Abstract
A previous screen of ∼200,000 compounds from the PubChem database identified 70 compounds possessing 50% effective concentrations (EC50s) below 1 μM against Leishmania major promastigotes that were not toxic to mammalian epithelial cancer cells at this concentration (E. Sharlow et al., PLoS Negl. Trop. Dis. 3:e540, 2009). Based on availability and chemical exclusion criteria, 31 of these compounds were purchased from commercial suppliers and evaluated for in vitro activity against intracellular L. donovani and L. amazonensis parasites. Benzothiazole cyanine compounds (PubChem 16196319 and 16196223) displayed potent activity against intracellular amastigotes, prompting a search for commercially available compounds that were structurally related. Pubchem 123859 (the cyanine dye thiazole orange) showed exceptionally potent activity against intracellular L. donovani in vitro (50% inhibitory concentration [IC50] = 21 ± 12 nM) and low cytotoxicity against Vero cells (IC50 = 7,800 ± 200 nM). Administration of 123859 and 16196319 at a dose of 1 mg/kg of body weight intraperitoneally (i.p.) daily for 5 days resulted in 44% ± 4% and 42% ± 3% suppression of liver parasitemia in L. donovani-infected BALB/c mice, respectively, compared to the untreated control group (the reductions in liver parasitemia were 30% ± 5% and 27% ± 4%, respectively, compared to the (2-hydroxypropyl)-β-cyclodextrin solution (HPβCD) vehicle control, which itself displayed some antileishmanial activity). Benzothiazole-containing cyanine dyes are thus potential lead compounds for the discovery of novel antileishmanial agents.
INTRODUCTION
Treatment options for visceral leishmaniasis (VL) have improved significantly over the past decade, most notably on the Indian subcontinent. Miltefosine, the first oral drug for treating VL, was registered in India in 2002, and paromomycin was approved as a parenteral VL drug by the Indian government in 2006. Combinations of existing antileishmanial drugs have also been studied. Different combinations of treatment with single-dose liposomal amphotericin B followed by short courses of miltefosine were extremely effective, with a single dose of liposomal amphotericin B at 5 mg/kg of body weight followed by a 10-day course of miltefosine resulting in a cure rate of 98% (32). This combination was evaluated together with other combinations consisting of (i) a single dose of 5 mg/kg liposomal amphotericin with a 10-day course of paromomycin and (ii) a 10-day course of both paromomycin and miltefosine, with all of these combinations providing a cure rate of 97% or greater (33). When given in a single 10 mg/kg dose, liposomal amphotericin B was shown to be highly effective against VL, producing a cure rate of 95.7% (30). Single-dose liposomal amphotericin B is proposed to play a central role in a VL elimination program in India, Nepal, and Bangladesh (12).
Despite these improvements in VL treatment on the Indian subcontinent, unmet needs in leishmaniasis chemotherapy still exist. In Africa, only the antimonial drug sodium stibogluconate and liposomal amphotericin B are registered for treating VL. Antimonial compounds, which have been replaced by the drugs listed above for treating VL on the Indian subcontinent due to widespread antimonial resistance (31), have been used for many years against leishmaniasis, and their use is plagued by the long duration of therapy and by the associated side effects (15, 18, 23, 31). Liposomal amphotericin B appears to be less effective against VL in Brazil (1) and in Africa (1, 14, 26) compared to India, and the expense of liposomal amphotericin B also limits its widespread use (9). Paromomycin is inexpensive and is thus a good candidate for VL treatment in African countries, but a 15 mg/kg/day regimen of paramomycin for 21 days was inferior to standard treatment with sodium stibogluconate (20 mg/kg/day for 30 days) (9). However, higher doses and a longer duration of treatment with paromomycin did result in increased efficacy against Sudanese VL (16). The standard of care in treating VL in countries of endemicity of the New World is poorly defined (24) but most likely relies on injectable pentavalent antimonial drugs (18).
Regarding the treatment of cutaneous leishmaniasis (CL), no single drug is recommended in all cases due to the existence of many causative species of Leishmania, and the evidence indicating the optimal therapy to be employed has been described as “patchy” (19). Pentavalent antimonials are most frequently employed to treat CL either by systemic administration or by intralesional injection (10, 38). Parenteral pentamidine is recommended to treat L. guyanensis infections (40) but is not as widely used against other Leishmania species causing CL. Oral miltefosine is not widely used against CL and has exhibited variable efficacy in the CL trials reported to date (29, 41). The use of topical paromomycin against CL has the advantage of lower cost and reduced side effects compared to pentavalent antimonial treatment. However, recent reviews have concluded that topical paromomycin is inferior to pentavalent antimonials for treating both Old World and New World CL (10, 38). Considering the current status of leishmaniasis treatment, it is clear that an effective, safe, and inexpensive oral drug would be a welcomed tool in treating VL in Africa and the Americas and in treating CL in all regions, either as monotherapy or in combination with existing agents.
Cell-based phenotypic screening has been useful in identifying new chemotypes against pathogenic protozoa (7, 8, 11, 28). Sharlow et al. reported the screening of 196,146 publicly available compounds for activity against Leishmania major promastigotes and identified 17,629 compounds as primary hits (27). By applying structural clustering and secondary assays to determine potency and selectivity, 70 compounds were identified that possessed 50% effective concentrations (EC50s) below 1 μM and that did not inhibit the growth of mammalian epithelial cancer cells at this concentration. Given the need for a new small-molecule candidate for the oral treatment of leishmaniasis and our continuing efforts in this area (42–44), we decided to study selected members from the set of 70 antileishmanial molecules reported by Sharlow et al. to determine whether any were candidates for further development as antileishmanial drug candidates. Several of the molecules that we evaluated displayed activity against intracellular Leishmania in vitro, and members of one class showed efficacy in a murine VL model.
MATERIALS AND METHODS
Chemicals and reagents.
A total of 35 potential antileishmanial molecules were obtained from commercial vendors: compounds 3117, 5455, 123859, and 6423245 (Sigma); 3242548 and 3236501 (ChemDiv); 3769434 (Vitas-M Laboratory, Ltd.); 6604199 (Fluka); 6261459 and 6603247 (Ambinter); 11293258 (Pharmeks); 15945170 (Life Chemicals Inc.); 16196001, 16196223, 16196319, 2999476, 2308258, and 2312703 (Enamine); 549148 and 1471937 (Interchim Inc.); and 89966, 298107, 645317, 728862, 757789, 760847, 786799, 2194030, 2846928, 2851545, 2855568, 2923184, 2937831, 2946668, and 2964134 (ChemBridge). These compounds were dissolved in dimethyl sulfoxide (DMSO) at a 2.5 mM working concentration and further diluted in medium for the in vitro assays. Amphotericin B, podophyllotoxin, and miltefosine were purchased from Sigma as reference compounds.
Cell culture. (i) Transgenic parasites.
Transgenic L. donovani promastigotes expressing the β-lactamase reporter gene were generously provided by Frederick Buckner (University of Washington, Seattle). These parasites were cultured at 26°C in medium M199 supplemented with 20% heat-inactivated fetal bovine serum (FBS), penicillin (50 U/ml), streptomycin (50 μg/ml), and nourseothricin (50 μg/ml) (to provide selection pressure). Transgenic β-lactamase-expressing L. amazonensis promastigotes were cultured in RPMI 1640 medium supplemented with 0.1 mM adenosine, 1 μg/ml folate, 50 U/ml penicillin, 50 μg/ml streptomycin, 1× RPMI 1640 vitamins, and 10% FBS (2). Transgenic β-lactamase-expressing L. major promastigotes were cultured in the medium described above with 20% FBS. Cultures were subjected to passage twice a week using approximately 200 μl of the old culture in 6 ml of fresh medium.
(ii) Wild-type parasites.
L. donovani LV82 promastigotes cultured in Schneider's medium supplemented with 25% FBS, penicillin (50 U/ml), and streptomycin (50 μg/ml) were used as the reference control for the in vitro assay and were also employed to assess in vivo efficacy.
β-Lactamase assay.
To evaluate the β-lactamase activity of transgenic L. donovani, stationary-phase promastigotes were collected, washed once with phosphate-buffered saline (PBS), and then suspended in β-lactamase assay buffer (0.1% Triton X-100, 100 μM nitrocefin, PBS) at a final concentration of 5 × 106 cells/ml. The suspension was diluted serially by 2-fold with the buffer described above and seeded at 100 μl per well into 96-well plates. L. amazonensis β-lactamase-expressing promastigotes and LV82 wild-type promastigotes were used as controls. After 2 h of incubation at 37°C, plates were read at 490 nm using a SPECTRAmax PLUS 384 spectrophotometer (Molecular Devices, Sunnyvale, CA).
Infection of macrophages.
Macrophages were elicited by intraperitoneal (i.p.) injection of 6-to-8-week-old female CD-1 mice with 2 ml of 2% starch suspension–PBS. After 24 h, mice were euthanized by CO2 asphyxiation and peritoneal macrophages were harvested by lavage using ice-cold macrophage medium (RPMI 1640 Glutamax [Gibco] containing 10% FBS, 50 units/ml penicillin, and 50 μg/ml streptomycin [pH 7.4]). Macrophages (1 × 105) were added to either Lab-Tek chamber slides (Nunc) or 96-well plates in a volume of 100 μl in the medium described above and were allowed to adhere overnight at 37°C and 5% CO2. Host cells were then infected with stationary-phase β-lactamase-expressing L. donovani promastigotes at 34°C and 5% CO2 at a ratio of 10 parasites to 1 host cell. On the following day, extracellular parasites were removed by two washes with HBSS (Hank's buffered salt solution) and then 200 μl of fresh medium was added to each well of the plate. After 24, 48, 72, and 96 h of infection, the chamber slides were washed once with PBS, fixed with methanol, and stained with Giemsa (distilled water [977 μl], Giemsa diluent [3.1 mM dibasic potassium phosphate, 8.3 mM monobasic sodium phosphate] [23 μl], Giemsa stain [50 μl]). The percentage of infected macrophages in each chamber was determined microscopically at 1,000× magnification by counting 200 macrophage nuclei. The cells in the 96-well plate were washed once with PBS and then lysed with the β-lactamase assay buffer as described above, allowing nitrocefin to react with the β-lactamase enzyme expressed by the parasite. After incubation at 34°C for 5 h, plates were read at 490 nm to quantitate β-lactamase activity. The infectivity of L. donovani LV82 promastigotes in peritoneal macrophages was assessed as the control.
Compound susceptibility assays using intracellular amastigotes.
Peritoneal macrophages (1 × 105 cells) obtained from CD-1 mice as described earlier were added to individual wells of 96-well plates in a volume of 100 μl. The next day, stationary-phase β-lactamase-expressing L. donovani promastigotes were added to the plates described above in a volume of 100 μl in macrophage medium at a ratio of 10 parasites to 1 macrophage. The following day, plates were washed twice with HBSS to remove those noninternalized promastigotes and then a 3-fold serial dilution of test compounds from 25 μM to 0.31 μM were added to the plate described above. After 3 days of incubation, the β-lactamase activity of intracellular amastigotes was determined. Testing was repeated from a 5 μM starting concentration if the 50% inhibitory concentration (IC50) of a given compound was lower than 0.31 μM. Amphotericin B was used as the reference drug in this assay. The activity of compounds against intracellular L. amazonensis or L. major β-lactamase-transfected parasites within peritoneal macrophages was determined as described previously (3).
Cytotoxicity assay. (i) Murine peritoneal macrophage toxicity.
The toxicity of compounds was tested against murine peritoneal macrophages by the method of Wang et al. (42).
(ii) Vero cell toxicity.
Selected compounds were evaluated for toxicity against Vero cells (African green monkey kidney cells) as described previously (25).
In vivo studies.
Compounds that displayed potent activity against intracellular L. donovani β-lactamase-expressing amastigotes were tested in a murine VL model as described previously (3). Briefly, BALB/c mice were infected with L. donovani LV82 promastigotes on day 0, treated with test compounds from day 7 to 11, and then sacrificed on day 14 for the in vivo efficacy evaluation. (2-Hydroxypropyl)-β-cyclodextrin solution (HPβCD) (45% [wt/vol]) was used as the vehicle control, and miltefosine was used as the reference drug control. Test compounds were dissolved in 45% HPβCD and were administered by the i.p. route. Liver parasitemia was evaluated according to a previously published method (3).
Statistical analysis.
For all experiments described above, statistical significance was determined using one-way analysis of variance with a post hoc Tukey's multiple-comparison test calculated with GraphPad InStat. A P value ≤ 0.05 was considered significant.
RESULTS
Development of an intracellular assay using β-lactamase-expressing L. donovani.
We desired to evaluate hit compounds from the L. major promastigote screen reported by Sharlow et al. for activity against intracellular parasites causing both VL and CL. While an intracellular assay using β-lactamase-expressing L. amazonensis parasites was conducted routinely in our laboratory (3, 42), intracellular L. donovani assays were performed by tedious microscopic enumeration of Giemsa-stained samples. We felt that the number of hits from the L. major promastigote screen to be evaluated against intracellular L. donovani was too large to use the microscopic enumeration assay, so we sought to develop a straightforward intracellular L. donovani assay with improved throughput. Transgenic L. donovani promastigotes were tested for β-lactamase expression by a colorimetric assay. A nearly linear correlation was observed between the number of promastigotes and β-lactamase activity (see Fig. S1 in the supplemental material). To compare the β-lactamase expression levels between different transgenic Leishmania species, L. amazonensis β-lactamase-expressing promastigotes were tested and showed similar results. Wild-type L. donovani LV82 promastigotes employed as a negative control gave a low background absorbance at 490 nm.
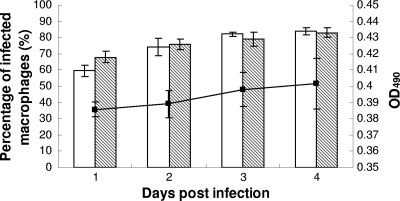
L. donovani β-lactamase-expressing promastigotes were also used to infect murine peritoneal macrophages at a ratio of 10:1. At the end of the incubation period (24, 48, 72, or 96 h postinfection), the percentage of infected macrophages was determined by microscopy and the β-lactamase activity of intracellular amastigotes was tested by the colorimetric assay. With the transgenic L. donovani strain, infection rates of 68%, 76%, 79%, and 83% were observed by Giemsa staining at 24, 48, 72, and 96 h postinfection, respectively, showing similar infectivity to the murine peritoneal macrophages when compared with LV82 parasites. Furthermore, the percentage of infected macrophages at 96 h postinfection increased significantly compared to that observed at 24 h for both species as determined microscopically (P < 0.05). The β-lactamase activity of transgenic intracellular amastigotes (expressed as optical density at 490 nm [OD490]) at 24, 48, 72 and 96 h postinfection was also determined. A good correlation was observed between the percentage of infected macrophages and β-lactamase activity (Fig. 1).
Fig 1.
Relationship between the percentage of infected macrophages and the β-lactamase activity of intracellular amastigotes. L. donovani transgenic promastigotes and L. donovani LV82 wild-type promastigotes were used to infect murine peritoneal macrophages. The percentage of infected macrophages was determined by Giemsa staining and microscopic analysis as indicated in Materials and Methods. The β-lactamase activity of intracellular L. donovani transgenic amastigotes (expressed as OD490) was determined as described in Materials and Methods. Results represent the means and standard deviations of the results of three independent experiments. White bars, the percentage of infected macrophages with LV82; shaded bars, the percentage of infected macrophages with transgenic L. donovani promastigotes; solid squares, OD490.
Selection of compounds for screening in the intracellular assay.
Compounds shown to be active in a high-throughput screen of Leishmania major promastigotes (27) were used as a starting point to reveal compounds with activity against intracellular Leishmania. We employed several exclusion criteria to narrow the list of 70 selective compounds possessing EC50s of less than 1 μM against L. major promastigotes for antileishmanial screening in our laboratory as mentioned in the introduction. Molecules were excluded from further consideration if they possessed a selectivity index for L. major promastigotes of <5-fold compared to that of the A549 cell line described in the original paper or if they contained polyaromatic ring systems, aromatic nitro groups, chloromethyl ketones, or α,β-unsaturated ketones. Members of classes of compounds such as azoles and diamidines whose antileishmanial activity has been well characterized were also excluded. Commercial availability further narrowed the list of potential compounds for screening. Thus, of the 70 compounds mentioned above, 31 were purchased from commercial suppliers for evaluation against intracellular L. donovani and L. amazonensis parasites in vitro. The structures of these 31 compounds are given in Fig. S2 in the supplemental material.
In vitro assay.
Thirty of the compounds mentioned above were tested in the intracellular Leishmania assay against both L. donovani and L. amazonensis parasites (compound 2937831 was not assayed due to solubility problems). Sixteen of these molecules displayed IC50s of <25 μM against L. donovani or L. amazonensis or both parasites. Five compounds (3117, 5455, 6604199, 16196223, and 16196319) exhibited IC50s of lower than 0.5 μM against L. donovani, and two of them (compounds 16196223 and 16196319) also displayed submicromolar potency against intracellular L. amazonensis and selectivity for intracellular parasites compared to mammalian cells (Table 1).
Table 1.
Antileishmanial potency and cytotoxicity of selected Pubchem compounds
| Pubchem compound | IC50 (μM) for indicated targeta |
|||
|---|---|---|---|---|
| Intracellular L. donovani transgenic amastigotes | Intracellular L. amazonensis transgenic amastigotes | Peritoneal macrophages | Vero cells | |
| 3117 | 0.043 ± 0.006 | >25 | 0.27 ± 0.04 | ND |
| 5455 | 0.14 ± 0.03 | 16 ± 9b | 0.37 ± 0.036 | ND |
| 89966 | >25 | >25 | >25 | ND |
| 298107 | >25 | >25 | >25 | ND |
| 645317 | >25 | >25 | >25 | ND |
| 728862 | >25 | >25 | >25 | ND |
| 757789 | 4.7 ± 2.3 | 14 ± 1 | 9.9 ± 1.1 | 17 ± 2 |
| 760847 | 2.1 ± 1.8 | 7.8 ± 0.2 | >25 | ND |
| 786799 | >25 | >25 | >25 | ND |
| 1471937 | 1.5 ± 0.2 | 9.5 ± 3.6 | 3.3 ± 0.5 | 3.5 ± 0.3 |
| 2194030 | 12 ± 5 | 12 ± 1 | 9.9 ± 1.2 | 3.4 ± 0.2 |
| 2846928 | 1.1 ± 0.2 | 1.2 ± 0.1 | 0.39 ± 0.02 | 3.4 ± 0.6 |
| 2851545 | 11 ± 3 | >25 | >25 | ND |
| 2855568 | >25 | >25 | >25 | ND |
| 2923184 | >25 | 15 ± 0 | >25 | ND |
| 2946668 | >25 | >25 | >25 | ND |
| 2964134 | >25 | >25 | >25 | ND |
| 2999476 | >25 | >25 | >25 | ND |
| 3236501 | >25 | >25 | >25 | ND |
| 3242548 | >25 | >25 | >25 | ND |
| 3769434 | 5.3 ± 1.1 | 20 ± 6 | >25 | ND |
| 6261459 | 0.53 ± 0.19 | 0.99 ± 0.27 | >25 | ND |
| 6603247 | 3.5 ± 2.0 | >25 | >25 | ND |
| 6604199 | 0.38 ± 0.22 | >25 | 2.6 ± 0.5 | 0.12 ± 0.03b |
| 9549148 | >25 | >25 | >25 | ND |
| 11293258 | 1.1 ± 0.1 | 1.0 ± 0.3 | 0.82 ± 0.14 | 5.0 ± 1.0 |
| 15945170 | 20 ± 6 | >25 | >25 | ND |
| 16196001 | 3.6 ± 1.4 | 7.8 ± 1.1 | >25 | ND |
| 16196223 | 0.18 ± 0.05 | 0.20 ± 0.04 | 4.1 ± 0.6 | 2.0 ± 0.1 |
| 16196319 | 0.26 ± 0.09 | 0.64 ± 0.32 | 5.2 ± 0.3 | ND |
| AmB | 0.046 ± 0.016 | 0.097 ± 0.012 | ND | ND |
| Podophyllotoxin | ND | ND | ND | 0.016 ± 0.001 |
Data represent means ± standard errors of the results of at least three independent determinations unless noted otherwise. ND, not determined.
Values represent the mean ± range of the results of two independent determinations.
The related dithiocarbamates 3117 (disulfiram) and 5455 (thiram) displayed potent in vitro activity against intracellular L. donovani but were surprisingly inactive against intracellular L. amazonensis. Considering that 3117 also showed in vivo efficacy in the L. major-infected murine footpad model reported by Sharlow et al. (27), both compounds were tested in our intracellular L. major assay. While 3117 and 5455 displayed submicromolar potency against intracellular L. major parasites (IC50 = 69 ± 15 nM and IC50 = 65 ± 10 nM, respectively), they also showed toxicity against peritoneal macrophages, disqualifying them from further consideration.
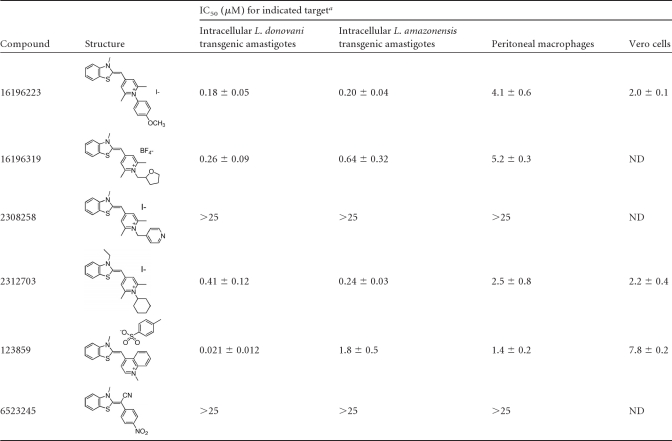
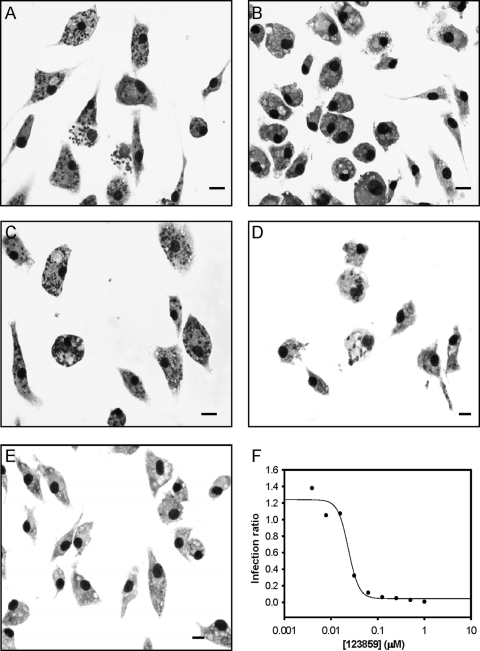
Given the striking potency of benzothiazole cyanine compounds 16196223 and 16196319, we searched the Pubchem database and identified four other commercially available analogs. These analogs were also tested for activity against intracellular L. donovani and intracellular L. amazonensis, and the structures and potencies of the six structurally related molecules are provided in Table 2. All of these compounds (except for 2308258 and 6523245) displayed potent activity against both Leishmania species. The cyanine dye 123859 (thiazole orange) showed exceptional in vitro antileishmanial activity, particularly against intracellular L. donovani (IC50 = 21 ± 12 nM). The selectivity indexes of 123859 were 67 and 371 for L. donovani compared to murine peritoneal macrophages and Vero cells, respectively. The activity of 123859 against intracellular L. donovani was also confirmed by Giemsa staining (42) as shown in Fig. 2.
Table 2.
Structure, potency, and cytotoxicity of benzothiazole compounds
Data represent means ± standard errors of the results of at least three independent determinations. ND, not determined.
Fig 2.
Antileishmanial potency of 123859 against intracellular L. donovani as determined by Giemsa staining of LV82-infected murine peritoneal macrophages. (A) Infected macrophage control; (B) noninfected macrophage control; (C) infected macrophages treated with 3 nM 123859; (D) infected macrophages treated with 24 nM 123859 (which inhibits about 50% of parasitemia as confirmed by microscopic enumeration); (E) infected macrophages treated with 500 nM 123859 (which clears parasitemia); (F) antileishmanial dose-response curve for 123859 from a representative experiment as assessed by microscopic enumeration. Infection ratio was calculated as described by Siqueira-Neto et al. (28). Bar, 10 μm.
In vivo efficacy of candidate compounds in L. donovani-infected BALB/c mice.
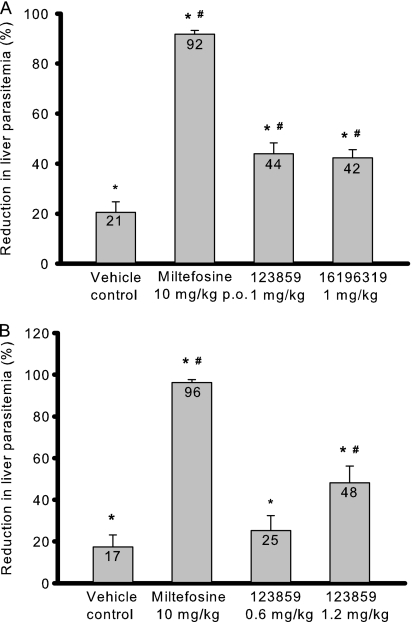
Due to their high in vitro antileishmanial activity and selectivity, compounds 123859, 16196223, and 16196319 were candidates for evaluation in the murine VL model. Compounds 123859 and 16196319 were tested at 1 mg/kg of body weight/day i.p. Significantly higher doses could not be administered due to the slowed breathing observed in an acute toxicity study in uninfected mice when tested at a dose of 2 mg/kg/day i.p. The respiratory tract was also reported as one of the target organs for thiazole orange toxicity on material safety data sheets for this compound. Compound 16196223 displayed adverse effects at doses of 1 mg/kg/day i.p. in uninfected mice and was therefore not evaluated for in vivo antileishmanial efficacy.
Results for the compounds tested in the murine VL model are summarized in Fig. 3. When given at a dose of 1 mg/kg/day i.p. for 5 days, compounds 123859 and 16196319 caused 44% and 42% suppression of liver parasitemia, respectively, compared to the untreated control (Fig. 3A). Interestingly, efficacy was also observed in the vehicle control group. The 45% (wt/vol) HPβCD vehicle itself resulted in 21% inhibition compared to the untreated control, consistent with the previously reported antileishmanial effect of methyl-β-cyclodextrin (20). Hence, there were 30% (P < 0.05) and 27% (P < 0.05) reductions of liver parasitemia with compounds 123859 and 16196319, respectively, compared to the HPβCD vehicle control. To confirm that the administration of 123859 results in a dose-dependent effect in L. donovani-infected mice, this compound was further tested at doses of 0.6 and 1.2 mg/kg/day i.p. for 5 days, resulting in 25% and 48% reductions of liver parasitemia, respectively, compared to the untreated group and 10% and 37% (P < 0.05) reductions of liver parasitemia, respectively, compared to the HPβCD vehicle control group (Fig. 3B). As a positive control, miltefosine treatment at 10 mg/kg/day for 5 days reduced the hepatic parasite burden by 92% when administered orally (Fig. 3A) and by 96% when administered i.p. (Fig. 3B), consistent with our previous data determined using this control drug in the murine VL model (data not shown).
Fig 3.
In vivo efficacy of benzothiazole cyanine compounds against Leishmania donovani. (A) Initial evaluation of 123859 and 16196319. (B) Dose-response study with 123859. Results are presented as the percent reduction in liver parasitemia versus the untreated control; all compounds were given by the i.p. route unless otherwise indicated. p.o., oral administration. Horizontal lines and error bars denote the means and standard deviations, respectively, of the results determined with experimental groups containing four animals. *, P < 0.05 compared to untreated control; #, P < 0.05 compared to vehicle control.
DISCUSSION
The identification of new antileishmanial chemotypes presents inherent challenges due to the biology of the Leishmania parasite. Since the symptoms of both VL and CL in the mammalian host are caused by intracellular parasites, assays to identify new antileishmanial candidate molecules should employ infected mammalian cells. Considering that the throughput of traditional intracellular Leishmania assays is low, the screening of existing chemical libraries for new antileishmanial chemotypes would be facilitated by technical improvements in the intracellular Leishmania assays. De Muylder et al. screened a library consisting of 909 biologically active molecules by the use of both a promastigote assay and an intracellular L. donovani screen employing image analysis, leading those investigators to conclude that the promastigote assay generates a high percentage of hits that do not show activity in the intracellular assay (5). The approach taken by Siqueira-Neto et al. in their antileishmanial evaluation of a 26,500-member chemical library was to use Leishmania promastigotes in a prescreen and then employ a cytotoxicity counterscreen and an intracellular Leishmania assay to identify promising antileishmanial candidates (28). Those investigators also used an intracellular Leishmania assay employing image analysis to evaluate 124 potential hit compounds that emerged from their primary and secondary assays. We took an approach similar to that of Siqueira-Neto et al. with the set of hits identified from the promastigote screen reported by Sharlow et al. (27), but we instead used an intracellular assay employing β-lactamase-expressing L. donovani and L. amazonensis parasites that did not require specialized microscopy and software to determine the efficacy of 35 potential antileishmanial compounds. While this approach was suitable for the number of compounds assessed in our current study, further improvements in this intracellular Leishmania assay would be needed before it could be used for true high-throughput applications.
In agreement with the previous antileishmanial screens cited above, our present study revealed that many compounds displayed selective activity against Leishmania promastigotes in the absence of a selective effect on intracellular amastigotes. Of 30 active compounds identified by the L. major promastigote screen reported by Sharlow et al. (27), 14 hits (47%) were identified in the intracellular L. donovani assay and 5 hits (17%) were discovered in the intracellular L. amazonensis assay that possessed IC50s below 10 μM with a selectivity index > 2. However, the species of Leishmania employed in the promastigote screen was different from those employed in the intracellular assays, so species differences could in part account for the distinctions observed. Fifty-nine active compounds were identified in the promastigote screen by De Muylder et al. (5), of which 26 were also active in the intracellular assay (2). The screening effort of Siqueira-Neto et al. identified 124 compounds that were selectively active against L. major promastigotes; only 5 of those compounds displayed selective activity against intracellular L. major (28). Taken together, these results indicate that the promastigote assay tends to identify many hits for compounds that are inactive against intracellular Leish-mania.
The most potent analogs identified in this study are the benzothiazole-containing cyanine compounds (a review of the chemistry of cyanines is cited) (13). In addition to the antileishmanial activity reported in the study by Sharlow et al., a series of papers have also described the antiprotozoal activity of cyanine dyes (21, 22, 34–36, 45). These reports mainly focused on the antimalarial effects of rhodanine-containing cyanines (rhodacyanines) and showed that these agents display nanomolar potency against Plasmodium falciparum in vitro (21, 22, 34, 35) and good in vivo efficacy against murine P. berghei infections (21, 22, 35). Only one cyanine compound lacking the rhodanine ring similar to those reported in our study, 1-methyl-2-[(3-methyl-1,3-benzothiazol-2(3H)-ylidene)methyl]pyridinium tosylate, was included in these papers (34, 36). Presumably, this class was not pursued further because the sole cyanine compound investigated was slightly less potent in vitro against P. falciparum erythrocyte-stage parasites and L. major promastigotes compared to some tricyclic rhodacyanines studied. Two rhodacyanines displayed midnanomolar potencies against intracellular L. donovani, and treatment of L. donovani-infected mice with one of these rhodacyanines at a dose of 1.3 mg/kg/day × 5 by the intravenous route resulted in 95% inhibition of liver parasitemia (45). In our study, cyanines 2312703, 16196319, and 16196223 all displayed midnanomolar potency against both intracellular L. donovani and intracellular L. amazonensis, while 123859 was highly active against intracellular L. donovani but less potent against intracellular L. amazonensis. Inactive benzothiazole 6523245, unlike the other compounds shown in Table 2, does not possess a positive charge; this may explain the inactivity of this molecule. The lack of activity of benzothiazole cyanine 2308258 is difficult to rationalize at this time but could indicate that specific molecular targets for these compounds in Leishmania are not permissive for the terminal pyridine ring present in this molecule. It has been speculated that the antileishmanial rhodacyanines may disrupt parasite membrane potential (36, 45), although no supporting data were given. This mechanistic hypothesis deserves further investigation in Leishmania.
While our current data together with the previous work cited above indicate that cyanines and rhodacyanines possess potent activity against intracellular Leishmania and promising in vivo activity, the toxicity of these compounds must be addressed. We were unable to administer compound 123859 or 16196319 at higher doses because of toxicity observed at a dose of 2 mg/kg/day i.p., and earlier in vivo studies with rhodacyanines also indicated that in vivo toxicity was an issue (35). Given the potency of cyanines and rhodacyanines against intracellular Leishmania and the structural diversity of active compounds in these classes, a medicinal chemistry program focused on determining the precise structural features required for antiparasitic potency while avoiding host toxicity is necessary. Based on the structure-activity relationship that is currently available, such an effort would likely begin with the 1-methyl-4-[(3-methyl-1,3-benzothiazol-2(3H)-ylidene)methyl] pyridinium core present in the active compounds shown in Table 2 and involve structural modifications to this core that produce the desired biological effects. We are optimistic that an appropriate therapeutic index can be achieved through lead optimization efforts.
Other potent analogs identified in our study are the alcohol addiction drug 3117 (disulfiram) and the pesticide 5455 (thiram). These structurally related dithiocarbamates exhibited good efficacy in our intracellular L. donovani and L. major assays, in agreement with the midnanomolar potency of 3117 observed against L. major axenic amastigotes (27). Surprisingly, these compounds were inactive against intracellular L. amazonensis. Distinct responses in different species of Leishmania have also been previously demonstrated in vitro with miltefosine (4, 6) as well as with other compounds (17, 28) and could be due to biochemical differences among Leishmania species (6). Species-dependent differences in compound susceptibility highlight the difficulty of developing a single drug with efficacy against all forms of leishmaniasis. Compound 3117 did not show toxicity against several other mammalian cells (A549, IMR-90, HeLa, PC-3, and MDA-MB-231) at 50 μM and was tolerated in L. major-infected mice at a dose of 160 mg/kg (27), but both 5455 and 3117 were toxic in our hands at submicromolar concentrations against murine peritoneal macrophages in vitro. Interestingly, they both had a lower IC50 in the cytotoxicity assay than in the intracellular L. amazonenesis assay. This is in accordance with the findings of De Muylder et al., who found that 5455 and several other compounds had a lower IC50 in uninfected host cells compared to infected cells (5). The Leishmania species infecting macrophages may therefore affect the toxicity profile of a particular compound on the host cell. Compounds 5455 and 3117 are also known teratogens (37, 39). Considering the latter point, it is difficult to imagine a scenario where these dithiocarbamates would present an improvement over miltefosine for the systemic treatment of either VL or diffuse CL. We are thus not pursuing investigation of 3117, 5455, or related dithiocarbamates as antileishmanial candidates at this time.
Several of the other compounds listed in Table 1 display antileishmanial activity in the intracellular assay. Although 6604199 (cycloheximide) showed selectivity for intracellular L. donovani compared to THP-1 macrophages in De Muylder's study (5), this compound was toxic to peritoneal macrophages and Vero cells in the present report, indicating that THP-1 cells are less sensitive to this compound. Therefore, 6604199, along with benzothiazole dimer 2194030 and amphipathic cations 2846928 and 11293258, was excluded from further consideration due to lack of selectivity against one or both of the mammalian cell lines employed in this study. Quinoxalinone 1471937 displays modest selectivity for intracellular L. donovani over peritoneal macrophages and Vero cells and no selectivity for intracellular L. amazonensis. The remaining active compounds may prove to be more interesting antileishmanial leads. Quinolone 760847 exhibits selectivity for intracellular Leishmania, especially L. donovani. The high-throughput screen reported by Siqueira-Neto et al. also discovered an antileishmanial quinolone, designated CH872, that displays midnanomolar efficacy against intracellular L. donovani (28). Substituted pyridinimine compound 6603247 and arylvinyl pyridinium compound 16196001 showed moderate potency against intracellular L. donovani but low cytotoxicity and could serve as potential starting points for the discovery of agents of greater potency. Isoindolamine 6261459 may be even more promising, given that it exhibits similar potency against L. donovani and L. amazonensis and ≥25-fold selectivity for intracellular parasites compared to murine peritoneal macrophages.
Conclusion.
The present study describes an intracellular Leishmania donovani assay with improved throughput compared to traditional microscopic assays that could be used to screen small libraries for antileishmanial activity. We have also followed up on the hits reported in an earlier high-throughput Leishmania promastigote assay and have identified a few classes of compounds with activity against intracellular parasites; the benzothiazole cyanines are worthy of lead optimization efforts, and other scaffolds may also merit investigation. Further improvements in the intracellular Leishmania assay are also desirable to permit the evaluation of large chemical libraries for effects on the more clinically relevant host form of the parasite.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the Bill and Melinda Gates Foundation.
We are grateful to Frederick Buckner for providing the β-lactamase-expressing Leishmania parasites, Susan Kilgore Jones, Shanshan He, and Julian Richard for assistance in obtaining selected compounds for testing, and Sihui Long and members of the Consortium for Parasitic Drug Development for helpful discussions regarding this project.
Footnotes
Published ahead of print 5 December 2011
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Berman J, et al. 1998. Efficacy and safety of liposomal amphotericin B (AmBisome) for visceral leishmaniasis in endemic developing countries. Bull. World Health Organ. 76:25–32 [PMC free article] [PubMed] [Google Scholar]
- 2. Delfín D, Bhattacharjee A, Yakovich A, Werbovetz K. 2006. Activity of and initial mechanistic studies on a novel antileishmanial agent identified through in silico pharmacophore development and database searching. J. Med. Chem. 49:4196–4207 [DOI] [PubMed] [Google Scholar]
- 3. Delfín D, Morgan R, Zhu X, Werbovetz K. 2009. Redox-active dinitrophenylthioethers against Leishmania: synthesis, structure-activity relationships and mechanism of action studies. Bioorg. Med. Chem. 17:820–829 [DOI] [PubMed] [Google Scholar]
- 4. de Morais-Teixeira E, Damasceno Q, Galuppo M, Romanha A, Rabello A. 2011. The in vitro leishmanicidal activity of hexadecylphosphocholine (miltefosine) against four medically relevant Leishmania species of Brazil. Mem. Inst. Oswaldo Cruz 106:475–478 [DOI] [PubMed] [Google Scholar]
- 5. De Muylder G, et al. 2011. A screen against Leishmania intracellular amastigotes: comparison to a promastigote screen and identification of a host cell-specific hit. PLoS Negl. Trop. Dis. 5:e1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Escobar P, Matu S, Marques C, Croft S. 2002. Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH3 (edelfosine) and amphotericin B. Acta Trop. 81:151–157 [DOI] [PubMed] [Google Scholar]
- 7. Gamo F, et al. 2010. Thousands of chemical starting points for antimalarial lead identification. Nature 465:305–310 [DOI] [PubMed] [Google Scholar]
- 8. Guiguemde W, et al. 2010. Chemical genetics of Plasmodium falciparum. Nature 465:311–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hailu A, et al. 2010. Geographical variation in the response of visceral leishmaniasis to paromomycin in East Africa: a multicentre, open-label, randomized trial. PLoS Negl. Trop. Dis. 4:e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khatami A, Firooz A, Gorouhi F, Dowlati Y. 2007. Treatment of acute Old World cutaneous leishmaniasis: a systematic review of the randomized controlled trials. J. Am. Acad. Dermatol. 57:335.e1-e29. [DOI] [PubMed] [Google Scholar]
- 11. Mackey A, et al. 2006. Discovery of trypanocidal compounds by whole cell HTS of Trypanosoma brucei. Chem. Biol. Drug Des. 67:355–363 [DOI] [PubMed] [Google Scholar]
- 12. Matlashewski G, et al. 2011. Visceral leishmaniasis: elimination with existing interventions. Lancet Infect. Dis. 11:322–325 [DOI] [PubMed] [Google Scholar]
- 13. Mishra A, Behera R, Behera P, Mishra B, Behera G. 2000. Cyanines during the 1990s: a review. Chem. Rev. 100:1973–2011 [DOI] [PubMed] [Google Scholar]
- 14. Mueller M, et al. 2007. Unresponsiveness to AmBisome in some Sudanese patients with kala-azar. Trans. R. Soc. Trop. Med. Hyg. 101:19–24 [DOI] [PubMed] [Google Scholar]
- 15. Murray H, Berman J, Davies C, Saravia N. 2005. Advances in leishmaniasis. Lancet 366:1561–1577 [DOI] [PubMed] [Google Scholar]
- 16. Musa A, et al. 2010. Paromomycin for the treatment of visceral leishmaniasis in Sudan: a randomized, open-label, dose-finding study. PLoS Negl. Trop. Dis. 4:e855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neal R, Allen S, McCoy N, Olliaro P, Croft S. 1995. The sensitivity of Leishmania species to aminosidine. J. Antimicrob. Chemother. 35:577–584 [DOI] [PubMed] [Google Scholar]
- 18. Oliveira A, et al. 2009. Severe adverse reactions to meglumine antimoniate in the treatment of visceral leishmaniasis: a report of 13 cases in the southwestern region of Brazil. Trop. Doct. 39:180–182 [DOI] [PubMed] [Google Scholar]
- 19. Palumbo E. 2009. Current treatment for cutaneous leishmaniasis: a review. Am. J. Ther. 16:178–182 [DOI] [PubMed] [Google Scholar]
- 20. Pucadyil T, Tewary P, Madhubala R, Chattopadhyay A. 2004. Cholesterol is required for Leishmania donovani infection: implications in leishmaniasis. Mol. Biochem. Parasitol. 133:145–152 [DOI] [PubMed] [Google Scholar]
- 21. Pudhom K, et al. 2009. Synthesis and biological properties of a rhodacyanine derivative, SSJ-127, having high efficacy against malaria protozoa. Heterocycles 77:207–210 [Google Scholar]
- 22. Pudhom K, et al. 2006. Synthesis of three classes of rhodacyanine dyes and evaluation of their in vitro and in vivo antimalarial activity. Bioorg. Med. Chem. 14:8550–8563 [DOI] [PubMed] [Google Scholar]
- 23. Rijal S, et al. 2003. Sodium stibogluconate cardiotoxicity and safety of generics. Trans. R. Soc. Trop. Med. Hyg. 97:597–598 [DOI] [PubMed] [Google Scholar]
- 24. Romero G, Boelaert M. 2010. Control of visceral leishmaniasis in Latin America—a systematic review. PLoS Negl. Trop. Dis. 4:e584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salem M, Werbovetz K. 2005. Antiprotozoal compounds from Psorothamnus polydenius. J. Nat. Prod. 68:108–111 [DOI] [PubMed] [Google Scholar]
- 26. Seaman J, et al. 1995. Liposomal amphotericin B (AmBisome) in the treatment of complicated kala-azar under field conditions. Clin. Infect. Dis. 21:188–193 [DOI] [PubMed] [Google Scholar]
- 27. Sharlow E, et al. 2009. Identification of potent chemotypes targeting Leishmania major using a high-throughput, low-stringency, computationally enhanced, small molecule screen. PLoS Negl. Trop. Dis. 3:e540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siqueira-Neto J, et al. 2010. Antileishmanial high-throughput drug screening reveals drug candidates with new scaffolds. PLoS Negl. Trop. Dis. 4:e675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Soto J, et al. 2004. Miltefosine for New World cutaneous leishmaniasis. Clin. Infect. Dis. 38:1266–1272 [DOI] [PubMed] [Google Scholar]
- 30. Sundar S, Chakravarty J, Agarwal D, Rai M, Murray H. 2010. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N. Engl. J. Med. 362:504–512 [DOI] [PubMed] [Google Scholar]
- 31. Sundar S, et al. 2000. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin. Infect. Dis. 31:1104–1107 [DOI] [PubMed] [Google Scholar]
- 32. Sundar S, et al. 2008. New treatment approach in Indian visceral leishmaniasis: single-dose liposomal amphotericin B followed by short-course oral miltefosine. Clin. Infect. Dis. 47:1000–1006 [DOI] [PubMed] [Google Scholar]
- 33. Sundar S, et al. 2011. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. Lancet 377:477–486 [DOI] [PubMed] [Google Scholar]
- 34. Takasu K, et al. 2002. Rhodacyanine dyes as antimalarials. 1. Preliminary evaluation of their activity and toxicity. J. Med. Chem. 45:995–998 [DOI] [PubMed] [Google Scholar]
- 35. Takasu K, Pudhom K, Kaiser M, Brun R, Ihara M. 2006. Synthesis and antimalarial efficacy of aza-fused rhodacyanines in vitro and in the P. berghei mouse model. J. Med. Chem. 49:4795–4798 [DOI] [PubMed] [Google Scholar]
- 36. Takasu K, et al. 2004. Antileishmanial activities of rhodacyanine dyes. Heterocycles 64:215–221 [Google Scholar]
- 37. Tilton F, La Du J, Vue M, Alzarban N, Tanguay R. 2006. Dithiocarbamates have a common toxic effect on zebrafish body axis formation. Toxicol. Appl. Pharmacol. 216:55–68 [DOI] [PubMed] [Google Scholar]
- 38. Tuon F, et al. 2008. Treatment of New World cutaneous leishmaniasis—a systematic review with a meta-analysis. Int. J. Dermatol. 47:109–124 [DOI] [PubMed] [Google Scholar]
- 39. van Boxtel A, et al. 2010. Dithiocarbamates induce craniofacial abnormalities and downregulate sox9a during zebrafish development. Toxicol. Sci. 117:209–217 [DOI] [PubMed] [Google Scholar]
- 40. van der Meide W, et al. 2009. Evaluation of treatment with pentamidine for cutaneous leishmaniasis in Suriname. Int. J. Dermatol. 48:52–58 [DOI] [PubMed] [Google Scholar]
- 41. Vélez I, et al. 2010. Efficacy of miltefosine for the treatment of American cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 83:351–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang M, et al. 2010. Novel arylimidamides for the treatment of visceral leishmaniasis. Antimicrob. Agents Chemother. 54:2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wasan E, et al. 2010. A novel tropically stable oral amphotericin B formulation (iCo-010) exhibits efficacy against visceral leishmaniasis in a murine model. PLoS Negl. Trop. Dis. 4:e913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wasan K, et al. 2009. Highly effective oral amphotericin B formulation against murine visceral leishmaniasis. J. Infect. Dis. 200:357–360 [DOI] [PubMed] [Google Scholar]
- 45. Yang M, et al. 2010. Fluorinated rhodacyanine (SJL-01) possessing high efficacy for visceral leishmaniasis (VL). J. Med. Chem. 53:368–373 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.