Abstract
The use of antibiotics as a supplement to bone cement for the purposes of providing a local release of antibiotics is common practice in arthroplasty surgery and the kinetics of elution of the antibiotics in such systems have been investigated previously. However, in these previous studies no account was taken of the potential effects that wear may have on the elution kinetics of the antibiotic. Here, we have modified an existing wear testing rig to allow the simultaneous study of the elution kinetics of bone cement samples containing antibiotics being subjected to immersion only and immersion and conjoint wear. The results show contrasting effects with two commonly used antibiotics. Bone cement containing daptomycin showed no substantial change in antibiotic elution due to wear, while cement containing gentamicin (the most commonly used antibiotic in this application) in contrast demonstrated a substantial reduction in the rate of antibiotic elution when wear was applied. Scanning electron microscopy revealed a possible explanation for these diverse results, due to wear-induced “sealing” of the surface in conjunction with the crystal morphology of the antibiotic.
INTRODUCTION
Infection is a major complication of arthroplasty surgery, with a reported incidence after primary surgery of 0.2 to 1% (3, 10). The surgical management of prosthetic joint infection includes a direct exchange procedure where after removal of the prosthesis a new prosthesis is implanted in the same operative session or a two-stage exchange procedure where there is an interval of several weeks or months before the new prosthesis is implanted. The only difference between the two procedures is the presence of the interval phase. While similar results for the eradication of infection have been reported with both techniques, the two-stage exchange is more commonly used. In both techniques, radical debridement is essential, including removal of all infected tissue, implants, and cement. Irrespective of what technique is used, additional antibiotic needs to be used to treat the existing infection and also for prophylaxis to prevent infection with new bacteria. If performing a direct exchange procedure, additional antibiotic is added to the bone cement that is used to secure the new implant. Systemic antibiotics can also be given. In the case of a staged exchange, there is also the opportunity during the interval phase to administer additional antibiotic locally by cement in the form of beads or a spacer. The use of a spacer maintains the patient's soft tissue tension and also allows some mobility of the joint before the new prosthesis is inserted at the time of second stage. Systemic antibiotics can also be given during this interval phase. One advantage of antibiotic elution from cement is that it produces a much higher local concentration of antibiotic than could be safely given systemically. Local levels of antibiotic can be up to 100 times higher than the MIC of the causative bacteria (1, 15). During the interval phase of the staged procedure, the patient may mobilize and, as the articulating surfaces of the joint have effectively been replaced by cement, these surfaces abrade against each other and the adjacent bone. The resulting effect of wear on the cement surfaces may influence the rate of antibiotic elution as a new cement surface is constantly being produced.
In principle, any antibiotic can be added to bone cement prior to polymerization as long as it is a sterile, heat-stable, water-soluble dry powder. The antibiotic is usually mixed into the cement powder by hand at the time of surgery, although some cement preparations preloaded with antibiotic are also available. The elution properties of the antibiotic relate directly to the water uptake by the bone cement with the antibiotic dissolving in the body fluid and eluting into the surrounding area. In clinical practice, the volume of antibiotic powder added to the cement does not usually exceed 10% (wt/wt) because above this level the biomechanical properties of the cement are affected (6). There are numerous studies in the literature looking at the rate of gentamicin elution from bone cement under different conditions, but to our knowledge none investigates the effect of wear, which one may hypothesize would occur with an articulating cement hip or knee spacer. Daptomycin, which is increasingly used as a local anti-infective in polymethylmethacrylate (PMMA) bone cement, has similarly to our knowledge not been investigated under wear conditions.
The aim of the present study was to assess the effect of wear on the rate of elution of two antibiotics, namely, gentamicin and daptomycin, from PMMA bone cement used as a spacer during two-stage revision arthroplasty surgery.
MATERIALS AND METHODS
Preparation of bone cement samples for elution and wear tests.
Gentamicin-containing bone cement samples were prepared by mixing 40 g of Biomet Plus bone cement (preloaded with 0.6 g of gentamicin sulfate) with 20 ml of Biomet monomer liquid in a manner consistent with the manufacturer's instructions (Biomet, Ltd., Swindon, United Kingdom). Daptomycin-containing bone cement samples were prepared by adding 0.19 g of daptomycin powder to 14.8 g of Biomet bone cement. The cement was then mixed with 7.5 ml of the PMMA monomer liquid in a Hi-Vac bone cement mixing bowl (Biomet). Both of these procedures are consistent with current practice in orthopedic surgery. In both cases, the bone cement was then placed into a mold (described below) with the aid of a spatula. A 2-kg mass was placed onto a ceramic tile on top of the mold to aid the removal of trapped air, and the antibiotic-loaded bone cement was then left to cure for 1 h.
The resulting antibiotic-loaded bone cement samples were annulus-shaped with a 40-mm outer diameter, 8-mm inner diameter, and 10-mm thickness. Once removed from the mold, samples were stored in a UV-sealed desiccator at −10°C until required. A total of eight samples were produced in this manner.
Calibration of antibiotic solutions. (i) Gentamicin.
Gentamicin was quantified by means of a high-performance liquid chromatography-mass spectrometry (HPLC-MS) technique developed by applying the separation conditions and detection of the gentamicin fragment ion at m/z 322 described previously (5) into an HPLC-MS method to quantify the drug. Samples for the standard curve were made up in the same buffer solution used for the elution studies, which consisted of 0.1 M ammonium acetate adjusted to pH 7.4 with ammonia solution. Use of a volatile ammonium acetate buffer (rather than a standard buffer such as phosphate-buffered saline) allowed direct analysis of the eluate by HPLC using electrospray ionization MS, without the need for a desalting step. Standards and samples were acidified by adding formic acid (to 0.01% [vol/vol]) to aid ionization during MS analysis and then separated via HPLC using a PU980 system (JASCO UK, Great Dunmow, United Kingdom) fitted with a Luna C18 (2) column (150 by 1 mm; particle size, 5 μm; Phenomenex, Inc., Torrance, CA) and a manual injection valve with a 1-μl sample loop. The mobile phase was 48.5 mM trifluoroacetic acid (90%):methanol (10%), and the flow rate was 30 μl min−1. The on-line MS detector was a Finnigan LCQ mass spectrometer with an ion spray source operated in positive ion mode with an ion spray voltage of 4.5 kV. The sheath gas flow rate was 80 ml min−1 (Nitrogen), the auxiliary gas flow rate was zero, and the capillary temperature was 250°C. Commercial preparations of gentamicin contain several isoforms of the drug. It was found that reliable quantification of gentamicin in eluates and standard samples could be achieved by monitoring the ion at m/z (mass to charge ratio) of 322 as a fragment ion of protonated gentamicin C1 (m/z 478).
Calibration samples with concentrations ranging from 0.196 to 300 μg ml−1 were used to produce a calibration curve to relate MS signal intensity to gentamicin sulfate solution concentration. Linear correlation regression analysis revealed a confident linear relationship (R2 = 0.993) between 0.39 and 300 μg of gentamicin sulfate ml−1 (see Fig. S1a in the supplemental material), which was used to determine gentamicin concentrations released from bone cement samples during the subsequent experiments.
(ii) Daptomycin.
Daptomycin, which (unlike gentamicin) has a substantial absorbance in the UV region, was quantified by HPLC with on-line UV detection by a modification of the published method (13), as follows. The stationary phase was an Ultra-Carb 5 ODS (30) column (150 by 4.6 mm; Phenomenex, Torrance, CA). The daptomycin standards were dissolved in a mobile phase that consisted of 70% 0.2 M phosphate buffer at pH 5.5 and 30% acetonitrile. The HPLC system consisted of a Perkin-Elmer series 200 pump and UV/Vis detector and was operated using Total Chrom software (Perkin-Elmer, Waltham, MA). The flow rate was set to 1.5 ml min−1, and the sample loop had a volume of 20 μl. The absorbance of the eluate was measured continuously at a wavelength of 223 nm. Daptomycin standard concentrations of 0.5, 1.0, 5.0, 10.0, 20.0, 50.0, and 100 μg ml−1 were analyzed to calibrate the HPLC output. When the concentration of daptomycin was plotted against peak area on the HPLC trace, a very strong linear relationship between in the UV absorbance peak areas and the concentration of daptomycin was observed, with an R2 value of close to 1 (see Fig. S1b in the supplemental material). Intraday percent coefficient of variation (%CV) values for both assays are also given in the supplemental material as an additional measure of the precision of the methods.
Effect of wear on antibiotic elution kinetics.
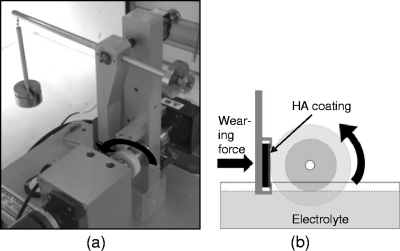
To evaluate the effect of wear on the rate of elution of the antibiotics from the bone cement, it was necessary to devise an experimental setup that would allow submersion of the cement sample, while also imposing a measurable and constant wear regime. These requirements were achieved through modification of the TE-66 microscale abrasive wear tester (Phoenix Tribology, Ltd., Newbury, United Kingdom), as described below and as shown in Fig. 1.
Fig 1.
(a) Modified TE-66 test rig with annular cement sample and HA-coated disc fitted and 2.5 N force applied via lever arm; (b) schematic diagram of the wear system. A partially submerged cement sample rotates against an HA-coated disc under a 2.5-N load.
Controlled wear was generated by use of an HVOF-VPD hydroxyapatite (HA)-coated Ti disc (30-mm diameter by 3-mm thick; Rz [mean peak to valley height] = 40 μm), which was placed into the TE-66 lever arm specimen holder. The cement sample was secured into the coaxial shaft of the TE-66, by way of a two-piece tapered collar fitment. The sample was orientated such that its flat 10-mm-thick outer perimeter was offered square to abrade against the HA-coated face of the Ti disc counter-face. The lever arm holding the HA-coated disc was then balanced so that only slight contact was made between the wearing cement sample and the counter-face. The system geometries and mechanical properties (Young's modulus [E] and Poisson's ratio [v]) of the two interacting surfaces were used (as detailed in the supplemental material) to calculate the required load necessary (2.5 N) to generate a target mean contact pressure of 2.79 MPa. This pressure value was chosen because it exceeded the mean cartilage contact pressure of 2.14 MPa generated by a 2.4 × body-weight force at the hip (appropriate for the single-leg support phase of level walking) in a 556-N (∼57 kg) body-weight individual as previously reported (11). The force of 2.5 N was imposed by the counter-face on the outer perimeter of the wearing cement sample by placing calibrated weights onto the lever arm of the TE-66.
A solution bath was placed beneath the assembly and filled with test solution (0.1 M ammonium acetate buffered test solution [pH 7.4]) until the lower portion of the cement sample was submerged (Fig. 1a). The solution in the bath was mixed at 300 rpm by a magnetic stirrer.
An automated sequence program was applied under the control of the Compend 2000 software package (Phoenix Tribology) to rotate the sample at 60 rpm for 51 h. The HA counter-face was repositioned every 10 h to ensure a sufficiently abrasive counter-face throughout the duration of the test. An extension shaft was fitted to the TE-66 to allow simultaneous rotation of the control (unworn) sample at the same speed, again partially submerged in a separate container filled with an identical buffer solution to that to which the worn sample was exposed.
The TE-66 wear rig was placed into a UV-sealed air-tight container for the duration of the test in order to permit environmental stability and to avoid contamination. The temperature and relative humidity within the container were continuously monitored and recorded. To monitor the elution levels of the antibiotic from the cement, periodic sampling was performed by removing 50-μl samples of the test solutions from the worn and unworn test baths using sterile pipettes. Each vial was given an identification mark and wrapped in aluminum foil to protect it from UV-induced degradation. Samples were stored at −15°C in the dark until analyzed.
Surface characterization.
To observe the effect of the 0.1 M ammonium acetate buffer (pH 7.4) solution on the surfaces, samples were prepared as previously described and then either directly sputter coated and imaged or immersed in ammonium acetate buffer for 24 h before sputter coating and imaging. The surfaces of the specimens were characterized using a Phillips XL40 scanning electron microscope (SEM). SEM specimens were sputter coated with carbon (to prevent surface charging) for topographical examination using secondary electron imaging and compositional analysis in backscattered electron (BSE) mode. An accelerating voltage between 11 and −20 kV was used for all SEM analyses.
Subsequent cross-sectional SEM analysis was performed to characterize both the PMMA bone cement's microstructure and the morphology and distribution of its constituent parts (including the gentamicin sulfate crystals). Details of image analysis to determine crystal sizes and distribution are given in the supplemental material.
RESULTS
Antibiotic elution behavior under normal (no-wear) and wear conditions.
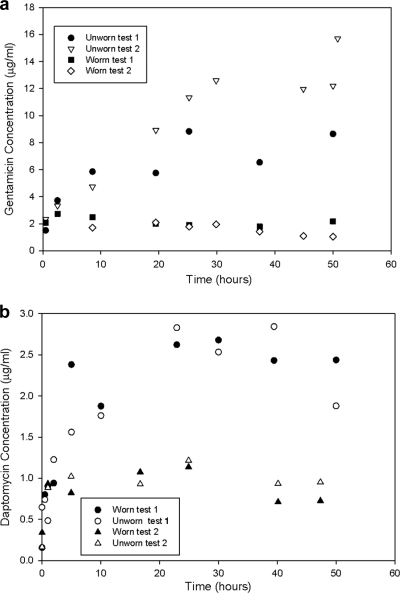
The results from two separate experiments to investigate the effect of wear on elution behavior of cement containing 1.25% (wt/wt) gentamicin are shown in Fig. 2a. In the unworn condition, antibiotic eluted over a period of ∼30 h to give a final concentration of eluted antibiotic of 9 to 15 μg ml−1. While there was an ∼1.7-fold difference in the amount of antibiotic eluted in the two tests without wear, in both cases much lower elution rates were observed for the bone cement samples which were subject to wear, leading to a reduction in the peak eluted gentamicin concentration of 3- to 7.5-fold between unworn and worn samples.
Fig 2.
Bone cement elution characteristics of gentamicin (a) and daptomycin (b) for unworn and worn conditions in duplicate tests.
Moreover, in the absence of wear, the concentration of the eluted gentamicin increased throughout the 51-h test period, while the samples subject to wear showed no significant increase in antibiotic concentration in the elution buffer after the first measurement. The incremental changes in eluted antibiotic concentration were seen to vary as a function of time reflecting the increasing degree of surface deformation that occurred over the duration of the test.
For the cement system loaded with daptomycin (1.25% wt/wt), elution took place over a similar timescale to that with gentamicin in the absence of wear. However, unlike that of the gentamicin, no substantial difference in elution kinetics was observed between that of the no-wear and wear conditions (Fig. 2b).
Morphology and dissolution characteristics of antibiotic crystals.
Microscopic analysis of the antibiotic-loaded bone cement samples showed that the morphology of the gentamicin crystals were significantly different from that of the daptomycin crystals (see Fig. S2 in the supplemental material). The range of crystal diameters of the gentamicin in the cement was 102 ± 69 μm (mean size ± the standard deviation [SD]) compared to 56 ± 14 μm in the cement loaded with daptomycin.
The particle size difference of the two antibiotics leads, in turn, to a difference in the distribution of the antibiotic within the bone cement, which may be the cause of the differences in the elution behavior. In the case of the daptomycin, there is uniform dissolution of the antibiotic since it is homogeneously distributed within the bone cement (with a mean “nearest-neighbor” [MNN] distance of 45.6 ± 17.3 μm). In contrast, the large crystals of gentamicin are heterogeneously distributed (MNN distance of 280 ± 136 μm) and, since the concentration of gentamicin in the bone cement is equal to that of daptomycin used in the cement in the parallel tests (1.25% [wt/wt]), the probability of an antibiotic crystal being exposed at the surface is reduced for the gentamicin system. Furthermore, for an equal amount of antibiotic the surface area of the gentamicin exposed to the solution is lower than that of the daptomycin. This, in turn, will affect the antibiotic dissolution characteristics.
Influence of wear on surface the morphology of bone cement.
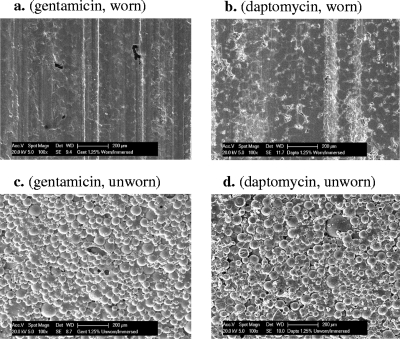
In order to investigate the origin of the very different effects of wear on elution of the two antibiotics, the surfaces of the samples were examined in detail using an SEM. The images from the gentamicin-loaded cement revealed that wear of the cement had resulted in a much smoother and more homogeneous surface than observed for the unworn samples and further illustrate the deformation that occurs at the surface (Fig. 3). These micrographs offer an explanation for the much lower rate of elution of gentamicin from the worn (compared to unworn) sample; notably, the smoother surface resulting from plastic deformation of the cement leads to a “sealing,” i.e., reduction in porosity of the surface, which in turn prevents solvent from penetrating into cavities previously formed by the elution of antibiotic crystals in the cement.
Fig 3.
SEM images of worn and unworn antibiotic-loaded bone cement surfaces after elution experiments. (a) Gentamicin, worn; (b) daptomycin, worn; (c) gentamicin, unworn; (d) daptomycin, unworn. Plastic deformation of the PMMA cement can be seen on the worn samples. Scale bars, 200 μm.
DISCUSSION
The observation that antibiotics elute from bone cement in the absence of wear is in agreement with previous studies (2, 4, 7–9, 14) and is consistent with the addition of gentamicin and other antibiotics to bone cement for local delivery of anti-infectives at the site of fixation of prostheses. In the case of gentamicin and the worn samples, we observed similar elution behavior in the first 200 min, but then a dramatic difference beyond this period. The difference between the worn and unworn gentamicin-loaded samples is that whereas elution of antibiotic from the unworn sample continues for at least 51 h, in the presence of wear no significant release of antibiotic is observed after the first 200 min of the experiment, and the total amount of gentamicin released is no more than one-third of that released from the unworn sample. Note that the observed drop in antibiotic concentration seen for the worn samples (Fig. 2a) may be attributed in part to a degradation of the gentamicin during the storage period prior to analysis. What is evident is that there is no major increase in antibiotic release as a function of time and increasing wear. The observed elution kinetics, together with the images observed using the SEM, indicate a superficial layer of cement from which the gentamicin elutes quickly on immersion in aqueous solution. In the absence of wear, aqueous solvent can then penetrate deeper into the cement generating pore pathways to allow continued release of the antibiotic. In the presence of wear, sealing over of crevices and cavities on the surface occurs and elution of more deeply buried gentamicin is greatly retarded. An obvious question is “why is this behavior also not seen with the daptomycin-loaded samples?” The principal reason may lie in the role of the gentamicin crystal size and distribution, and the resulting pore pathways that develop during the early stages of antibiotic elution (Fig. 2a and 3). This in turn limits both the surface and the volume ratios of pore distribution that develop in the early stages of the wear process. In the case of the gentamicin the elution of large crystals will result in a low number of large voids in the surface. These voids will be much more susceptible to deformation (Fig. 3) because of the low mechanical constraint around the void, which permits plastic deformation and “sealing” of the surface. In contrast, the smaller crystal size and higher surface area levels of the daptomycin create, on subsequent elution, a higher degree of porosity within the cement (Fig. 3). Much greater surface deformation is now required to “seal” this porous structure in order for the wear to have a major effect on the elution characteristics.
Although the simple elution/wear system used here may not totally reflect the local concentration of antibiotic achieved in the tissues around a prosthesis, it is interesting that the gentamicin concentration of ∼2 μg ml−1 that was observed at this point in both worn and unworn samples is twice the MIC breakpoint for gentamicin-susceptible coagulase-negative staphylococci, a major cause of periprosthetic infections (12).
These unexpected observations indicate the need for a reevaluation of the use of antibiotic-loaded cement spacers for the articulating spacers during two-stage arthroplasty. Although sustained antibiotic release may occur from unworn surfaces of the cement, the previous presumption that the wearing of the cement surfaces provided a continued enhanced release of antibiotic to help eradicate any persistent infection must now be called into question. The extent to which wear at the articulating surfaces retards release of an antibiotic is expected to depend on the load applied to the joint, the number of cycles of movement, the range of sizes of antibiotic particles, and their distribution throughout the cement.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. Hague and M. Clench for expert technical assistance and advice with HPLC-MS and H. Jones for support with the wear tests. The support and access to facilities within the Materials Engineering Research Institute, BioMedical Research Centre, and the Northern General Hospital are also gratefully acknowledged.
This study was supported by funding contributions from Biomet PLC and Novartis PLC.
Footnotes
Published ahead of print 12 December 2011
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Adams K, Couch L, Cierny G, Calhoun J, Mader JT. 1992. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic impregnated polymethylmethacrylate beads. Clin. Orthopaed. Rel. Res. 278:244–252 [PubMed] [Google Scholar]
- 2. Baker AS, Greenham LW. 1988. Release of gentamicin from acrylic bone cement. J. Bone Joint Surg. Am. 70:1551–1557 [PubMed] [Google Scholar]
- 3. Blom AW, Taylor AH, Pattison G, Whitehouse S, Bannister GC. 2003. Infection after total hip arthroplasty: the Avon experience. J. Bone Joint Surg. 85B:956–959 [DOI] [PubMed] [Google Scholar]
- 4. Buchholz HW, Elson RA, Heinert K. 1984. Antibiotic-loaded acrylic cement: current concepts. Clin. Orthop. 190:96–108 [PubMed] [Google Scholar]
- 5. Clarot I, Chaimbault P, Hasdenteufel F, Netter P, Nicholas A. 2004. Determination of gentamicin sulfate and related compounds by high-performance liquid chromatography with evaporative light scattering detection. J. Chromatogr. A 1031:281–287 [DOI] [PubMed] [Google Scholar]
- 6. Frommelt L. 2007. Antibiotic choices in bone surgery: local therapy using antibiotic-loaded bone cement, p 59–63 In Walenkamp GH. (ed), Local antibiotics in arthroplasty. Thieme, New York, NY [Google Scholar]
- 7. Hall EW, et al. 2004. Release of daptomycin from polymethylmethacrylate beads in a continuous flow chamber. Diagn. Microbiol. Infect. Dis. 50:261–265 [DOI] [PubMed] [Google Scholar]
- 8. Klekamp J, Dawson JM, Hass DW, DeBoer D, Christie M. 1999. The use of vancomycin and tobramycin in acrylic bone cement: biomechanical effects and elution kinetics for use in joint arthroplasty. J. Arthroplasty 14:339–346 [DOI] [PubMed] [Google Scholar]
- 9. Penner MJ, Masri BA, Duncan CP. 1996. Elution characteristics of vancomycin and tobramycin combined in acrylic bone cement. J. Arthroplasty 11:939–944 [DOI] [PubMed] [Google Scholar]
- 10. Phillips CB, et al. 2003. Incidence rates of dislocation, pulmonary embolism and deep infection during the first six months after elective total hip replacement. J. Bone Joint Surg. 85A:20–26 [DOI] [PubMed] [Google Scholar]
- 11. Rushfeldt PD, Mann RW, Harris WH. 1983. Improved techniques for measuring in vitro the geometry and pressure distribution in the human acetabulum. II. instrumented endoprosthesis measurement of articular surface pressure distribution. J. Biomechanics 14:315–323 [DOI] [PubMed] [Google Scholar]
- 12. Stockley I, Mockford BJ, Hoad-Reddick A, Norman P. 2008. The use of two-stage exchange arthroplasty with depot antibiotics in the absence of long-term antibiotic therapy in infected total hip replacement. J. Bone Joint Surg. Br. 90-B:145–148 [DOI] [PubMed] [Google Scholar]
- 13. Tobin CM, Darville JM, Lovering AM, MacGowan AP. 2008. An HPLC assay for daptomycin in serum. J. Antimicrob. Chemother. 62:1462–1463 [DOI] [PubMed] [Google Scholar]
- 14. Wahlig H, Dingeldein E, Bergmann R, Ruess K. 1978. The release of gentamicin from polymethylmethacrylate beads: an experimental and pharmacokinetic study. J. Bone Joint Surg. Br. 60-B:270–275 [DOI] [PubMed] [Google Scholar]
- 15. Walenkamp GH. 1997. Chronic osteomyelitis. Acta Orthopaed. Scand. 68:497–506 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.