Abstract
Mycobacterial resuscitation-promoting factors (RPFs) have been of great interest since the discovery that they promote the growth of nonculturable Mycobacterium tuberculosis cells. Yet, their precise role in mycobacterial survival and infection has remained elusive. We performed a chemical screen to identify molecules that show preferential killing of a Mycobacterium tuberculosis mutant lacking RPFs over wild-type bacilli and found that the mutant has enhanced sensitivity to the β-lactam class of antibiotics. By monitoring β-lactam diffusion across the mycobacterial outer membrane, we found that the RPFs are required to maintain the outer membrane integrity, as their deletion results in an increase in outer membrane permeability.
TEXT
Mycobacterium tuberculosis, the causative agent of tuberculosis, resists killing by many antibiotics. This resistance arises partly from the unique mycobacterial long-chain mycolic acids that are covalently bound to the cell wall peptidoglycan by intermediate arabinogalactan polymers, encasing the bacterium in a poorly permeable, hydrophobic shell. Bacteria regulate growth and division using many classes of peptidoglycan-modifying enzymes to remodel the cell wall, including, in M. tuberculosis, the five resuscitation-promoting factors (RPFs; rpfA to -E), which are thought to correspond to peptidoglycan lytic transglycosylases in Gram-negative organisms. Like lytic transglycosylases, RPFs are not essential for in vitro growth. Thus, deletion of all five RPF homologues (H37RvΔrpfACDEB, here RPFnull) in M. tuberculosis has no effect on bacterial viability in broth culture, although on solid agar delayed colony formation has been observed (5). In contrast, the RPFs are essential for growth and persistence in a mouse model of M. tuberculosis infection (5). Since cell wall growth and division can apparently proceed in vitro in the absence of RPFs, it is unclear what their essential role is in vivo.
Homology studies, demonstrated enzymatic activity (8), and interactions with other cell wall-modifying enzymes (3) all suggest that RPFs can function to modify peptidoglycan. Because of the covalent interaction between the peptidoglycan and the mycolic acids, alterations to peptidoglycan may affect the outer membrane, the integrity of which contributes to hallmark mycobacterial characteristics, including clumping of cells in culture, impermeability, and broad antibiotic tolerance. RPFs may therefore contribute to these characteristics.
In order to elucidate further roles of the RPFs in M. tuberculosis, we performed a high-throughput chemical screen of 26,000 compounds to identify molecules that preferentially inhibit growth of an M. tuberculosis mutant in which all 5 rpf genes have been deleted (RPFnull) but not wild-type M. tuberculosis (RPFWT). RPFnull, a generous gift of V. Mizrahi (University of the Witwatersrand, Johannesburg, South Africa), was engineered to constitutively express red fluorescent protein and grown to logarithmic phase in Middlebrook 7H9 broth, centrifuged at 58 × g to remove large clumps, diluted to an optical density at 600 nm (OD600) of 0.05 in fresh medium, and dispensed into 384-well plates containing dimethyl sulfoxide (DMSO) stocks of individual small molecules (final concentration, ∼25 μM). The library (collection at the Broad Institute) contained known bioactive compounds, natural products, and synthetic compounds. After incubating for 4 days, outgrowth was determined by measuring fluorescence at an excitation wavelength of 544 nm and a detection cutoff of 580 nm.
We compared the results from this screen to a parallel screen against RPFWT and selected for follow-up the top 210 compounds for which there was a significant difference in Z score between RPFWT and RPFnull. Of these, 147 (70%) had repeatable activity and were carried on for further analysis. Some compounds with significant differences in activity against the two strains may not have been identified because the screening concentration exceeded the inhibitory concentration of both strains. Compounds with general cytotoxicity in an erythrocyte lysis assay were eliminated.
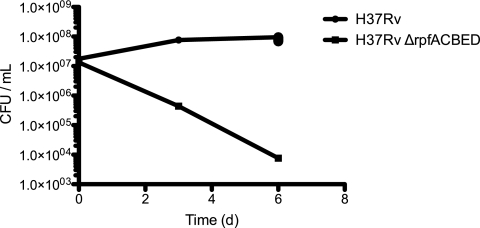
We measured the 90% inhibitory concentration (IC90) of each compound for the RPFnull and RPFWT strains by OD600. We also verified each compound's activity against an alternatively derived RPFnull strain, H37RvΔrpfACDEB, in which all five RPF genes had been genetically knocked out in a different sequence. This ensured that each compound's activity was not associated with any non-RPF-related mutations acquired during the construction of the RPF deletion strains. We focused on 11 compounds (Table 1) with the most dramatic differences in activity against RPFnull compared to the RPFWT. Of the 11 lead compounds, five were β-lactams, with four being cephalosporins (cefotaxime, cefditoren, ceftriaxone, and cefmetazole) and a fifth being a penicillin (dicloxacillin). We found that the IC90 for RPFnull against cefotaxime was ∼8-fold lower than in RPFWT (Table 2), consistent with our screening results. We observed similar sensitizations of the mutant for ceftriaxone and cefamandole. CFU measurement for RPFWT and RPFnull verified that observed effects on OD600 arose from bactericidal activity (Fig. 1).
Table 1.
Additional hits from RPFnull screen
aChemBank is a public cheminformatics resource available at http://chembank.broad.harvard.edu/ (7).
Table 2.
IC90s of β-lactams against H37Rv, RPFnull, and related strains
| β-Lactam | Straina | IC90 (μM)b |
|---|---|---|
| Cefotaxime | H37Rv (RPFWT) | 26 |
| H37RvΔrpfA | 13–26 | |
| H37RvΔrpfB | 13–26 | |
| H37RvΔrpfC | 26 | |
| H37RvΔrpfD | 26 | |
| H37RvΔrpfE | 26–52 | |
| H37RvΔrpfAB | 26 | |
| H37RvΔrpfACBED::pHrpfCDE | 6.5–26 | |
| H37RvΔrpfACBE | 6.5 | |
| H37RvΔrpfACBED (RPFnull) | 1.6–3.3 | |
| H37RvΔrpfACDEBc | 0.78 | |
| Cefamandole | H37Rv (RPFWT) | 390–780 |
| H37RvΔrpfACBED (RPFnull) | 98 | |
| With potassium clavulanate (1 μM) | H37Rv (RPFWT) | 24 |
| H37RvΔrpfACBED (RPFnull) | 1.5 | |
| Ceftriaxone | H37Rv (RPFWT) | 3.2–6.3 |
| H37RvΔrpfACBED (RPFnull) | 1.6 |
From reference 5.
Differences in IC90s are representative of at least two independent experiments containing at least four duplicates each; for cefamandole, amounts of clavulanate were varied across duplicates.
RPFs were deleted from this strain in a different order than that for RPFnull.
Fig 1.
RPFWT and RPFnull were grown in 3 μM cefotaxime, and surviving bacteria were enumerated at the indicated time points by plating for CFU on 7H10 agar. The result shown is representative of the experiment performed in biological triplicate.
To determine which RPFs contributed to RPFnull's increased β-lactam sensitivity, we measured the cefotaxime IC90 against a range of mutants lacking one or more RPFs (Table 2). No individual RPF gene was singlehandedly responsible for RPFWT-level cefotaxime resistance. The biggest increases in sensitivity came with the loss of rpfA and rpfB. In general, with the progressive loss of RPFs, cefotaxime sensitivity gradually increased. The additive effects observed suggest functional redundancy of the RPFs. Restoration of rpfC, rpfD, and rpfE by integration of a plasmid carrying these genes at the mycobacteriophage L5 attB site suppressed cefotaxime sensitivity in RPFnull.
To test whether increased β-lactam sensitivity in RPFnull could be due to loss of β-lactamase activity with sequential deletion of the RPFs, we measured the cefamandole IC90 of RPFWT and RPFnull in the presence of 1 μM clavulanate, a β-lactamase inhibitor. If differences in β-lactamase activity account for the differences in cephalosporin sensitivity in the two strains, its inhibition by clavulanate should cause the cefamandole IC90 values for the RPFWT and RPFnull to converge. In fact, treatment with clavulanate did not cause this convergence; instead, a small divergence was observed (Table 2), thus demonstrating that loss of β-lactamase activity does not account for the increased β-lactam sensitivity in RPFnull.
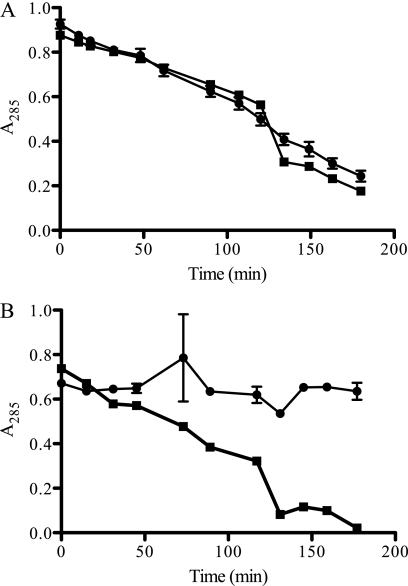
Since RPFnull is also hypersensitive to SDS and macrolide antibiotics relative to RPFWT (5), we hypothesized that it may have a general permeability defect underlying both these phenotypes as well as cephalosporin sensitivity. To test this, using previously reported methods (9), we measured the relative rates of cefamandole hydrolysis by intact RPFWT and RPFnull cells (Fig. 2). When these rates are normalized to their respective rate of hydrolysis in lysates, they provide a comparison of the relative rates of diffusion across the intact outer membrane and of their relative permeabilities (provided that the concentration of cefamandole internal to the permeability barrier is below the order of the β-lactamase Km). In highly permeable cells, intracellular hydrolysis of cefamandole is assumed to be rate determining, while in poorly permeable cells, diffusion through the permeability barrier is assumed to be rate limiting.
Fig 2.
Kinetics of cefamandole hydrolysis. Absorption at 285 nm of H37Rv (●) or H37RvΔrpfACDEB (■). Samples were mechanically lysed (A) or resuspended in phosphate-buffered saline intact (B) and exposed to 20 mM cefamandole nafate. Subsequently, absorption measurements were taken at the indicated times. The illustrated values are averages of two technical replicates for each strain. Parameters vlysed and vintact are the slope and error calculated from the aggregated data for each strain.
We calculated a vintact/vlysed ratio of 1.09 ± 0.04 for RPFnull, compared to an RPFWT vintact/vlysed ratio of 0.07 ± 0.09. We also found that the loss of cefamandole sensitivity observed with the restoration of rpfC, rpfD, and rpfE in RPFnull was reflected in a correspondingly lower vintact/vlysed ratio of 0.19 ± 0.04, indicative of decreased permeability. These results show that RPFnull has increased permeability to cefamandole.
We have found that the deletion of RPFs in the M. tuberculosis H37Rv strain results in increased sensitivity to cephalosporins because of a resulting increased permeability in its outer membrane. While this work provides a clear demonstration of the change in permeability with respect to cephalosporins, it is possible that the reported sensitivity of RPFnull to other small molecules, including SDS (5), the macrolide class of antibiotics, vancomycin, and rifampin (6), may also be due to this same permeability defect. This finding suggests that changes in mycobacterial cell physiology at the peptidoglycan level may have far-reaching effects on the essential mycolic acid permeability barrier and that such alterations may affect the ability of the mutant bacilli to survive in vivo. Specifically, loss of particular cell wall-modifying enzymes may decouple the rates of peptidoglycan and outer membrane synthesis, as suggested in a model by Heidrich et al. (2), thus causing defects in the outer membrane that permit a wide range of molecules to diffuse in and out of the cell.
Our work shows that genetic ablation of RPF activity can potentiate a number of small molecules against mycobacteria, including known antibiotics presently unused in therapy. The use of RPF inhibitors should have similar effects. Recently, Demina et al. (1) have identified several small molecules with promising RPF-inhibitory activity, while more generally bulgecin is a known inhibitor of lytic transglycosylases. β-Lactams are historically one of the most successful antibiotic classes, and the use of the β-lactamase inhibitor clavulanate to potentiate them against mycobacteria has recently been proposed (4). This work provides a mechanistically orthogonal approach to potentiating β-lactam antibiotics and other antibiotics.
ACKNOWLEDGMENTS
We thank the members of the Hung lab for their helpful comments. We thank B. Gordhan, B. Kana, and V. Mizrahi (University of the Witwatersrand, Johannesburg, South Africa) for their generous gift of the strains and plasmid used in this work. We thank the Broad Institute Chemical Biology Platform for their assistance in performing the chemical screen.
Footnotes
Published ahead of print 12 December 2011
REFERENCES
- 1. Demina GR, et al. 2009. Finding of low molecular weight inhibitors of resuscitation promoting factor enzymatic and resuscitation activity. PLoS One 4:e8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heidrich C, Ursinus A, Berger J, Schwarz H, Höltje J. 2002. Effects of multiple deletions of murein hydrolases on viability, septum cleavage, and sensitivity to large toxic molecules in Escherichia coli. J. Bacteriol. 184:6093–6099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hett EC, et al. 2007. A partner for the resuscitation-promoting factors of Mycobacterium tuberculosis. Mol. Microbiol. 66:658–668 [DOI] [PubMed] [Google Scholar]
- 4. Hugonnet J, Tremblay LW, Boshoff HI, Barry CE, Blanchard JS. 2009. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 323:1215–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kana BD, et al. 2008. The resuscitation-promoting factors of Mycobacterium tuberculosis are required for virulence and resuscitation from dormancy but are collectively dispensable for growth in vitro. Mol. Microbiol. 67:672–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kana BD, Mizrahi V, Gordhan BG. 2010. Depletion of resuscitation-promoting factors has limited impact on the drug susceptibility of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 65:1583–1585 [DOI] [PubMed] [Google Scholar]
- 7. Seiler KP, et al. 2008. ChemBank: a small-molecule screening and cheminformatics resource database. Nucleic Acids Res. 36:D351–D359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Telkov MV, et al. 2006. Proteins of the rpf (resuscitation-promoting factor) family are peptidoglycan hydrolyases. Biochemistry (Mosc.) 71:414–422 [DOI] [PubMed] [Google Scholar]
- 9. Zimmermann W, Rosselet A. 1977. Function of the outer membrane of Escherichia coli as a permeability barrier to β-lactam antibiotics. Antimicrob. Agents Chemother. 12:368–372 [DOI] [PMC free article] [PubMed] [Google Scholar]