Abstract
The potent antiretroviral pyrimidinediones IQP-0528 (PYD1) and IQP-0532 (PYD2) were formulated in polyurethane intravaginal rings (IVRs) as prophylactic drug delivery systems to prevent the sexual transmission of HIV-1. To aid in the selection of a pyrimidinedione candidate and the optimal loading of the drug in the IVR delivery system, four pyrimidinedione IVR formulations (PYD1 at 0.5 wt% [PYD10.5wt%], PYD11wt%, PYD24wt%, and PYD214wt%) were evaluated in pigtail macaques over 28 days for safety and pyrimidinedione vaginal biodistribution. Kinetic analysis of vaginal proinflammatory cytokines, native microflora, and drug levels suggested that all formulations were safe, but only the high-loaded PYD214wt% IVR demonstrated consistently high pyrimidinedione vaginal fluid and tissue levels over the 28-day study. This formulation delivered drug in excess of 10 μg/ml to vaginal fluid and 1 μg/g to vaginal tissue, a level over 1,000 times the in vitro 50% effective concentration. The in vitro release of PYD1 and PYD2 under nonsink conditions correlated well with in vivo release, both in amount and in kinetic profile, and therefore may serve as a more biologically relevant means of evaluating release in vitro than typically employed sink conditions. Lastly, the pyrimidinediones in the IVR formulation were chemically stable after 90 days of storage at elevated temperature, and the potent nanomolar-level antiviral activity of both molecules was retained after in vitro release. Altogether, these results point to the successful IVR formulation and vaginal biodistribution of the pyrimidinediones and demonstrate the usefulness of the pigtail macaque model in evaluating and screening antiretroviral IVR formulations prior to preclinical and clinical evaluation.
INTRODUCTION
The HIV/AIDS public health crisis urgently needs preventative technologies to protect the 1 million women who contract the virus each year in sub-Saharan Africa, where certain countries report infection rates exceeding 20% in young women (44). Recently, the CAPRISA 004 clinical trial evaluated the antiretroviral tenofovir in a coitally dependent vaginal gel and demonstrated a significant reduction in HIV-1 infections (1). However, user adherence was generally low and declined with time, likely due to the inconvenience of the dosage regimen. Since high rates of user adherence correlated with a reduced HIV infection rate, delivery systems with higher patient adherence may be crucial for maximizing topical microbicide effectiveness. Intravaginal rings (IVRs) offer a number of advantages over vaginal gels, including a preference by women, ease of use, and sustained therapeutic delivery for up to 90 days (15). With all formulations, effectiveness is dependent on user adherence, potency of the active pharmaceutical ingredient (API), and delivery of API to the target site. Therefore, it is anticipated that microbicide IVRs will demonstrate greater effectiveness than frequently applied or coitally dependent dosage forms such as gels or condoms (16, 47).
The significant reduction in HIV infections in the CAPRISA 004 trial validates the use of reverse transcriptase (RT) inhibitors (RTIs) as microbicides and provides the rationale for an RTI-based IVR to enable long-term topical delivery of RTIs (29). The 2,4(1H,3H)-pyrimidinediones (PYDs) comprise an important RTI class, with several analogs demonstrating both nanomolar-level HIV-1 RT and cell entry inhibition in vitro (4). Microbicides that can inhibit HIV-1 at multiple steps in its replication cycle are attractive since they may prevent initial infection and thereby reduce selection of drug-resistant virus (12). Additionally, the PYDs are generally chemically stable, have few or no chiral centers, and are synthesizable in four steps from readily available precursors (5). Of the available PYDs, analogs IQP-0528 (PYD1) and IQP-0532 (PYD2) have been chosen for microbicide formulation due to their favorable calculated log partition coefficient (C log P), photostability, therapeutic index, and HIV-1 inhibition (Fig. 1 and Table 1) (24).
Fig 1.
Pyrimidinedione analogs PYD1 and PYD2. C log P, calculated log partition coefficient.
Table 1.
Antiviral activity of PYD1 and PYD2, unformulated and after in vitro release
| Compound | EC50 (nM)a |
|||
|---|---|---|---|---|
| Unformulated |
Day 30 nonsink condition in vitro |
|||
| CPE inhibition assay with CEM-SS/HIV-1IIIB | SFU assay with CEM-SS/CEM-SS-HIV-1RF | CPE inhibition assay with CEM-SS/HIV-1IIIB | SFU assay with CEM-SS/CEM-SS-HIV-1RF | |
| PYD1 | 4 | 5 | 60 | 7 |
| PYD2 | 10 | 7 | 40 | 7 |
The mean values presented are from a single representative antiviral assay with control compounds evaluated in parallel and derived from three replicate wells.
Comprehensive IVR formulation requires relevant in vivo studies to optimize and evaluate API pharmacokinetics, biodistribution, and safety. Two parallel in vivo human pharmacokinetic studies with the RTI dapivirine formulated in silicone IVRs have recently been reported (31, 38). Although these first microbicide IVR pharmacokinetic studies demonstrated the feasibility and safety of an IVR for sustained release of antiretroviral agents, proceeding directly to clinical evaluation without prior animal studies can be risky, since toxicity or unacceptable drug pharmacokinetics may create significant setbacks, resulting in wasted time, effort, and cost. Animal models evaluating microbicide pharmacokinetics and safety would thus be useful at an earlier stage of microbicide IVR development when IVR design and composition are not yet finalized.
Selection of an appropriate animal model to test product safety and efficacy is difficult and the subject of debate in the microbicide field because animal models to replicate HIV-1 transmission and pathogenesis vary with the question asked (12, 45). However, nonhuman primate safety and pharmacokinetic studies can assist in the selection and assessment of microbicide candidates (34, 43). The pigtail macaque model is particularly relevant since it closely models human vaginal anatomy, physiology, and bacterial microflora (45, 46). Moreover, female pigtail macaques have a menstrual cycle length and frequency similar to those of women and therefore do not require progesterone administration to synchronize their menstrual cycle, a treatment which also thins the vaginal epithelium and thus increases API and virus permeation (25, 33). Numerous gel-based microbicide candidates have therefore been evaluated for safety and pharmacokinetics in the pigtail macaque model (34), including a tenofovir gel viral challenge study which predicted effectiveness prior to the CAPRISA 004 clinical trial (33). However, only recently has an IVR macaque model been developed (36). Therefore, to aid in the selection of a lead PYD molecule, to determine its concentration in the IVR, and to identify overall safety and vaginal biodistribution, we formulated PYD1 and PYD2 at various concentrations in polyurethane (PU) IVRs and compared the in vitro release, chemical stability, and antiviral activity to the in vivo 28-day safety and biodistribution in pigtail macaques.
MATERIALS AND METHODS
Materials.
Medical-grade Tecoflex EG-85A PU was purchased from Lubrizol (Wickliffe, OH). Dyna-Purge purging compound was purchased from Shuman Plastics (Buffalo, NY). Single lots of micronized IQP-0528 and IQP-0532 (purity, >99.8%; mean particle size, 1.5 μm) were provided by ImQuest BioSciences (Frederick, MD). 19-Norethindrone and zidovudine (AZT) (purities, >98%) were purchased from Sigma-Aldrich (St. Louis, MO). Solutol HS-15 solubilizer was purchased from BASF (Ludwigshafen, Germany). All solvents and reagents were American Chemical Society grade unless noted.
Ring fabrication.
PYD-loaded PU rods were fabricated in a two-step process: PU pellets were frozen under liquid nitrogen, ground in a Pulverisette 14 stainless steel variable-speed rotor mill (Fritsch, Germany) to a particle size of roughly 500 μm, and then roll mixed on a jar mill with micronized PYD1 or PYD2. Upon thorough mixing, the PU-PYD powder mixture was manually fed into a benchtop twin-screw extruder (Thermo Haake, Waltham, MA) set at 140°C and 50-rpm screw speed and extruded continuously through a 4.3-mm-diameter aluminum die. Upon exiting the die, the compressed cylindrical rod was allowed to swell to approximately 5 mm in cross-sectional diameter and wrapped around a tube to form a circular ring shape. Upon extrusion of the entire sample, the machine was purged using 20 g of Dyna-Purge.
A total of four PYD formulations were made; the final PYD loadings in the polymer, as determined by solvent extraction, were 0.5 and 1 wt% PYD1 (PYD10.5wt% and PYD11wt%, respectively) and 4 and 14 wt% PYD2 (PYD24wt% and PYD214wt%, respectively). The upper loading of 14 wt% API was selected, as it is approximately the maximum amount at which PYD2 is soluble in the hydrophobic PU; since the API release rate increases linearly with API loading until maximum solubility is attained in the polymer (given that dissolution into the vaginal fluid is not rate limiting), loading-dependent release was expected over the loadings evaluated (7, 14). Placebo IVRs were also made as controls. IVRs were fabricated by cutting the extruded rods to 6.6 cm in length using a PU butt-welding kit (Fenner Drives, Manheim, PA) to melt and subsequently join the ends together to form an IVR with an outer diameter of 25 mm. The 5- by 25-mm IVR dimensions were selected on the basis of previous work concluding that a 5- by 25-mm IVR is well tolerated and retained in the pigtail macaque vaginal tract (36).
In vitro release.
To simulate the in vivo drug elution from the ring into the surrounding vaginal fluid, IVRs in triplicate were placed in either sink or nonsink in vitro release medium solutions which were replaced every 24 h. The sink release medium consisted of the 2 wt% nonionic surfactant Solutol in pH 4.2 25 mM sodium acetate buffer, whereas the nonsink release medium contained 0.05 wt% surfactant. A 0.05 wt% surfactant was selected as the nonsink release medium since it was above the critical micelle concentration of approximately 0.02 wt% and therefore had increased PYD solubility compared to water but still resulted in PYD saturation (2, 42). The volume of release medium was adjusted over the course of the study so that the concentration for either PYD in the 2 wt% surfactant release medium was roughly 1/10 to 1/15 the maximum solubility. On days 1, 3, 7, 10, 15, 20, 25, and 30, an aliquot of release medium from each sample was transferred to a high-pressure liquid chromatography (HPLC) vial for drug content analysis.
Antiviral activity.
The antiviral activities of PYD1 and PYD2, both unformulated and formulated in the 0.05 wt% surfactant release medium from the 30-day IVR in vitro release assay, were evaluated by the cytopathic effect (CPE) inhibition and the cell-cell HIV transmission (syncytium-forming unit [SFU]/RT) assays. The CPE inhibition assay was performed in CEM-SS cells with HIV-1IIIB. Briefly, 6 serial dilutions of test or control compound in RPMI 1640 medium supplemented with 10% fetal bovine serum, 1% l-glutamine, and 1% penicillin-streptomycin (10% complete medium) was added to appropriate wells of a 96-well round-bottom microtiter plate. CD4-expressing CEM-SS cells at a concentration of 2.5 × 103 cells per well and HIV-1IIIB at an appropriate predetermined titer yielding 90% or greater cell killing in virus control wells were sequentially added to the microtiter plate. The cultures were incubated in 5% CO2 at 37°C for 6 days. Following incubation, the microtiter plates were stained with 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) dye to evaluate the efficacy and toxicity of the test compound(s). XTT dye was metabolized to a colored formazan product by active mitochondria in viable cells. The HIV-1 RTI AZT was evaluated in parallel as a positive control for HIV inhibition. Cell, virus, precipitation/color, and medium controls were evaluated in parallel on each plate. Each test concentration was evaluated in triplicate and then averaged to determine the 50% effective concentration (EC50).
Assessment of the inhibition of cell-to-cell transmission of HIV was performed using a syncytial inhibition (SFU/RT) assay (41). CEM-SS cells chronically infected with HIV-1RF (CEM-SSRF cells) were cocultivated in serial logarithmic dilutions (range, 105 to 101 cells) with uninfected CD4+ CEM-SS cells at 5 × 105 cells per well along with test compound in flat-bottom 96-well microtiter plates at 37°C in 5% CO2. Following cocultivation for 24 h, the number of syncytia in each well was counted by microscopic observation. Cell-to-cell transmission was also evaluated by quantification of the burst of virus production, which occurs at between 24 and 48 h after the cocultivation of the infected and uninfected cells. Virus production in the cell-free supernatant for determination of RT activity was quantified using a standard radioactive incorporation polymerization assay (3). Toxicity of the test compounds was determined using the XTT dye assay as described above. Dextran sulfate was used as a positive control for the assay. Each test concentration was evaluated in triplicate and then averaged to determine the EC50.
PYD chemical stability and quantification of IVR drug content.
To determine the chemical stability of the PYDs in the IVR formulations, extruded rods were cut into approximately 50-mg pieces and randomly selected, weighed, and placed into separate aluminum pouches. The pouches were then sealed using a heated impulse sealer (recycle setting = 3, sealing setting = 7, 155°C) and placed in a stability chamber set at 40°C with 75% relative humidity (Caron, Marietta, OH). At 0, 30, and 90 days, the pouches were removed and stored at −80°C until completion of the 90-day time point. PYD1 and PYD2 were then extracted from IVR segments at each drug loading concentration using a previously developed PU dissolution method (21). Briefly, the segments were dissolved in a 5 ml dimethyl acetamide-dichloromethane mixture along with the internal standard progestogen 19-norethindrone. One milliliter was transferred to a centrifuge tube, and methanol was added to precipitate the polymer. The sample was then centrifuged and the supernatant was quantified via HPLC. PYD extraction efficiency, as determined using spike blank samples, was 97.3% ± 0.4% (n = 5).
Drug content analysis for in vitro studies.
The following methods were used to quantify and detect PYD1 and PYD2 in vitro. Samples were injected on an Agilent 1200 series HPLC with an Agilent Zorbax octyldecyl silane (ODS) column (particle size, 5 μm; 4.6 by 250 mm). For PYD1, a 12-min isocratic method was used with 35/65 (vol/vol) double-deionized water-acetonitrile at a flow rate of 1.5 ml/minute. Typical retention times for PYD1 and norethindrone were 6.4 and 3.0 min, respectively. For PYD2, a 15-min gradient water-acetonitrile method was used at a flow rate of 1 ml/minute. Typical retention times for PYD2 and norethindrone were 13.0 and 6.3 min, respectively. PYD1 and PYD2 were detected at 267 nm, whereas norethindrone was detected at 245 nm.
Nonhuman primate studies.
Two 28-day IVR safety and vaginal biodistribution studies using pigtail macaque monkeys were performed following procedures approved by the CDC Institutional Animal Care and Use Committee according to the Guide for the Care and Use of Laboratory Animals as previously described (36). A total of six macaques divided into three groups with two animals per group were used for each study. PYD1 and PYD2 were evaluated since they both possess promising microbicide IVR attributes. A wide range of drug loadings was evaluated, as there are no microbicide IVR macaque studies reported in literature and therefore we were unsure what loading might achieve acceptable safety and pharmacokinetics from this type of delivery system. Furthermore, the PYDs have not been extensively studied in vivo, and therefore, their vaginal and systemic toxicological profile was uncertain. For the PYD1 study, the three groups were placebo IVR, PYD10.5wt% IVR, and PYD11wt% IVR. For the PYD2 study, the three groups were placebo IVR, PYD24wt% IVR, and PYD214wt IVR. IVRs were inserted in the posterior vagina (ectocervix) on day 0 and left in place for 28 days. All vaginal fluid and tissue samples were collected at both the vaginal introitus (distal to the ring) and the ectocervix (proximal to the ring). Upon completion of the 28-day time point, IVRs were removed and analyzed for remaining drug content as described above.
Safety.
Prior to IVR insertion, baseline safety, including evaluation of colposcopy samples, plasma and vaginal cytokine levels, and vaginal microflora levels, were established in all animals to allow inter- and intra-animal comparisons. IVRs were then inserted and the safety parameters were monitored for any changes. Vaginal microflora was characterized for all macaques on days 0, 7, and 21, as well as 10 days after IVR removal (day 38) as previously described (35). The aerobic and anaerobic organisms Lactobacillus species, viridans group streptococci, and Gardnerella vaginalis were identified to the genus, species, or group of bacteria level using phenotypic tests, Gram stain and colony morphology, and aerotolerance, as described in the Manual of Clinical Microbiology (30). Lactobacillus species and viridans group streptococci were tested for hydrogen peroxide production in a qualitative assay on tetramethylbenzidine agar plates (37).
Blood plasma and vaginal swab specimens were collected from each macaque to analyze the induction of any mucosal or systemic cytokines. All macaque-specific antibodies were used for plasma cytokine analysis, including gamma interferon (IFN-γ), interleukin-2 (IL-2), tumor necrosis factor alpha (TNF-α), IL-6, macrophage inflammatory proteins 1α and 1β (MIP-1α and MIP-1β), RANTES, eotaxin, IL-15, IL-12p40, granulocyte colony-stimulating factor (G-CSF), IL-8, and interleukin-1 receptor antagonist (IL-1Ra). Likewise, all vaginal cytokines available for analysis included IL-1β, IL-6, IL-8, G-CSF, RANTES, IL-1Ra, TNF-α, MIP-1α, and MIP-1β. Both plasma and vaginal cytokines were analyzed using fluorescent multiplexed bead-based assays (Invitrogen, Carlsbad, CA, and Bio-Rad, Hercules, CA) as previously described (36). All cytokine assays utilized individual cytokine standard curves created by serial dilution and the in-house internal positive control of phorbol myristate acetate-ionomycin-stimulated pigtail macaque peripheral blood mononuclear cell supernatant. Introitus and ectocervix vaginal swab specimens were collected from each animal at days −11, 0, 3, 7, 14, 21, 28, and 38. The swabs were weighed prior to and after vaginal fluid collection to determine vaginal fluid recovery. The swabs were then immersed in 200 μl of a 1:100 dilution of a protease inhibitor cocktail (1 ml; P8340; Sigma) in phosphate-buffered saline (PBS) for 30 min at 4°C. A Costar Spin-x centrifuge filter unit (catalog no. CLS8160; Sigma) was used to separate the extracted medium from the swab. Samples were centrifuged at 12,000 rpm for 20 min at 4°C. In calculating the final concentration of the cytokines, a dilution factor was calculated on the basis of a dilution factor of (x + y)/x, where x equals the volume of the material collected (assuming a vaginal fluid density of 1.0 g/ml) and y is the volume of the extraction buffer added. This dilution factor was used to calculate the final concentration of the cytokines in the mucosal secretions. Systemic cytokine quantification was carried out in the same manner using blood plasma collected at the same time points as vaginal swabs.
The surfaces of the IVRs postimplantation were examined for cellular and noncellular adhesion and drug surface crystallization. A 1% Alcian blue mucin stain was prepared in water, and 200 μl was placed on top of the ring segment for 10 s and subsequently rinsed with water and examined. A 4′,6-diamidino-2-phenylindole (DAPI) cell nuclear stain was prepared at 1 μM, 200 μl was added on top of ring segments, and the segments were viewed under a fluorescence microscope. Lastly, crystallization of either PYD on the IVR surface was observed using a polarized light microscope.
PYD measurement in plasma, vaginal fluid, and vaginal tissue.
Plasma and vaginal fluid (days −11, 0, 3, 7, 14, 21, 28, and 38) and vaginal tissue (days −11, 7, and 38 for PYD1; days −11, 7, 21, and 38 for PYD2) were taken from the animals for drug content analysis. Weck-Cel wicks (Medtronic Xomed, Inc.) were used to collect vaginal fluid at both the introitus and ectocervix. Wicks were inserted and allowed to absorb fluid for approximately 5 min prior to removal. Biopsy samples were taken at the introitus and ectocervix using a Miltex Townsend model 30-1445 minibite cervical biopsy punch forceps with a bite of 4.2 by 2.3 by 1 mm.
PYD in biological samples was extracted and analyzed by modifying a previously developed liquid chromatography-tandem mass spectrometry method (23). Briefly, each sample was extracted with acetonitrile, followed by concentration and resuspension in mobile phase A, which was composed of 0.04% formic acid, 0.04% ammonium hydroxide, and 0.057% acetic acid in water. The final processed sample was then injected onto a 150- by 2-mm Ascentis phenyl reverse-phase column (Supelco, Bellefonte, PA) connected to a Shimadzu Prominence HPLC system (Durham, NC) and eluted with a water-acetonitrile mobile-phase gradient. A Model 3200 QTrap mass spectrometer (Applied Biosystems, Foster City, CA) in multiple-reaction-monitoring mode was used to monitor the transitions for the compounds of interest. For PYD1, m/z 341 to 287 and 341 to 133 were monitored, while m/z 343 to 275 and 343 to 122 were monitored for PYD2. The lower limit of quantification (LLOQ) was defined as the minimum level at which quantitative results could be obtained and was calculated to be 10 times the standard deviation of injections at the lowest concentration which were statistically different from blank injections using a 99% confidence interval. The LLOQ of both PYDs for this assay was determined to be 5 ng/ml. The amount of vaginal fluid absorbed onto the wick was measured by weighing the wicks before and after fluid absorption. All biopsy samples were also weighed after collection. The vaginal tissue LLOQ was determined to be 5 ng/sample. The vaginal fluid LLOQ was 170 ng, for an average collected vaginal fluid mass of 5.5 mg. To convert weight/weight concentrations of PYD (ng of PYD/mg of vaginal fluid or tissue) to molarity (μM), vaginal fluid and tissue densities of 1.0 g/ml were assumed.
Statistical analysis.
Statistical difference between unpaired microflora groups was determined using the Mann-Whitney U test. Chemical degradation of the PYDs as a function of time was determined using one-way analysis of variance (ANOVA). For both tests, a P value of <0.05 was considered significant.
RESULTS
In vitro release.
PYD-PU hot-melt extrusion produced amorphous, molecularly dissolved PYD in PU whose cylindrical rod ends were subsequently butt welded to create macaque IVRs (Fig. 2). The in vitro release profile was determined for both the lowest loading (PYD10.5wt%) and highest loading (PYD214wt%), where the cumulative percentage of PYD released (Q) under sink conditions was significantly higher than that under nonsink conditions (Fig. 3). The cumulative amounts released from PYD10.5wt% and PYD214wt% at 30 days were 3 and 71 mg, respectively, under sink conditions and 1 and 14 mg, respectively, under nonsink conditions. The cumulative release profiles were quite different: the sink condition exhibited matrix-controlled kinetics of Q ∝ time1/2, whereas the nonsink condition exhibited partition-controlled kinetics of Q ∝ time.
Fig 2.
Human IVR (left) and macaque IVR (right).
Fig 3.
Percent cumulative release of PYD1 and PYD2 in vitro over 30 days (sink and nonsink conditions) compared to 28-day in vivo data. Mean ± SD, n = 3 for all points.
Antiviral activity and chemical stability.
To confirm that the two PYDs maintained their nanomolar-level activity against HIV-1 after being released from the IVRs, we compared the antiviral activity of PYD1 and PYD2 before formulation and after in vitro release from the IVRs. The activity of both unformulated PYDs in the CPE and SFU assays ranged from 4 to 10 nM (Table 1). The in vitro release samples exhibited diminished (4 to 15 times) nanomolar-level activity in the CPE assay but maintained similar nanomolar-level activity in the cell-to-cell transmission assay. Likewise, the chemical stability of PYD1 and PYD2 in the IVR formulation was evaluated under the accelerated stability condition of 40°C for 90 days (Table 2). There was no significant chemical degradation of either compound over the 90-day period (one-way ANOVA, P > 0.05), suggesting that these formulations are chemically stable for an extended period of time. Ninety-day HPLC chromatograms of the extracted PYD showed no new peaks to suggest chemical degradation, thus providing further support that no degradation of these compounds occurred under the stability conditions tested (see the figure in the supplemental material).
Table 2.
Chemical stability of formulated PYD1 and PYD2 stressed at 40°C
| Formulation | % recovery after the following no. of days at 40°Ca: |
||
|---|---|---|---|
| 0 | 30 | 90 | |
| PYD1 | 100.0 ± 1.8 | 100.0 ± 3.4 | 99.4 ± 0.7 |
| PYD2 | 100.0 ± 0.9 | 100.5 ± 1.0 | 99.6 ± 1.6 |
| Spike blank | 97.3 ± 0.4 | ||
Data are means ± standard deviations (n = 5). Percent recovery was determined by HPLC and normalized to that at day 0.
Safety of PYD IVRs in pigtail macaques.
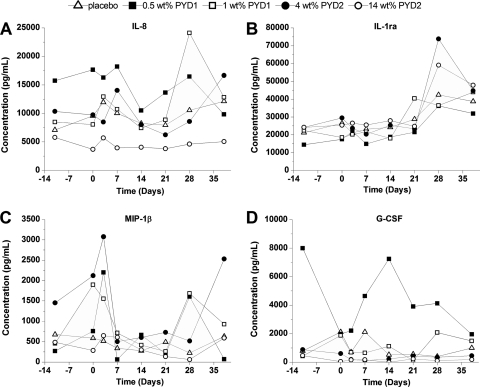
The in vivo safety of the PYD formulations was evaluated over the study duration by analyzing colposcopy specimen, plasma, and vaginal cytokine levels and microflora levels. Colposcopies revealed no obvious tissue inflammation or abrasions that would indicate IVR irritation (data not shown). Cytokine levels fluctuated greatly between time points and animals both in plasma (see Table S1 in the supplemental material) and vaginally (displaying four cytokines whose levels were consistently above the detection limit to allow comparisons between the formulations) (Fig. 4; see Table S2 in the supplemental material). However, there were no discernible trends in cytokine levels when comparing the level for each animal to its corresponding day −11 or day 0 level (intra-animal) or between groups (placebo versus drug loaded). Likewise, there was no significant difference (Mann-Whitney test, P > 0.05) in vaginal microflora levels between placebo and IVR groups (Fig. 5). Altogether, these results suggest that the placebo and PYD-loaded IVRs did not perturb the vaginal tissue and native microflora.
Fig 4.
Mean vaginal cytokine levels as a function of time of IL-8 (A), IL-1ra (B), MIP-1β (C), and G-CSF (D) for placebo IVRs, PYD1 IVRs, and PYD2 IVRs. IVRs were inserted at day 0 and removed at day 28. The mean data point was the average of single measurements in each animal (n = 4 and 2 animals for placebo and PYD IVRs, respectively).
Fig 5.
Vaginal microflora levels (numbers of CFU) of Lactobacillus species, viridans group streptococci, and G. vaginalis from animals with placebo and PYD IVRs (bars, mean value for all four time points; n = 27 and 21 for placebo and PYD IVRs, respectively).
To further assess device performance and biocompatibility, the surfaces of the removed IVRs were examined for cellular and macromolecular adhesion as well as PYD surface crystallization. The IVRs changed little in appearance, maintaining a transparent look, except for the slight presence of blood on IVRs removed from menstruating macaques (data not shown). Additionally, the PYD214wt% IVR showed API surface crystallization, as observed by polarized light microscopy (data not shown). DAPI cell nucleus stains revealed considerable cellular adhesion on all IVRs, and all IVRs stained positive for mucin using an Alcian blue stain (data not shown).
PYD vaginal fluid levels.
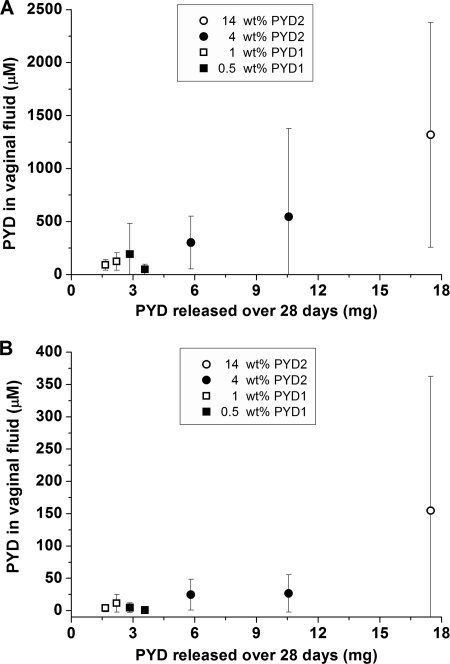
PYD vaginal fluid levels were quantified over the 28-day period to aid in evaluation of release kinetics and biodistribution. Wicks collected and analyzed for drug content prior to IVR placement (days −11 and 0) or 10 days after IVR removal (day 38) revealed no detectable drug for any of the four drug-loaded formulations. With the exception of one sample, PYD vaginal fluid levels at the ectocervix exceeded micromolar concentrations across all PYD IVR loadings, animals, and time points during ring insertion, although the higher-drug-loaded IVRs generally maintained higher respective PYD vaginal fluid levels (Fig. 6A). The highest-loaded IVRs (14 wt%) also exhibited a significant burst (day 3 PYD levels several log units higher than day 7 levels) yet still maintained near millimolar PYD vaginal fluid levels at 28 days. At the introitus, PYD vaginal fluid levels were roughly an order of magnitude lower than those in the ectocervix but demonstrated the similar trend of greater PYD concentrations with higher IVR drug loadings (Fig. 6B). Although most vaginal fluid samples contained micromolar PYD levels, at least one introitus vaginal fluid sample for each loading had levels below the LLOQ, highlighting the in vivo variability. Overall, however, increased PYD IVR loading correlated with increased vaginal fluid concentrations at both the introitus and ectocervix, with Pearson correlation coefficients (R values) of 0.57 and 0.65, respectively. A positive correlation also existed between the cumulative amount of PYD released at 28 days in vivo, as determined by API solvent extraction from IVRs after study completion at 28 days, and time-averaged PYD concentrations in vaginal fluid (Fig. 7), with Pearson correlation coefficients at the introitus and ectocervix of 0.53 and 0.64, respectively. The cumulative percent PYD released in vivo was more similar to that under nonsink than sink in vitro release conditions (Fig. 3).
Fig 6.
PYD levels in vaginal fluid at the ectocervix (proximal) (A) and introitus (distal) (B) for PYD2 IVRs and PYD1 IVRs. n = 2 animals per group, except on days 21 and 28 of 14 wt% PYD2 loading (n = 1). PYD1 and PYD2 levels were below the LLOQ prior to IVR insertion and after IVR removal (days −11 and 38; data not shown).
Fig 7.
In vivo correlation between PYD levels in vaginal fluid and mg PYD released from the individual IVRs after the 28-day pharmacokinetic study at both the ectocervix (A) and introitus (B) for PYD2 IVRs and PYD1 IVRs. Vaginal fluid levels were averaged over the 28-day implantation period (mean ± SD, n = 5 time points). The amount (mg) of PYD1 or PYD2 released in vivo from the IVR was determined by drug solvent extraction from the IVR following the 28-day vaginal implantation period.
Vaginal tissue drug levels.
Vaginal tissue biopsied and analyzed for PYD concentration prior to IVR placement (day −11) and 10 days after IVR removal (day 38) revealed no detectable API (data not shown). All ectocervical tissues exposed to PYD IVRs (collected at day 7 for PYD1 and days 7 and 21 for PYD2) contained micromolar PYD levels, except for the lowest-loading PYD10.5wt% group, where the tissue concentration was below the LLOQ (Table 3). However, at the introitus, only the highest-loading PYD214 wt% IVR group gave detectable drug levels, with 2 of 3 biopsy samples having micromolar drug levels.
Table 3.
Ectocervical and introitus PYD vaginal tissue levels for each individual biopsy specimen
| Location | Formulation | Concna |
|||
|---|---|---|---|---|---|
| Day 7 |
Day 21 |
||||
| Animal 1 | Animal 2 | Animal 1 | Animal 2 | ||
| Ectocervix | 0.5 wt% PYD1 | 6.4 (2.2) | <3.5 (1.2) | ||
| 1 wt% PYD1 | 16.6 (5.6) | 1.3 (0.4) | |||
| 4 wt% PYD2 | 27.4 (9.3) | 20.9 (7.1) | 10.5 (3.6) | 12.3 (4.2) | |
| 14 wt% PYD2 | 8.7 (3.0) | 9.1 (3.1) | Sample lost | 7.3 (2.5) | |
| Introitus | 0.5 wt% PYD1 | <4.2 (1.4) | <1.5 (0.5) | ||
| 1 wt% PYD1 | <4.2 (1.4) | <2.9 (1.0) | |||
| 4 wt% PYD2 | <3.2 (1.1) | <3.9 (1.3) | <8.6 (2.9) | <5.4 (1.8) | |
| 14 wt% PYD2 | 14.0 (4.8) | <4.5 (1.5) | Sample lost | 4.2 (1.4) | |
Data are in μM (μg/g). For PYD1, biopsy specimens were collected only on day 7, whereas for PYD2, biopsy specimens were collected on both day 7 and day 21. In tissue samples where drug was not detected, the individual sample LLOQ is given (indicated by <), as it differs for each biopsy specimen on the basis of its tissue mass. PYD1 and PYD2 levels were less than LLOQ prior to IVR insertion (day −11) and after IVR removal (day 38) and are not shown.
DISCUSSION
The PYD-PU powder blend hot-melt extrusion method was an improvement over previous multistep fabrication techniques employing a solvent (14, 21). The in vitro PYD release from IVRs under nonsink conditions compared well with the in vivo release, and the antiviral activity and chemical stability of the PYDs in the formulation were maintained. We observed an increase in PYD vaginal fluid concentrations with increasing PYD IVR loadings (and amount of PYD released from the IVR). The vaginal fluid and tissue drug levels attained in the highest-loaded group were greater than 1,000 times the in vitro EC50, suggesting that the PYD214 wt% IVR may sufficiently load PYD in vaginal tissue along the length of the vaginal tract at concentrations sufficient to prevent HIV-1 infection.
In vitro studies.
Typically, in vitro drug release testing utilizes sink conditions, wherein the API concentration in the release medium never exceeds one-third the equilibrium saturation concentration (42). Conversely, nonsink conditions exist when the concentration of API in the release medium nears its maximum solubility, resulting in attenuated drug release due to a smaller concentration gradient as well as API partitioning between the polymer and medium (6, 17). Although utilization of sink conditions does in fact classify the device type—the most common being time dependent (matrix devices) or time independent (reservoir devices)—it seldom reflects in vivo pharmacokinetics. This and other studies utilizing novel delivery platforms have shown a disparity between in vitro release under sink conditions and in vivo release, particularly for poorly water soluble compounds, which constitute the majority of antiretrovirals being developed as microbicides (20, 42). With hydrophobic compounds such as the PYDs, achieving sink conditions requires large volumes of aqueous release medium (up to hundreds of milliliters per day) and/or a high concentration of surfactants or organic solvents to increase the API's solubility in the medium.
We performed in vitro release under sink and nonsink conditions to determine an in vitro/in vivo release correlation that may be beneficial for future PYD IVR development. The cumulative percentages of PYD1 and PYD2 released in nonsink release medium was quite similar to those observed in vivo, whereas sink conditions overestimated drug release. In vitro, the 14 wt% PYD2 IVR released 23 and 11 times more API than the 0.5 wt% PYD1 IVR under sink and nonsink conditions, respectively. The lower differential release between the 14 wt% PYD2 and 0.5 wt% PYD1 IVRs under nonsink conditions was likely due to rapid PYD saturation of the release medium with the high loading, therefore hindering further drug release from the IVR. Given the low volume and variable production of vaginal fluid, it is not surprising that sink conditions did not predict in vivo pharmacokinetics for either hydrophobic PYD at the loadings evaluated (28, 32). Therefore, the nonsink release condition established an in vivo/in vitro release correlation that may be useful for future hydrophobic API microbicide formulation development.
All formulations pose the potential problem of API chemical instability and subsequent loss of biological activity upon storage (19). A successful microbicide formulation must be able to withstand elevated temperatures, which may occur during storage, without causing chemical degradation of the API. We stressed the PYD IVRs at 40°C for 90 days and observed no PYD1 or PYD2 chemical degradation, as evaluated by HPLC. Similarly, the PYD1 and PYD2 released from the IVRs in vitro showed nanomolar-level antiviral activity, as evaluated in CPE inhibition and SFU assays. Although the CPE inhibition assay showed diminished (4 to 15 times) activity compared to that of the unformulated PYD, this relatively subtle change could be due to inherent error in the assay and/or presence of surfactant in the release medium. Therefore, the PU carrier and processing conditions were able to maintain the chemical stability of both drugs upon fabrication and storage, and the released compounds maintained nanomolar-level antiviral activity.
In vivo safety and PYD biodistribution.
Safety is an essential component of a microbicide's efficacy since irritation, inflammation, or disruption of the vaginal epithelium can increase the susceptible immune cell population in the cervicovaginal mucosa (22). Similarly, suppression of lactic acid-secreting microflora such as Lactobacillus spp. and viridans group streptococci may be deleterious to maintenance of an acidic pH, which can neutralize pathogens such as HIV-1 (8). We monitored epithelial integrity, microflora, and plasma and vaginal cytokines throughout the 28-day in vivo studies. Although the inter- and intra-animal cytokine and microflora variability was quite high, we observed no clear indication that the PYDs were detrimental locally or systemically. Furthermore, cytokine levels were similar to those described in previously published work comparing naïve and placebo IVR exposure (36).
Thorough examination of the IVR surface postimplantation revealed dispersed adherent cells and Alcian blue staining of cervicovaginal mucus. Biological matter has been observed on the surface of IVRs in other in vivo IVR studies and has not noticeably increased vaginal inflammation (13, 27, 40). The impact of biological matter adhesion on drug release has not been reported in literature but could potentially affect drug elution and the corresponding pharmacokinetics. However, since the diffusion coefficient of small-molecule APIs through mucus is comparable to that of IVR polymers (approximately 10−7 cm2/s [10, 17, 26]), it is unlikely that a thin layer of biological material on the ring surface significantly impedes drug transport. This assumption holds true only for uncharged molecules such as the PYDs, as mucus is known to bind charged molecules and thereby hinder transport (9, 10). Crystallized PYD was also observed on the surface of the 14 wt% PYD2 IVR after removal, suggesting partition-controlled drug release where dissolution into vaginal fluid is the rate-limiting step as a result of the PYDs' high polymer solubility and low aqueous solubility (6). This phenomenon may be advantageous, as the matrix IVR could provide relatively constant drug release throughout the dosage duration, as suggested with the PYD214wt% IVR vaginal fluid levels.
Also in regard to API transport, the impact of biopsy specimen collection and frequency of collection on drug release and pharmacokinetics from sustained-delivery devices has not been studied. Potentially, large biopsy specimen and/or frequent biopsy specimen collection could create significant tissue trauma, inducing a local wound healing or inflammatory response and possibly altering drug release and local biodistribution. To mitigate this risk, we used a miniature-sized biopsy punch forceps (average tissue mass, 5 mg) and limited biopsy frequency to 14 days to minimize any local tissue trauma and associated alteration of PYD pharmacokinetics.
Since PYDs primarily act to inhibit RT inside susceptible immune cells, they must be sufficiently concentrated in vaginal tissue, where initial infection of these cells is thought to occur (18). The efficacy of an RTI formulation therefore likely hinges on the API's ability to diffuse into the surrounding vaginal fluid and subsequently partition into the local vaginal tissue. We collected vaginal fluid and tissue samples at two anatomical sites (introitus and ectocervix) to evaluate PYD distribution. Most ectocervical vaginal fluid and tissue samples attained micromolar PYD concentrations for all of the PYD IVR loadings, yet at the introitus, only the highest loading (14 wt%) demonstrated PYD levels above the LLOQ. Encouragingly, PYD vaginal tissue concentrations for this 14 wt% loading were quite similar to those measured in the dapivirine IVR clinical study (3.5 μg PYD per g of tissue), whereas PYD vaginal fluid levels were higher than those of dapivirine (7.1 μg PYD per ml of fluid) (38). Considering that the PYDs and dapivirine have nanomolar-level EC50 activity against HIV-1 RT, the micromolar PYD concentrations attained in the vaginal tract—over 1,000 times the EC50—suggest that this formulation may be effective and warrants further evaluation.
In both vaginal fluid and tissue, PYD levels at the ectocervix were greater than those at the introitus, a trend also observed in the dapivirine IVR clinical trial (38). Despite this gradient, the near steady-state PYD levels attained at the introitus after 3 days of IVR implantation argue for significant drug transport by vaginal fluid convection, an advantageous phenomenon that IVR efficacy likely depends upon for adequate biodistribution (11, 39). Collecting vaginal fluid and tissue at earlier time points, such as hours after IVR insertion, would be beneficial for determining when the PYDs are sufficiently concentrated along the length of the vaginal tract to prevent viral infection. Likewise, attaining the vaginal washout kinetics of the PYDs after IVR removal (no PYD was detected 10 days after IVR removal in our study) would provide insight as to how long an IVR can be absent from the vagina before protection is lost.
In sum, the nonsink in vitro release medium condition accurately predicted PYD in vivo release and may prove useful for many of the hydrophobic APIs formulated in microbicide IVRs. The potent nanomolar-level activity of the PYDs was maintained after in vitro release, and the PYDs were chemically stable in the PU IVR formulation. None of the four IVR formulations evaluated in the pigtail macaque model caused any obvious vaginal irritation, inflammation, or toxicity. The highest-loading IVR formulation appeared to be the most promising, as it was the only formulation that achieved drug levels in excess of 1,000 times the in vitro EC50 along the entire length of the vaginal tract 3 days after insertion and maintained these levels throughout the 28-day study. Furthermore, since only approximately 10% of loaded drug was released by day 28, it may be possible to increase the dose duration and thus reduce the daily cost of the device in resource-poor areas. Due to the inherent data variability when utilizing a low number of animals in a pilot safety and pharmacokinetic study, statistical analyses and depth of conclusions were limited. A follow-on study with the lead formulation using a larger number of animals and improved bioanalytical methods with lower detection limits is planned to confirm safety and pharmacokinetics. Despite these limitations, the reported study was beneficial, as it demonstrated successful PYD formulation in an IVR and promising in vivo safety and pharmacokinetic evaluation in pigtail macaques to justify further development of the PYD IVR as a sustained-dosage prophylactic to prevent HIV infection.
Supplementary Material
ACKNOWLEDGMENTS
Work conducted at the University of Utah was financially supported by National Institutes of Health grants U19AI076980 and U19AI077289. This project has been funded in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, U.S. Department of Health and Human Services, under interagency agreement no. Y1-AI-0681-01.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
We have no commercial or other associations that might pose a conflict of interest.
The use of trade names is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services, the Public Health Service, or the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print 12 December 2011
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Abdool Karim Q, et al. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bravo Gonzalez RC, Boess F, Durr E, Schaub N, Bittner B. 2004. In vitro investigation on the impact of Solutol HS 15 on the uptake of colchicine into rat hepatocytes. Int. J. Pharm. 279:27–31 [DOI] [PubMed] [Google Scholar]
- 3. Buckheit RW, Jr, et al. 1994. Biological and biochemical anti-HIV activity of the benzothiadiazine class of nonnucleoside reverse transcriptase inhibitors. Antiviral Res. 25:43–56 [DOI] [PubMed] [Google Scholar]
- 4. Buckheit RW, Jr, Hartman TL, Watson KM, Chung SG, Cho EH. 2008. Comparative evaluation of the inhibitory activities of a series of pyrimidinedione congeners that inhibit human immunodeficiency virus types 1 and 2. Antimicrob. Agents Chemother. 52:225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buckheit RW, Jr, et al. 2007. The structure-activity relationships of 2,4(1H,3H)-pyrimidinedione derivatives as potent HIV type 1 and type 2 inhibitors. Antivir. Chem. Chemother. 18:259–275 [DOI] [PubMed] [Google Scholar]
- 6. Chien YW, Lambert HJ. 1974. Controlled drug release from polymeric delivery devices. II. Differentiation between partition-controlled and matrix-controlled drug release mechanisms. J. Pharm. Sci. 63:515–519 [DOI] [PubMed] [Google Scholar]
- 7. Chien YW, Lin S. 2002. Optimisation of treatment by applying programmable rate-controlled drug delivery technology. Clin. Pharmacokinet. 41:1267–1299 [DOI] [PubMed] [Google Scholar]
- 8. Clarke JG, et al. 2002. Microflora changes with the use of a vaginal microbicide. Sex. Transm. Dis. 29:288–293 [DOI] [PubMed] [Google Scholar]
- 9. Cone RA. 2009. Barrier properties of mucus. Adv. Drug Deliv. Rev. 61:75–85 [DOI] [PubMed] [Google Scholar]
- 10. Cu Y, Saltzman WM. 2009. Mathematical modeling of molecular diffusion through mucus. Adv. Drug Deliv. Rev. 61:101–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geonnotti AR, Katz DF. 2010. Compartmental transport model of microbicide delivery by an intravaginal ring. J. Pharm. Sci. 99:3514–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grant RM, et al. 2008. Whither or wither microbicides? Science 321:532–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gunawardana M, et al. 2011. Microbial biofilms on the surface of intravaginal rings worn in non-human primates. J. Med. Microbiol. 60:828–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gupta KM, et al. 2008. Polyurethane intravaginal ring for controlled delivery of dapivirine, a nonnucleoside reverse transcriptase inhibitor of HIV-1. J. Pharm. Sci. 97:4228–4239 [DOI] [PubMed] [Google Scholar]
- 15. Hardy E, Hebling EM, Sousa MH, Almeida AF, Amaral E. 2007. Delivery of microbicides to the vagina: difficulties reported with the use of three devices, adherence to use and preferences. Contraception 76:126–131 [DOI] [PubMed] [Google Scholar]
- 16. Heise LL, et al. 2011. Apples and oranges? Interpreting success in HIV prevention trials. Contraception 83:10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Helbling IM, Luna JA, Cabrera MI. 2011. Mathematical modeling of drug delivery from torus-shaped single-layer devices. J. Control. Release 149:258–263 [DOI] [PubMed] [Google Scholar]
- 18. Hladik F, Hope TJ. 2009. HIV infection of the genital mucosa in women. Curr. HIV/AIDS Rep. 6:20–28 [DOI] [PubMed] [Google Scholar]
- 19. Huynh-Ba K. (ed). 2009. Handbook of stability testing in pharmaceutical development. Springer, New York, NY [Google Scholar]
- 20. Iyer SS, Barr WH, Karnes HT. 2006. Profiling in vitro drug release from subcutaneous implants: a review of current status and potential implications on drug product development. Biopharm. Drug Dispos. 27:157–170 [DOI] [PubMed] [Google Scholar]
- 21. Johnson TJ, Gupta KM, Fabian J, Albright TH, Kiser PF. 2010. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur. J. Pharm. Sci. 39:203–212 [DOI] [PubMed] [Google Scholar]
- 22. Klasse PJ, Shattock R, Moore JP. 2008. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu. Rev. Med. 59:455–471 [DOI] [PubMed] [Google Scholar]
- 23. Kuklenyik Z, et al. 2009. Effect of mobile phase pH and organic content on LC-MS analysis of nucleoside and nucleotide HIV reverse transcriptase inhibitors. J. Chromatogr. Sci. 47:365–372 [DOI] [PubMed] [Google Scholar]
- 24. Mahalingam A, et al. 2011. Vaginal microbicide gel for delivery of IQP-0528, a pyrimidinedione analog with a dual mechanism of action against HIV-1. Antimicrob. Agents Chemother. 55:1650–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malcolm K, et al. . 2010. Pre-treatment with Depo-Provera modifies the pharmacokinetics of CMPD167 in rhesus macaques following vaginal ring administration, abstr. 37. Abstr. Microbicides 2010, Pittsburgh, PA [Google Scholar]
- 26. Malcolm K, et al. 2003. Influence of silicone elastomer solubility and diffusivity on the in vitro release of drugs from intravaginal rings. J. Control. Release 90:217–225 [DOI] [PubMed] [Google Scholar]
- 27. Miller L, MacFarlane SA, Materi HL. 2005. A scanning electron microscopic study of the contraceptive vaginal ring. Contraception 71:65–67 [DOI] [PubMed] [Google Scholar]
- 28. Mitchell C, et al. 2011. Estimating volume of cervicovaginal secretions in cervicovaginal lavage fluid collected for measurement of genital HIV-1 RNA levels in women. J. Clin. Microbiol. 49:735–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morris GC, Lacey CJ. 2010. Microbicides and HIV prevention: lessons from the past, looking to the future. Curr. Opin. Infect. Dis. 23:57–63 [DOI] [PubMed] [Google Scholar]
- 30. Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Landry ML. (ed). 2007. Manual of clinical microbiology, 9th ed ASM Press, Washington, DC [Google Scholar]
- 31. Nel A, et al. 2009. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women. J. Acquir. Immune Defic. Syndr. 51:416–423 [DOI] [PubMed] [Google Scholar]
- 32. Owen DH, Katz DF. 1999. A vaginal fluid simulant. Contraception 59:91–95 [DOI] [PubMed] [Google Scholar]
- 33. Parikh UM, et al. 2009. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J. Virol. 83:10358–10365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patton DL, Cosgrove Sweeney YT, Paul KJ. 2008. A summary of preclinical topical microbicide vaginal safety and chlamydial efficacy evaluations in a pigtailed macaque model. Sex. Transm. Dis. 35:889–897 [DOI] [PubMed] [Google Scholar]
- 35. Patton DL, Sweeney YC, Rabe LK, Hillier SL. 1996. The vaginal microflora of pig-tailed macaques and the effects of chlorhexidine and benzalkonium on this ecosystem. Sex. Transm. Dis. 23:489–493 [DOI] [PubMed] [Google Scholar]
- 36. Promadej-Lanier N, et al. 2009. Development and evaluation of a vaginal ring device for sustained delivery of HIV microbicides to non-human primates. J. Med. Primatol. 38:263–271 [DOI] [PubMed] [Google Scholar]
- 37. Rabe LK, Hillier SL. 2003. Optimization of media for detection of hydrogen peroxide production by Lactobacillus species. J. Clin. Microbiol. 41:3260–3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Romano J, et al. 2009. Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res. Hum. Retroviruses 25:483–488 [DOI] [PubMed] [Google Scholar]
- 39. Saltzman WM. 2001. Drug delivery: engineering, principles for drug therapy. Oxford University Press, New York, NY [Google Scholar]
- 40. Sivin I, et al. 1997. Contraceptives for lactating women: a comparative trial of a progesterone-releasing vaginal ring and the copper T 380A IUD. Contraception 55:225–232 [DOI] [PubMed] [Google Scholar]
- 41. Srivastava P, et al. 2004. Optimization of unique, uncharged thioesters as inhibitors of HIV replication. Bioorg. Med. Chem. 12:6437–6450 [DOI] [PubMed] [Google Scholar]
- 42. Tang L, Khan SU, Muhammad NA. 2001. Evaluation and selection of bio-relevant dissolution media for a poorly water-soluble new chemical entity. Pharm. Dev. Technol. 6:531–540 [DOI] [PubMed] [Google Scholar]
- 43. Thomas C. 2009. Roadblocks in HIV research: five questions. Nat. Med. 15:855–859 [DOI] [PubMed] [Google Scholar]
- 44. UNAIDS 2010. Report on the global AIDS epidemic. UNAIDS, Geneva, Switzerland [Google Scholar]
- 45. Van Rompay KK. 2010. Evaluation of antiretrovirals in animal models of HIV infection. Antiviral Res. 85:159–175 [DOI] [PubMed] [Google Scholar]
- 46. Veazey RS. 2008. Microbicide safety/efficacy studies in animals: macaques and small animal models. Curr. Opin. HIV AIDS 3:567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Woolfson AD, et al. 2006. Potential use of vaginal rings for prevention of heterosexual transmission of HIV: a controlled-release strategy for HIV microbicides. Am. J. Drug Deliv. 4:7–20 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.