observed specificity of the C-terminal aaPGS domain from B. anthracis is in good agreement with a recent study where an overall amount of approximately 10% L-PG was determined due to the presence of the B. anthracis ORF BAS1375 (77).
The recombinant overproduction of the C-terminal aaPGS domain of S. thermophilus did not allow for the determination of additional modified polar lipids. It was concluded that the employed protein fragment does not display catalytic activity analogously, as it was recently shown for the orthologous C-terminal domain from the S. aureus enzyme (19). However, the analysis of the lipid composition of the native S. thermophilus strain revealed 10% of L-PG compared to the overall content of polar lipids (see Fig. S2B in the supplemental material). The integrity of the L-PG molecule was confirmed by tandem mass spectroscopy as described earlier (for details, see Fig. S3A and B in the supplemental material) (87). Since BLAST analyses revealed only a single orthologous aaPGS in S. thermophilus LMG 18311, we conclude that the stu1256 ORF codes for the related L-PGS enzyme.
Drastically increased cellular aaPG levels under acidic growth conditions have been reported for several organisms (35, 43, 68). When B. licheniformis or B. thuringiensis was cultivated at pH 7.0 or pH 5.0, respectively, no varying L-PG concentrations were observed. The integrity of B. licheniformis L-PG was confirmed by mass spectroscopy as described elsewhere (data not shown) (87). The adaptation of these strains to acidic growth conditions does not involve an increase of the overall aaPG content as it was observed for P. aeruginosa (A-PG) and S. aureus (L-PG) (35, 43). This might indicate that L-PG-mediated lipid homeostasis is not a general response mechanism to acidic conditions. Since bacterial membranes of S. aureus are devoid of phosphatidylethanolamine, one might speculate that L-PG can partially substitute phosphatidylethanolamine function.
An overall L-PG content of 10% for B. anthracis cells was estimated from a recent study (77). For B. licheniformis and B. thuringiensis, overall contents of 3% and 10% L-PG were determined, respectively (Table 1; see also Fig. S2B in the supplemental material). These values indicate that L-PG contributes significantly to the overall lipid composition of these bacteria under standard growth conditions. Interestingly, S. thermophilus, a microorganism which is widely used in the dairy industry, also possesses a high L-PG content of 10%. The lactose metabolism of this organism results in high concentrations of lactate (13). Interestingly, A-PG formation in P. aeruginosa renders this organism resistant to sodium lactate (43). Obviously, the concept of L-PGS-mediated lipid modification is not directly related to the pathogenicity or the resistance of a specific organism. The synthesis of L-PG is rather an efficient strategy for the adaptation of the bacterial membrane to various requirements.
P. putida and P. aeruginosa PA14 contain high levels of A-PG in response to acidic conditions.
P. putida is a nonpathogenic organism that is widely utilized in biotechnology, whereas the P. aeruginosa PA14 strain is used as a reference strain for the investigation of bacterial pathogenicity. All aaPGS proteins from the genus Pseudomonas show a high degree of sequence identity (at least 79%), but A-PG formation has been described only for the P. aeruginosa PAO1 strain to date (43). Cultivation of P. putida and P. aeruginosa PA14 under acidic conditions (pH 5.3) revealed the dominant formation of A-PG (see Fig. S2B in the supplemental material) at a level of 5% and 4% according to the overall lipid content, whereas only minor amounts of <0.5% were determined when both strains were cultivated at a pH of 7.3. The two-dimensional TLC lipid analysis did not reveal any phospholipid at the specific position of L-PG. For both organisms, BLAST analyses revealed only a single orthologous aaPGS gene. Therefore, we concluded that ORFs PP_1202 (from P. putida) and PA14_52350 (from P. aeruginosa PA14) specifically code for A-PGS enzymes. In light of our previous study (43) and according to these new experiments, one might conclude that many Pseudomonads synthesize high levels of A-PG in response to an acidic environment.
The aaPGSs of B. phymatum, of K. radiotolerans, and of P. polymyxa catalyze the formation of A-PG.
To further identify new organisms showing a potential specificity for the formation of A-PG, an overall of three additional bacteria and their respective aaPGS enzymes have been analyzed in vivo and in vitro. B. phymatum is a Gram-negative betaproteobacterium which is capable of symbiotic nitrogen fixation (16). The analysis of B. phymatum cells revealed 0.5% A-PG compared to the overall cellular lipid content (see Fig. S2B in the supplemental material). The analysis of the enzymatic activity of the recombinantly overproduced aaPGS protein from B. phymatum in the presence of radioactively labeled amino acids solely revealed the formation of A-PG (see Fig. S2A).
K. radiotolerans is a Gram-positive organism showing exceptional survival characteristics in the presence of gamma radiation (5). For this organism, an overall cellular A-PG content of 0.5% was determined in vivo, and the recombinant protein showed a clear specificity for the formation of A-PG (see Fig. S2A and B in the supplemental material).
P. polymyxa is a Gram-positive diazotrophic bacilli with the ability to synthesize polypeptide antibiotics like polymyxins and fusaricidins (23, 66). This organism showed in vivo a cellular A-PG content of 4%. In vitro experiments using radioactively labeled alanine and lysine in the presence of the recombinantly overproduced aaPGS protein indicated a strict specificity for the formation of A-PG (see Fig. S2A and B in the supplemental material). This is one of the first descriptions of a Gram-positive organism employing one aaPGS enzyme showing a strict specificity for the formation of A-PG. According to the overall amount of 4% A-PG in P. polymyxa, one might propose that the lipid aminoacylation might help to resist the production of various polymyxin derivatives.
For B. phymatum, K. radiotolerans, and P. polymyxa, no increase of the overall A-PG content was observed when these strains were cultivated under acidic conditions (pH 7 and pH 5, respectively). It was concluded that these organisms make use of a different adaptation strategy. Despite the fact that B. phymatum is phylogenetically related to the Pseudomonads, no elevated A-PG amounts in response to acidic stress conditions were observed. Therefore, we conclude that acidic induction is not a general principle which is related to A-PG synthesis.
A-PG is also synthesized in the archaeon M. barkeri.
M. barkeri is a highly oxygen-sensitive methanogenic archaeon. Polar membrane lipids of M. barkeri contain diether and hydroxyldiether phospholipids instead of the glycerol diacyl diester molecules found in bacteria. The hydrophobic part of the molecule is composed of isoprene-based moieties instead of the unbranched fatty acid residues usually employed in bacteria. In contrast to this, bacteria and archaea share an architecture for their polar lipid head groups (phosphate bound to glycerol, serine, ethanolamine, or inositol) (46).
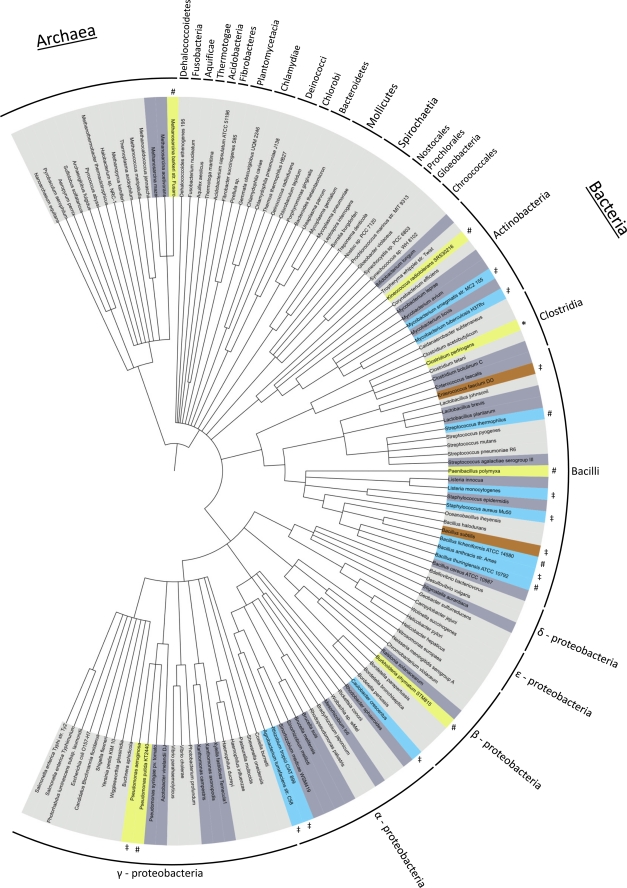
To date, there is no description of an aaPGS enzyme from the archaeal kingdom. We decided to analyze the protein from the anaerobic methanogen M. barkeri. This organism is extremely oxygen sensitive; therefore, we focused on the characterization of the specificity of the corresponding aaPGS protein in vitro. Our experiments in the presence of the PG substrate from E. coli clearly demonstrated a strict specificity for the formation of A-PG (see Fig. S2A in the supplemental material). This heterologous experiment might indicate that the alanyl modification of an M. barkeri phospholipid also takes place in vivo. These observations are strongly supported by a recent study dealing with the specific determinants of the PG substrate for A-PGS catalysis in P. aeruginosa. The analysis of various substrate derivatives revealed that the hydrophobic part of PG is of minor importance for substrate recognition. Interestingly, a substrate variant carrying two isoprenoid-like branched fatty acid ester side chains [1,2-diphytanoyl-sn-glycero-3-phospho-(1′rac-glycerol)] was also accepted as an A-PGS substrate (30). These results are indicative of an A-PGS-mediated lipid modification in M. barkeri. Theoretical analysis of archaeal genomes revealed only aaPGS enzymes for Methanosarcinaceae (Fig. 2). In this group, a lateral gene transfer from bacteria has been described for a variety of Methanosarcina genes (14, 57). According to this, the M. barkeri A-PGS gene might have derived from a bacterial ancestor, which is also indicated by high degree of sequence identity with bacterial A-PGS enzymes (45% C-terminal domains of M. barkeri and P. aeruginosa; see Fig. S4B in the supplemental material).
Fig 2.
Phylogenetic distribution of aaPGS enzymes in prokaryotes. A phylogenetic tree containing representative organisms was generated using the iTOL software (51). Microbes carrying aaPGS homologous genes are indicated in dark gray. The presence of L-PGS enzymes is indicated in blue, whereas organisms carrying A-PGS are shown in yellow. Strains in orange harbor an enzyme with broadened substrate specificity (the B. subtilis enzyme synthesizes A-PG and L-PG; the E. faecium protein produces A-PG, L-PG, and arginyl-PG). *, C. perfringens possesses an A-PGS and an L-PGS enzyme simultaneously. #, the corresponding activity has been characterized in the present study (see Table 1). ‡, data are taken from the literature (Table 2).
Determinants for aaPGS substrate specificity.
The newly investigated enzymes are an important prerequisite for the intended understanding of A-PGS and L-PGS substrate specificity. For the analysis of potential sequence-based characteristics relevant for A-PG or L-PG synthesis, several multiple sequence alignments were performed using all available aaPGS sequences which have been unambiguously assigned to date (Tables 1 and 2; Fig. 2). As described earlier, alignments of the full-length proteins revealed very low sequence identity values for the N-terminal part of the respective proteins (30, 65), e.g., 10% sequence identity for the N-terminal domains of Mycobacterium smegmatis and B. phymatum (data not shown). This observation is in accordance with the transmembrane topology of this part of the protein, which is relevant for the anchoring of the overall protein in the bacterial membrane (30, 65). In contrast to this, the C-terminal domains of aaPGS enzymes show more pronounced identity values of at least 16% (see Fig. S4B in the supplemental material). For this part of the P. aeruginosa protein, an overall of 19 key amino acid residues of aaPGS catalysis have been characterized with the help of an extended mutagenesis study (30). Not only this P. aeruginosa investigation but also additional experiments employing aaPGS enzymes from B. subtilis, C. perfringens, and S. aureus unambiguously indicated that the water-soluble C-terminal domain of the protein harbors all elements responsible for specific substrate recognition and catalysis (19, 73). According to these findings, we clearly focused on the analysis of sequence characteristics located on the C-terminal part of the respective enzymes. The corresponding sequence alignment, which is grouped according to the specificity of the individual enzymes together with the related sequence identity statistics, is depicted in Fig. S4 in the supplemental material. On the basis of the present data set, all L-PGS sequences revealed a sequence identity of at least 16% (compare values highlighted in light gray in Fig. S4B in the supplemental material). This value is smaller than the minimal sequence identity of 17% that was observed for the cross-comparison of A-PGS versus L-PGS sequences (see Fig. S4B, black rectangle). This initial finding might indicate that the specificity of L-PGS catalysis is not based on highly conserved sequence motifs that are reflected in the present alignment statistics. The comparison of all A-PGS sequences (values highlighted in dark gray in Fig. S4B) resulted in a minimal sequence identity of 31%. However, this value might rather reflect the limited amount of available sequences and their restricted phylogenetic distribution than the presence of a highly conserved sequence motif responsible for specific synthesis of A-PG. To substantiate these hypotheses, the A-PGS and L-PGS groups of the alignment were carefully inspected using the WebLogo software (11) to generate specific sequence logos visualizing the individual sequence variations in a quantitative manner for each group (see Fig. S4A). However, the analysis did not reveal any amino acid position in the sequence alignment, which is characteristic for a member of the A-PGS-specific or alternatively for a member of the L-PGS-specific group of sequences (compare lower and middle sequence logos in Fig. S4A in the supplemental material). As can be seen in Fig. S4A in the supplemental material, an almost identical conservation pattern for A-PGS and L-PGS enzymes was observed (compare the respective logo to the upper sequence logo). Due to this lack of characteristic determinants, it was also possible to allocate the B. subtilis and E. faecium aaPGS (showing broadened substrate specificity) into both the A-PGS and L-PGS groups without producing any mismatches according to the existing sequence variations (compare alignments in Fig. S4A in the supplemental material). Analogously, one might conclude that the obtained sequence logos efficiently highlight amino acid residues of prime importance for the catalysis of the overall transesterification process. This finding is supported by the results of a recent mutagenesis study which identified 15 amino acid residues with relevance for A-PGS activity (30). These residues (D579, K580, Y635, E657, E658, S709, D710, E720, S724, Y732, S763, D765, R768, Y831, K840) are depicted in the sequence of P. aeruginosa A-PGS in Fig. S4A in the supplemental material. Notably, the neighboring amino acid positions to these key residues do not show a variable pattern that can be attributed to the specificity of the respective enzymes.
DISCUSSION
Formation of L-PG versus A-PG: two different adaptation strategies?
The understanding of resistance mechanisms is of prime importance for the future development of new antimicrobial strategies. The deletion of orthologous A-PGS/L-PGS genes in Gram-positive organisms has been clearly correlated with the attenuated virulence of several pathogens, like S. aureus, Mycobacterium tuberculosis, and L. monocytogenes (59, 65, 87). A charge repulsion mechanism was proposed as a preferential model to explain, e.g., the L-PG-mediated resistance against positively charged CAMPs or defensin-like peptides in Gram-positive organisms. This is the very first investigation toward the understanding of the physiological consequences of A-PG- versus L-PG-dependent lipid modification. For this purpose, the present study employs the A-PG-mediated resistance of the model organism P. aeruginosa in the presence of an overall of seven antimicrobials from differing classes. We are focusing on complemented strains producing A-PG, L-PG, or A-PG concomitantly with L-PG as an in vivo model system for the investigation of particular functions of aaPG biosynthesis. It must be clear that this experimental strategy does not resolve the molecular mode of antimicrobial lipid interaction. For this purpose, sophisticated biophysical experiments in the presence of synthetic membrane systems might be favorable. However, for the detailed understanding of antimicrobial susceptibility, further aspects of phospholipid function must be taken into account. It has been shown that phospholipids have the ability to actively influence the functional properties of membrane-associated processes (15). Besides this, specific phospholipids might be arranged with high local concentrations in so-called microdomains (54) and the polar lipid extracts employed for standard TLC analysis contain both the cytoplasmic membrane (composed of various quantities from the inner and outer leaflet) and the inner leaflet of the outer membrane. Experimentally, it is quite challenging to deal with those aspects, so the optimal design of an appropriate in vitro experiment is quite illusive. This study employs an in vivo model which might reflect aaPG-mediated effects on an overall cellular level rather than on the molecular level. This setup allows for the answering of key questions for the understanding of A-PG- and L-PG-mediated antimicrobial resistance with relevance for the future development of aaPGS-related antimicrobial strategies.
According to the overall positive net charge of the artificially produced L-PG molecule, a more efficient alteration of the membrane charge was expected. However, in none of our experiments was a more resistant P. aeruginosa strain observed, which clearly indicates that, e.g., the L-PGS gene is not a classical virulence factor like an adhesin or a toxin.
Nevertheless, aaPG formation in P. aeruginosa drastically increases bacterial resistance under conditions of antimicrobial treatment. This becomes most evident in the presence of ampicillin, where a 5-fold-increased MIC for the wild-type strain in comparison to that of the deletion mutant was observed in the employed model system. For Gram-positive model organisms, the charge repulsion mechanism (65) has been most intensively studied in the presence of CAMP molecules. According to this model, a more positive bacterial membrane charge would also result in a reduced CAMP susceptibility for the tested chimeric P. aeruginosa strains. In our Gram-negative model system, none of the complemented strains had the ability to at least partially restore the wild-type resistance in the presence of the cationic molecules protamine sulfate, poly-l-lysine, or polymyxin E. In individual cases, the artificial lipid composition of the chimeric mutants even resulted in an increased susceptibility of individual strains (ΔSacomp, poly-l-lysine). These results might indicate that the cationic peptide resistance mechanism analyzed in the present investigation clearly differs from the related studies in Gram-positive organisms. According to this, it is important to take into account electrostatic as well as hydrophobic interactions of CAMP molecules with bacterial target sites. An alteration of the fluidity and/or permeability of the membrane might be responsible for the observed P. aeruginosa phenotypes. In the present literature, resistance phenotypes have been described which protect against a broad range of antimicrobial peptides or only against a certain type of CAMP molecules. According to this, one might expect a variety of resistance mechanisms even for a single organism. In our experiments, such differing mechanisms might be responsible for the differing effects upon complementation in the presence of ampicillin versus oxacillin. Such mechanisms might be dependent on an altered membrane potential, or the A-PG molecule might have an impact on membrane defect formation. Further experimental work is needed to elucidate consequences of various cellular A-PG concentrations. In this context, differing mechanisms for Gram-positive and Gram-negative organisms are to be expected.
Our phenotyping experiments in combination with our transcriptional analysis clearly indicate that aaPGS-mediated adaptation processes are highly relevant for the susceptibility of the Gram-negative model organism P. aeruginosa in the presence of antimicrobials. According to this, the specific inactivation of aaPGS enzymes in Gram-positive as well as in Gram-negative organisms is a promising strategy which might help to overcome bacterial resistance against our present-day antibiotics.
A diverse evolution is responsible for the specificity of A-PGS and L-PGS.
To date, the substrate specificity of an overall of 22 aaPGS enzymes has been characterized (Tables1 and 2). However, no characteristic determinants for the discrimination of Ala-tRNAAla versus Lys-tRNALys have been identified. Due to these observations, we propose a very diverse evolutionary origin for the individual A-PGS and L-PGS sequences. Even a significantly increased number of annotated aaPGS genes might not allow for the reliable prediction of aaPGS substrate specificity. A recent study employing a series of artificially modified aminoacyl-tRNA substrates clearly indicated that the overall integrity and sequence of the huge tRNA moiety is not essential for aaPGS catalysis (30). However, it was shown that the amino acid moiety is of prime importance for substrate recognition (30, 75). Due to these observations, one might speculate that A-PGS enzymes differ from L-PGS proteins due mainly to a smaller size of their respective amino acid binding pockets. Such alterations might imply only minor amino acid substitutions to restrict the overall size of this binding site. Obviously, the evolution of aaPGS enzymes did not develop a single, characteristic amino acid pattern to determine the required specificity. It seems reasonable that a series of amino acids are in a spatial position allowing for the tuning of the amino acid binding pocket. As observed for many mutagenesis experiments in the laboratory, a single amino acid mutation often does not change the specificity of the system entirely but rather results in a widening of the specificity. The well-characterized aaPGS enzymes showing broadened substrate specificity might represent such an intermediate situation, which in these cases enabled the respective organisms to efficiently fine-tune their membranes by using different aaPG molecules simultaneously (73). Interestingly, all aaPGS systems from the genus Bacillus show very pronounced sequence identity values higher than 63% (see Fig. S4B in the supplemental material). Nevertheless, among these sequences, only the B. subtilis enzyme shows dual specificity for A-PG and L-PG (73), whereas in B. anthracis, B. licheniformis, and B. thuringiensis, aaPGS-dependent lipid homeostasis is based solely on the formation of L-PG. This might indicate that the widening of the aaPGS specificity had occurred as a recent event, having no influence on the related Bacillus species.
To verify these evolutionary considerations, we highlighted the presence of aaPGS genes (dark gray) and the related specificities in a phylogenetic tree consisting of representative prokaryotes (Fig. 2). From this representation, it becomes clear that even the members of a specific class do not share an aaPGS-dependent lipid homeostasis. Organisms carrying A-PG-specific enzymes (colored yellow) and L-PG-specific proteins (colored blue) and also microbes employing enzymes with broadened substrate specificity (colored orange) do not indicate a phylogenetic clustering of the respective aaPGS systems. Based on the present-day experimental data, it becomes clear that the group of Bacilli and the group of Actinobacteria contain organisms making use of A-PG and L-PG, respectively. To date, enzymatic systems with a broadened substrate specificity have been found only in the Bacilli group (Fig. 2).
The analyses of aaPGS specificity and the related evolutionary considerations are relevant for the intended inhibition of aaPGS-mediated lipid homeostasis. To overcome the problem of the increasing number of highly resistant bacteria, it would be beneficial to find a rationale to interfere all classes of aaPGS enzymes with a single compound. The newly established enzyme specificities might be relevant for the process of identifying such inhibitors. The understanding of A-PGS and L-PGS substrate recognition on a structural level might give a future rationale for the development of such antibacterial strategies.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. MO 1749/1-1).
Special thanks go to our mentor Dieter Jahn for his continuous support.
Footnotes
Published ahead of print 20 January 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 [DOI] [PubMed] [Google Scholar]
- 2. Alvarez-Ortega C, Wiegand I, Olivares J, Hancock RE, Martinez JL. 2011. The intrinsic resistome of Pseudomonas aeruginosa to beta-lactams. Virulence 2:144–146 [DOI] [PubMed] [Google Scholar]
- 3. Andrews JM. 2001. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 48 (Suppl 1): 5–16 [DOI] [PubMed] [Google Scholar]
- 4. Aspedon A, Groisman EA. 1996. The antibacterial action of protamine: evidence for disruption of cytoplasmic membrane energization in Salmonella typhimurium. Microbiology 142 (Pt 12): 3389–3397 [DOI] [PubMed] [Google Scholar]
- 5. Bagwell CE, et al. 2008. Survival in nuclear waste, extreme resistance, and potential applications gleaned from the genome sequence of Kineococcus radiotolerans SRS30216. PLoS One 3:e3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brazas MD, Hancock RE. 2005. Using microarray gene signatures to elucidate mechanisms of antibiotic action and resistance. Drug Discov. Today 10:1245–1252 [DOI] [PubMed] [Google Scholar]
- 7. Coakley RD, et al. 2003. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc. Natl. Acad. Sci. U. S. A. 100:16083–16088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Conrad RS, Gilleland HE., Jr 1981. Lipid alterations in cell envelopes of polymyxin-resistant Pseudomonas aeruginosa isolates. J. Bacteriol. 148:487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Conte M, Aliberti F, Fucci L, Piscopo M. 2007. Antimicrobial activity of various cationic molecules on foodborne pathogens. World J. Microbiol. Biotechnol. 23:1679–1683 [DOI] [PubMed] [Google Scholar]
- 10. Contreras I, Shapiro L, Henry S. 1978. Membrane phospholipid composition of Caulobacter crescentus. J. Bacteriol. 135:1130–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crooks GE, Hon G, Chandonia JM, Brenner SE. 2004. WebLogo: a sequence logo generator. Genome Res. 14:1188–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davies J, Spiegelman GB, Yim G. 2006. The world of subinhibitory antibiotic concentrations. Curr. Opin. Microbiol. 9:445–453 [DOI] [PubMed] [Google Scholar]
- 13. Delorme C. 2008. Safety assessment of dairy microorganisms: Streptococcus thermophilus. Int. J. Food Microbiol. 126:274–277 [DOI] [PubMed] [Google Scholar]
- 14. Deppenmeier U, et al. 2002. The genome of Methanosarcina mazei: evidence for lateral gene transfer between bacteria and archaea. J. Mol. Microbiol. Biotechnol. 4:453–461 [PubMed] [Google Scholar]
- 15. Dowhan W, Bogdanov M. 2002. Chapter 1 functional roles of lipids in membranes, p 1–35 In Dennis JEV, Vance E. (ed), New comprehensive biochemistry, vol 36 Elsevier, Amsterdam, The Netherlands [Google Scholar]
- 16. Elliott GN, et al. 2007. Burkholderia phymatum is a highly effective nitrogen-fixing symbiont of Mimosa spp. and fixes nitrogen ex planta. New Phytol. 173:168–180 [DOI] [PubMed] [Google Scholar]
- 17. Enoch DA, Bygott JM, Daly ML, Karas JA. 2007. Daptomycin. J. Infect. 55:205–213 [DOI] [PubMed] [Google Scholar]
- 18. Epand RF, Maloy L, Ramamoorthy A, Epand RM. 2010. Amphipathic helical cationic antimicrobial peptides promote rapid formation of crystalline states in the presence of phosphatidylglycerol: lipid clustering in anionic membranes. Biophys. J. 98:2564–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ernst CM, et al. 2009. The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion. PLoS Pathog. 5:e1000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fischer W, Leopold K. 1999. Polar lipids of four Listeria species containing l-lysylcardiolipin, a novel lipid structure, and other unique phospholipids. Int. J. Syst. Bacteriol. 49:653–662 [DOI] [PubMed] [Google Scholar]
- 21. Fonseca AP, Sousa JC. 2007. Effect of shear stress on growth, adhesion and biofilm formation of Pseudomonas aeruginosa with antibiotic-induced morphological changes. Int. J. Antimicrob. Agents 30:236–241 [DOI] [PubMed] [Google Scholar]
- 22. Friedman L, Alder JD, Silverman JA. 2006. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 50:2137–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grau FH, Wilson PW. 1962. Physiology of nitrogen fixation by Bacillus polymyxa. J. Bacteriol. 83:490–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hachmann AB, Angert ER, Helmann JD. 2009. Genetic analysis of factors affecting susceptibility of Bacillus subtilis to daptomycin. Antimicrob. Agents Chemother. 53:1598–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hachmann AB, et al. 2011. Reduction in membrane phosphatidylglycerol content leads to daptomycin resistance in Bacillus subtilis. Antimicrob. Agents Chemother. 55:4326–4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hancock RE, Brinkman FS. 2002. Function of Pseudomonas porins in uptake and efflux. Annu. Rev. Microbiol. 56:17–38 [DOI] [PubMed] [Google Scholar]
- 27. Hancock RE, Woodruff WA. 1988. Roles of porin and beta-lactamase in beta-lactam resistance of Pseudomonas aeruginosa. Rev. Infect. Dis. 10:770–775 [DOI] [PubMed] [Google Scholar]
- 28. Hartmann W, Galla HJ. 1978. Binding of polylysine to charged bilayer membranes: molecular organization of a lipid. peptide complex. Biochim. Biophys. Acta 509:474–490 [DOI] [PubMed] [Google Scholar]
- 29. Harwood CR, Cutting SM. 1990. Modern microbiological methods: molecular biological methods for Bacillus subtilis. Wiley & Sons, Chichester, United Kingdom [Google Scholar]
- 30. Hebecker S, et al. 2011. Alanyl-phosphatidylglycerol synthase: mechanism of substrate recognition during tRNA-dependent lipid modification in Pseudomonas aeruginosa. Mol. Microbiol. 80:935–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heydorn A, et al. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395–2407 [DOI] [PubMed] [Google Scholar]
- 32. Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86 [DOI] [PubMed] [Google Scholar]
- 33. Hoang TT, Kutchma AJ, Becher A, Schweizer HP. 2000. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid 43:59–72 [DOI] [PubMed] [Google Scholar]
- 34. Houtsmuller UM, van Deenen L. 1963. Identification of a bacterial phospholipid as an O-ornithine ester of phosphatidyl glycerol. Biochim. Biophys. Acta 70:211–213 [DOI] [PubMed] [Google Scholar]
- 35. Houtsmuller UM, van Deenen LL. 1965. On the amino acid esters of phosphatidyl glycerol from bacteria. Biochim. Biophys. Acta 106:564–576 [DOI] [PubMed] [Google Scholar]
- 36. Ibrahim MA, Griko N, Junker M, Bulla LA. 2010. Bacillus thuringiensis: a genomics and proteomics perspective. Bioeng. Bugs 1:31–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Irie Y, et al. 2010. Pseudomonas aeruginosa biofilm matrix polysaccharide Psl is regulated transcriptionally by RpoS and post-transcriptionally by RsmA. Mol. Microbiol. 78:158–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johansen C, Verheul A, Gram L, Gill T, Abee T. 1997. Protamine-induced permeabilization of cell envelopes of Gram-positive and Gram-negative bacteria. Appl. Environ. Microbiol. 63:1155–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jones DE, Smith JD. 1979. Phospholipids of the differentiating bacterium Caulobacter crescentus. Can. J. Biochem. 57:424–428 [DOI] [PubMed] [Google Scholar]
- 40. Jones T, et al. 2008. Failures in clinical treatment of Staphylococcus aureus infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob. Agents Chemother. 52:269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Julian K, et al. 2007. Characterization of a daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus strain in a patient with endocarditis. Antimicrob. Agents Chemother. 51:3445–3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jung D, Powers JP, Straus SK, Hancock RE. 2008. Lipid-specific binding of the calcium-dependent antibiotic daptomycin leads to changes in lipid polymorphism of model membranes. Chem. Phys. Lipids 154:120–128 [DOI] [PubMed] [Google Scholar]
- 43. Klein S, et al. 2009. Adaptation of Pseudomonas aeruginosa to various conditions includes tRNA-dependent formation of alanyl-phosphatidylglycerol. Mol. Microbiol. 71:551–565 [DOI] [PubMed] [Google Scholar]
- 44. Koch C, Hoiby N. 2000. Diagnosis and treatment of cystic fibrosis. Respiration 67:239–247 [DOI] [PubMed] [Google Scholar]
- 45. Koehler TM. 2009. Bacillus anthracis physiology and genetics. Mol. Aspects Med. 30:386–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koga Y. 2011. Early evolution of membrane lipids: how did the lipid divide occur? J. Mol. Evol. 72:274–282 [DOI] [PubMed] [Google Scholar]
- 47. Komatsuzawa H, et al. 2001. Cloning and sequencing of the gene, fmtC, which affects oxacillin resistance in methicillin-resistant Staphylococcus aureus. FEMS Microbiol. Lett. 203:49–54 [DOI] [PubMed] [Google Scholar]
- 48. Koo SP, Bayer AS, Yeaman MR. 2001. Diversity in antistaphylococcal mechanisms among membrane-targeting antimicrobial peptides. Infect. Immun. 69:4916–4922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Koprivnjak T, Peschel A. 2011. Bacterial resistance mechanisms against host defense peptides. Cell. Mol. Life Sci. 68:2243–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Larkin MA, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 51. Letunic I, Bork P. 2007. Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics 23:127–128 [DOI] [PubMed] [Google Scholar]
- 52. Li M, et al. 2009. Staphylococcus aureus mutant screen reveals interaction of the human antimicrobial peptide dermcidin with membrane phospholipids. Antimicrob. Agents Chemother. 53:4200–4210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lim LM, et al. 2010. Resurgence of colistin: a review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacotherapy 30:1279–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lopez D, Kolter R. 2010. Functional microdomains in bacterial membranes. Genes Dev. 24:1893–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lyczak JB, Cannon CL, Pier GB. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. MacFarlane MG. 1962. Characterization of lipoamino-acids as O-amino-acid esters of phosphatidyl-glycerol. Nature 196:136–138 [Google Scholar]
- 57. Maeder DL, et al. 2006. The Methanosarcina barkeri genome: comparative analysis with Methanosarcina acetivorans and Methanosarcina mazei reveals extensive rearrangement within methanosarcinal genomes. J. Bacteriol. 188:7922–7931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Maloney E, et al. 2011. Alterations in phospholipid catabolism in Mycobacterium tuberculosis lysX mutant. Front. Microbiol. 2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Maloney E, et al. 2009. The two-domain LysX protein of Mycobacterium tuberculosis is required for production of lysinylated phosphatidylglycerol and resistance to cationic antimicrobial peptides. PLoS Pathog. 5:e1000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mishra NN, et al. 2009. Analysis of cell membrane characteristics of in vitro-selected daptomycin-resistant strains of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:2312–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mogi T, Kita K. 2009. Gramicidin S and polymyxins: the revival of cationic cyclic peptide antibiotics. Cell. Mol. Life Sci. 66:3821–3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Muraih JK, Pearson A, Silverman J, Palmer M. 2011. Oligomerization of daptomycin on membranes. Biochim. Biophys. Acta 1808:1154–1160 [DOI] [PubMed] [Google Scholar]
- 63. Nestorovich EM, Danelon C, Winterhalter M, Bezrukov SM. 2002. Designed to penetrate: time-resolved interaction of single antibiotic molecules with bacterial pores. Proc. Natl. Acad. Sci. U.S.A. 99:9789–9794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nishi H, Komatsuzawa H, Fujiwara T, McCallum N, Sugai M. 2004. Reduced content of lysyl-phosphatidylglycerol in the cytoplasmic membrane affects susceptibility to moenomycin, as well as vancomycin, gentamicin, and antimicrobial peptides, in Staphylococcus aureus. Antimicrob. Agents Chemother. 48:4800–4807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Peschel A, et al. 2001. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 193:1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Raza W, Xang W, Shen Q-R. 2008. Paenibacillus polymyxa: antibiotics, hydrolytic enzymes and hazard assessment. J. Plant Pathol. 90:419–430 [Google Scholar]
- 67. Reasoner DJ, Geldreich EE. 1985. A new medium for the enumeration and subculture of bacteria from potable water. Appl. Environ. Microbiol. 49:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Reeve WG, et al. 2006. The Sinorhizobium medicae WSM419 lpiA gene is transcriptionally activated by FsrR and required to enhance survival in lethal acid conditions. Microbiology 152:3049–3059 [DOI] [PubMed] [Google Scholar]
- 69. Riou M, et al. 2010. In vivo development of antimicrobial resistance in Pseudomonas aeruginosa strains isolated from the lower respiratory tract of intensive care unit patients with nosocomial pneumonia and receiving antipseudomonal therapy. Int. J. Antimicrob. Agents 36:513–522 [DOI] [PubMed] [Google Scholar]
- 70. Rompf A, et al. 1998. Regulation of Pseudomonas aeruginosa hemF and hemN by the dual action of the redox response regulators Anr and Dnr. Mol. Microbiol. 29:985–997 [DOI] [PubMed] [Google Scholar]
- 71. Rossetti FF, et al. 2004. Interaction of poly(l-lysine)-g-poly(ethylene glycol) with supported phospholipid bilayers. Biophys. J. 87:1711–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Roy H. 2009. Tuning the properties of the bacterial membrane with aminoacylated phosphatidylglycerol. IUBMB Life 61:940–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Roy H, Ibba M. 2009. Broad range amino acid specificity of RNA-dependent lipid remodeling by multiple peptide resistance factors. J. Biol. Chem. 284:29677–29683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Roy H, Ibba M. 2008. Monitoring Lys-tRNALys phosphatidylglycerol transferase activity. Methods 44:164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Roy H, Ibba M. 2008. RNA-dependent lipid remodeling by bacterial multiple peptide resistance factors. Proc. Natl. Acad. Sci. U.S.A. 105:4667–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sacre MM, El Mashak EM, Tocanne JF. 1977. A monolayer (π,ΔV) study of the ionic properties of alanylphosphatidylglycerol: effects of pH and ions. Chem. Phys Lipids 20:305–318 [DOI] [PubMed] [Google Scholar]
- 77. Samant S, Hsu FF, Neyfakh AA, Lee H. 2009. The Bacillus anthracis protein MprF is required for synthesis of lysylphosphatidylglycerols and for resistance to cationic antimicrobial peptides. J. Bacteriol. 191:1311–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 79. Schreiber K, et al. 2006. Anaerobic survival of Pseudomonas aeruginosa by pyruvate fermentation requires an Usp-type stress protein. J. Bacteriol. 188:659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sievers S, et al. 2010. Changing the phospholipid composition of Staphylococcus aureus causes distinct changes in membrane proteome and membrane-sensory regulators. Proteomics 10:1685–1693 [DOI] [PubMed] [Google Scholar]
- 81. Soboh F, Khoury AE, Zamboni AC, Davidson D, Mittelman MW. 1995. Effects of ciprofloxacin and protamine sulfate combinations against catheter-associated Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 39:1281–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sohlenkamp C, et al. 2007. The lipid lysyl-phosphatidylglycerol is present in membranes of Rhizobium tropici CIAT899 and confers increased resistance to polymyxin B under acidic growth conditions. Mol. Plant Microbe Interact. 20:1421–1430 [DOI] [PubMed] [Google Scholar]
- 83. Staubitz P, Neumann H, Schneider T, Wiedemann I, Peschel A. 2004. MprF-mediated biosynthesis of lysylphosphatidylglycerol, an important determinant in staphylococcal defensin resistance. FEMS Microbiol. Lett. 231:67–71 [DOI] [PubMed] [Google Scholar]
- 84. Stover CK, et al. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964 [DOI] [PubMed] [Google Scholar]
- 85. Strateva T, Yordanov D. 2009. Pseudomonas aeruginosa—a phenomenon of bacterial resistance. J. Med. Microbiol. 58:1133–1148 [DOI] [PubMed] [Google Scholar]
- 86. Taylor JR. 1997. An introduction to error analysis: the study of uncertainties in physical measurements, 2nd ed University Science Books, Sausalito, CA [Google Scholar]
- 87. Thedieck K, et al. 2006. The MprF protein is required for lysinylation of phospholipids in listerial membranes and confers resistance to cationic antimicrobial peptides (CAMPs) on Listeria monocytogenes. Mol. Microbiol. 62:1325–1339 [DOI] [PubMed] [Google Scholar]
- 88. Thoma S, Schobert M. 2009. An improved Escherichia coli donor strain for diparental mating. FEMS Microbiol. Lett. 294:127–132 [DOI] [PubMed] [Google Scholar]
- 89. Tocanne JF, Ververgaert PH, Verkleij AJ, van Deenen LL. 1974. A monolayer and freeze-etching study of charged phospholipids. II. Ionic properties of mixtures of phosphatidylglycerol and lysylphosphatidylglycerol. Chem. Phys Lipids 12:220–231 [DOI] [PubMed] [Google Scholar]
- 90. van Delden C, Iglewski BH. 1998. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis. 4:551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Veith B, et al. 2004. The complete genome sequence of Bacillus licheniformis DSM13, an organism with great industrial potential. J. Mol. Microbiol. Biotechnol. 7:204–211 [DOI] [PubMed] [Google Scholar]
- 92. Vidal S, Bredin J, Pages JM, Barbe J. 2005. Beta-lactam screening by specific residues of the OmpF eyelet. J. Med. Chem. 48:1395–1400 [DOI] [PubMed] [Google Scholar]
- 93. Wenzel M, Bandow JE. 2011. Proteomic signatures in antibiotic research. Proteomics 11:3256–3268 [DOI] [PubMed] [Google Scholar]
- 94. Wright AJ. 1999. The penicillins. Mayo Clin. Proc. 74:290–307 [DOI] [PubMed] [Google Scholar]
- 95. Yoshimura F, Nikaido H. 1985. Diffusion of beta-lactam antibiotics through the porin channels of Escherichia coli K-12. Antimicrob. Agents Chemother. 27:84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yousef AE, Carlstrom C. 2003. Food microbiology: a laboratory manual. John Wiley & Sons, Hoboken, NJ [Google Scholar]
- 97. Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389–395 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.