Abstract
In cystic fibrosis patients, chronic lung infection with Pseudomonas aeruginosa and the associated decline in lung function are the major cause of mortality. In this report, we show that pyocin S2 displays potent activity against P. aeruginosa biofilms, thus representing a potentially improved therapeutic option. Using an invertebrate model of P. aeruginosa infection, we also show that pyocin S2 is highly active in vivo.
TEXT
Due to the increasing prevalence of multidrug-resistant pathogenic bacteria and the poor efficacy of existing treatments against chronic bacterial infection, there is a critical requirement for the development of novel classes of antibiotics (4, 14). This is exemplified in cystic fibrosis (CF) patients, for whom, despite aggressive antibiotic therapy, chronic lung infection with P. aeruginosa and the concomitant intense inflammatory response leads to a progressive loss of lung function and is the major proven cause of mortality among this group of patients (8, 11).
An alternative strategy for antibiotic discovery is to utilize the narrow-spectrum antibiotics used by bacteria for intraspecies competition. In Gram-negative bacteria, these often take the form of high-molecular-weight protein antibiotics known as bacteriocins (6, 7, 13). In this report, we show that pyocin S2 displays potent activity in vitro against clinical isolates of P. aeruginosa growing in the biofilm state. Further to this, pyocin S2 is highly active in an invertebrate model of P. aeruginosa infection.
P. aeruginosa grows predominantly as a biofilm in the CF-infected lung, and this state is associated with high levels of resistance to small-molecule antibiotics (6, 9). To determine if pyocins display potent activity against P. aeruginosa growing in the biofilm state, we first cloned the genes for pyocin S2 (2) and its immunity protein ImS2 into an Escherichia coli expression vector and expressed and purified the protein in complex with its immunity protein.
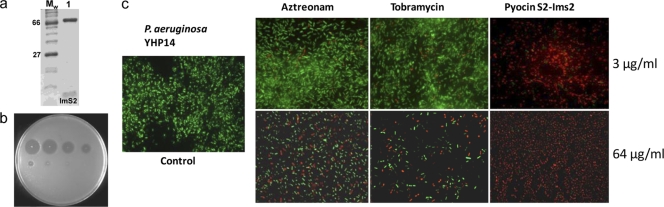
The pyocin S2-ImS2 complex was isolated by nickel affinity chromatography by virtue of an engineered C-terminal His6 tag on the immunity protein (Fig. 1a). The purified pyocin S2-ImS2 complex was highly active against P. aeruginosa growing on LB agar (Fig. 1b). We initially tested the activity of pyocin S2 against biofilms formed by P. aeruginosa isolate YHP14 treated with a single dose of pyocin S2 for 1 h at 3 μg/ml and 64 μg/ml and visualized by fluorescence microscopy using LIVE/DEAD cell viability staining (Fig. 1c). Parallel biofilm killing assays were also performed for tobramycin and aztreonam, approved antibiotics for treatment of chronic CF lung infection by inhalation (12). From the relative number of live (green) and dead (red) cells, it is apparent that pyocins are highly active against P. aeruginosa in the biofilm state, killing a larger proportion of cells than either tobramycin or aztreonam at equivalent concentrations (Fig. 1c).
Fig 1.
Purification of S-type pyocins and killing activity of the pyocin S2-Im2 complex against P. aeruginosa. (a) SDS-PAGE of purified pyocin S2-ImS2 complex (lane 1). (b) Activity of purified pyocin S2-ImS2 against P. aeruginosa. A 5-fold serial dilution (starting concentration, 2 mg/ml [top left]) of pyocin S2-ImS2 was spotted onto a lawn of growing P. aeruginosa strain YHP17 which was grown overnight at 37°C. Clear zones indicate cell death. (c) Activity of pyocin S2-ImS2 against P. aeruginosa biofilms. Biofilms (24 h) of P. aeruginosa YHP14 were grown on poly-l-lysine glass cover slides, treated with a pyocin S2 at 3 μg/ml and 64 μg/ml for 1 h, and visualized by fluorescence microscopy using LIVE/DEAD cell viability staining. Red, dead cells; green, live cells.
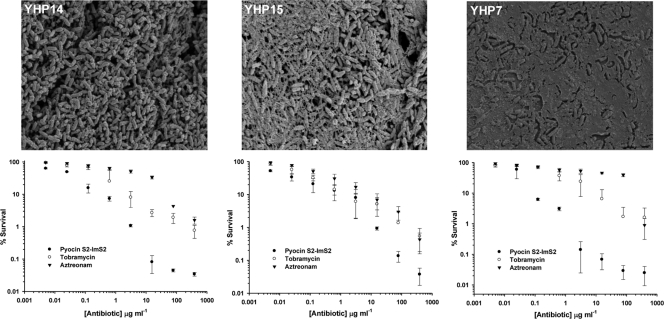
To obtain quantitative data on the ability of pyocins to kill P. aeruginosa biofilms, we treated YHP14 24-h biofilms, grown on the plastic pegs of the MBEC biofilm cultivation system, with pyocin S2, tobramycin, and aztreonam (0.001 to 390 μg/ml) for 1 h (Fig. 2). Cells were removed by sonication, and colonies were counted by plating serial dilutions of recovered cells on solid media. The ability of YHP14 to form structured multilayered biofilms under these conditions was first confirmed by electron microscopy (Fig. 2). In this assay, pyocin S2 was found to kill P. aeruginosa in the biofilm state considerably more effectively than aztreonam or tobramycin, with S2-treated biofilms showing a reduction of close to 4 log units in cell survival at the highest concentration of antibiotic tested.
Fig 2.
Biofilm-associated survival of P. aeruginosa strains YHP14, YHP15, and YHP7 after treatment with pyocin S2, aztreonam, or tobramycin. Upper panels show 24-h biofilms from each strain visualized by scanning electron microscopy. Lower panels show the percent survival after a single treatment with pyocin S2-ImS2, aztreonam, or tobramycin (0.001 μg/ml to 390 μg/ml) for 1 h. Error bars represent the standard deviations between replicate samples (n = 6). All P. aeruginosa clinical isolates were collected from sputum samples from children with cystic fibrosis at the Royal Hospital for Sick Children (Yorkhill, Glasgow, United Kingdom). P. aeruginosa biofilms were formed on an MBEC 96-peg plate platform (Innovotech, Edmonton, Canada), as previously described (1).
Since clinical isolates of P. aeruginosa are phenotypically diverse, in particular in terms of the level of production and incorporation of extracellular polysaccharide into the biofilm matrix, we compared the activity of pyocin S2 against isolates with different levels of mucoidy (Fig. 2). Despite different levels of extracellular polysaccharide production, the level of killing of pyocin S2 against YHP15 and YHP7 biofilms was remarkably similar to that shown against YHP14 and in all cases was greater than the level of killing shown by tobramycin and aztreonam. At an antibiotic concentration of 3 μg/ml, cell survival of pyocin S2-treated YHP7 biofilms was 0.3%, with tobramycin- and aztreonam-treated biofilms showing ≥100-fold increased survival rates of 30% and 60%, respectively.
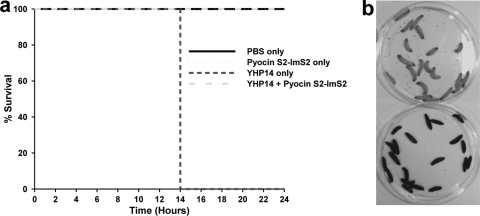
To determine if pyocins are active during infection, we tested the ability of pyocin S2 to provide protection against a lethal P. aeruginosa infection in a whole animal. For this purpose, we chose a nonvertebrate host, the Galleria mellonella caterpillar, in which P. aeruginosa has been shown to be highly virulent and in which pathogenesis is dependent on similar virulence factors that are essential for pathogenesis in mammals (10). Groups of 20 larvae were inoculated with 104 CFU of YHP14 and after 3 h injected with either phosphate-buffered saline (PBS) (control) or pyocin S2 (27 mg/kg). The larvae were monitored every 2 h between 10 and 72 h. Those in the control group (PBS injected after inoculation) died 12 to 14 h postinfection, while those treated with pyocin S2 postinfection survived until the experiment was stopped at 72 h (Fig. 3a). Lethal infection by P. aeruginosa is accompanied by the generation of melanin in larvae, leading to a distinct change in color from cream to dark brown in larvae close to death (note light and dark larvae in Fig. 3b). Larvae in additional control groups that were not infected with P. aeruginosa but were treated with PBS or pyocin also survived until the end of the experiment, indicating that pyocin S2 is not toxic to the host in the absence of infection (Fig. 3a). Colony counts from larvae confirmed that the PBS-only and pyocin-only controls contained no P. aeruginosa. The group that was infected with strain YHP14 contained between 5 × 108 and 1 × 109 CFU at time of death. The infected group treated with pyocin contained between 10 and 40 CFU.
Fig 3.
Ability of pyocin S2-ImS2 to provide protection against a lethal P. aeruginosa infection in the Galleria mellonella caterpillar. (a) Survival plot for groups of larvae infected with P. aeruginosa YHP14 and treated with pyocin S2-ImS2. Groups of 20 larvae were not infected or were inoculated with 104 CFU of P. aeruginosa YHP14 and after 3 h were injected with either PBS (control) or pyocin S2 (27 mg/kg). (b) Lethal infection by P. aeruginosa is accompanied by the generation of melanin in larvae leading to a distinct change in color from cream to dark brown in larvae close to death. Shown are larvae infected with YHP14 and treated with pyocin S2-ImS2 (top) or with PBS (bottom). Infection of Galleria mellonella larvae (Livefood, United Kingdom) was performed as previously described (5).
In this report, we have shown that pyocin S2 has potent activity against P. aeruginosa growing in the biofilm state and is capable of protecting against a lethal P. aeruginosa infection. In infections where the microbiology of infection is closely monitored, species-specific protein antibiotics may provide a useful therapeutic option.
ACKNOWLEDGMENTS
We thank Andrew Roe and Ashleigh Holmes for assistance with confocal microscopy.
This work was supported by funding from Medical Research Scotland and Tenovus Scotland. L.M. is supported by a Wellcome Trust studentship.
Footnotes
Published ahead of print 19 December 2011
REFERENCES
- 1. Ceri H, et al. 2001. The MBEC Assay System: multiple equivalent biofilms for antibiotic and biocide susceptibility testing. Methods Enzymol. 337:377–385 [DOI] [PubMed] [Google Scholar]
- 2. Denayer S, Matthijs S, Cornelis P. 2007. Pyocin S2 (Sa) kills Pseudomonas aeruginosa strains via the FpvA type I ferripyoverdine receptor. J. Bacteriol. 189:7663–7668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reference deleted.
- 4. Flamm RK, et al. 2004. Factors associated with relative rates of antibiotic resistance in Pseudomonas aeruginosa isolates tested in clinical laboratories in the United States from 1999 to 2002. Antimicrob. Agents Chemother. 48:2431–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harrison F, Browning LE, Vos M, Buckling A. 2006. Cooperation and virulence in acute Pseudomonas aeruginosa infections. BMC Biol. 4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kleanthous C. 2010. Swimming against the tide: progress and challenges in our understanding of colicin translocation. Nat. Rev. Microbiol. 8:843–848 [DOI] [PubMed] [Google Scholar]
- 7. Loftus SR, et al. 2006. Competitive recruitment of the periplasmic translocation portal TolB by a natively disordered domain of colicin E9. Proc. Natl. Acad. Sci. U. S. A. 103:12353–12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lyczak JB, Cannon CL, Pier GB. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mah TF, et al. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306–310 [DOI] [PubMed] [Google Scholar]
- 10. Miyata S, Casey M, Frank DW, Ausubel FM, Drenkard E. 2003. Use of the Galleria mellonella caterpillar as a model host to study the role of the type III secretion system in Pseudomonas aeruginosa pathogenesis. Infect. Immun. 71:2404–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murray TS, Egan M, Kazmierczak BI. 2007. Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr. Opin. Pediatr. 19:83–88 [DOI] [PubMed] [Google Scholar]
- 12. O'Sullivan BP, Yasothan U, Kirkpatrick P. 2010. Inhaled aztreonam. Nat. Rev. Drug Discov. 9:357–358 [DOI] [PubMed] [Google Scholar]
- 13. Parret AH, De Mot R. 2002. Bacteria killing their own kind: novel bacteriocins of Pseudomonas and other γ-proteobacteria. Trends Microbiol. 10:107–112 [DOI] [PubMed] [Google Scholar]
- 14. Souli M, Galani I, Giamarellou H. 2008. Emergence of extensively drug-resistant and pandrug-resistant Gram-negative bacilli in Europe. Euro Surveill. 13(47):pii=19045 [PubMed] [Google Scholar]