Abstract
Quinolones are increasingly favored over trimethoprim-sulfamethoxazole (TMP-SMX) for empirical treatment of uncomplicated urinary tract infection (UTI). This is associated with increasing resistance toward this broad-spectrum group of antibiotics. Our objective is to describe the prescribing patterns and identify determinants of the choice between TMP-SMX and quinolones for outpatient UTI treatment in Switzerland. An ongoing national Sentinel surveillance system was used to study 11,799 antibiotic prescriptions for UTI in adult outpatients and associated physician and patient factors between 2006 and 2008, to compare the prescription of quinolones versus that of TMP-SMX for treatment of UTI. Most UTI episodes were diagnosed as cystitis (90%). TMP-SMX was prescribed for one-fifth (22%) of UTIs. Independent predictors for prescribing quinolones were pyelonephritis and physicians with low thresholds for prescribing antibiotics for upper respiratory tract infections (“high prescribers”), whereas female patients were more likely to receive TMP-SMX. High-prescribing physicians also more often cared for patients who themselves favor antibiotic treatment (P < 0.001). Quinolones are commonly prescribed to outpatients with UTI. Nonclinical factors influence the choice of quinolones versus TMP-SMX, which may provide opportunities for interventions to improve prescribing patterns and control quinolone resistance.
INTRODUCTION
Urinary tract infection (UTI) is the most frequent bacterial infection encountered in adult primary care. As antibiotics are considered the standard treatment, UTIs account for about 15% of outpatient antibiotic prescriptions. Currently, trimethoprim-sulfamethoxazole (TMP-SMX) or a quinolone is the most commonly used drug for the treatment of UTIs (42). Increasing resistance trends among uropathogens, primarily Escherichia coli, complicate the choice of antibiotics in the outpatient management of UTI (16).
The Infectious Disease Society of America (IDSA) and European guidelines recommend TMP-SMX for 3 days as a standard primary therapy for uncomplicated cystitis, if the local prevalence of TMP-SMX resistance rate does not exceed 10 to 20% (36, 44). Some authorities even suggested continuing the use of TMP-SMX even if resistance rates are 30% or higher, in light of the benign, self-limited course of uncomplicated UTI and given the threat of emerging quinolone resistance (12, 16, 20, 28, 34, 35, 38). However, prescribing practices suggest that current guidelines are often not followed and that use of quinolones in UTI is in fact increasing (3, 11, 22, 23, 33, 42). Given the documented correlation between antibiotic use and resistance rate, this is likely to increase the problem of quinolone-resistant pathogens (9).
Antibiotic prescribing practices for respiratory tract infections have been carefully studied, but there is little corresponding literature for urinary tract infections (5, 10, 21, 24, 32, 40). Knowledge of prescribing patterns is essential for the development of interventions aimed at changing antibiotic prescribing behavior. Switzerland is among the European countries with relatively low outpatient antibiotic consumption rates (6). Nevertheless, use of quinolones is high, even though TMP-SMX resistance is below 30% (per the Swiss database on antibiotic resistance, http://www.search.ifik.unibe.ch). In the current study, we analyzed prescribing patterns for UTI in Switzerland and identified physician and patient characteristics that influenced the choice between TMP-SMX and quinolones in order to provide a basis upon which to develop specific interventions to reduce excessive quinolone usage.
MATERIALS AND METHODS
Swiss Sentinel Surveillance Network.
We used data on prescriptions of antibiotics collected within the Swiss Sentinel Surveillance Network between January 2006 and December 2008 (http://www.sentinella.ch).
The Swiss Sentinel Surveillance Network was established in 1986 and consists of a voluntary network of selected general practitioners. In the selection process the distributions of all physicians by their specialty, of the political states (cantons), and of rural and urban regions are considered. Participants are recruited in annual advertisement campaigns or are directly addressed by regional representatives. The participation in the Sentinel Network is on a voluntary basis. In 2008, the number of physicians participating was 195 (109 general practitioners, 57 internists, and 29 pediatricians) covering 3.1% of all Swiss practitioners in primary care.
Since 1 January 2006, Sentinel physicians have been asked to report data on all prescribed antibiotics, which include all oral and injected antibiotics, on a weekly basis to the Swiss Federal Office of Health (SFOH). Topical application of antibiotics is not reported in the weekly form. Additionally, information on patient demographics, indication for antibiotic treatment, and antibiotic class prescribed is recorded for each visit.
Sentinel physicians also report on other topics, such as activity of influenza-like illness (http://www.sentinella.ch).
Study population.
From a total of 87,629 antibiotic prescriptions during the study period, 15,013 prescriptions were for UTI. Excluded from the analysis were 26 physicians (829 prescriptions) in 2006, 12 physicians (398 prescriptions) in 2007, and 18 physicians (656 prescriptions) in 2008 who did not report regularly (reports for <39 weeks in a year) and 298 additional prescriptions, for which information about indication for antibiotic treatment, antibiotic class, sex, year of birth, or patient preference regarding antibiotic prescription was lacking. Subsequently, patients who were aged <17 years (n = 998) or who were treated by a pediatrician (n = 72) were excluded. Of the remaining 13,943 visits, we excluded 2,144 visits in which an antibiotic class other than TMP-SMX or quinolone was prescribed. This left a total of 11,799 prescriptions for analysis.
In the surveys, the attitude of the patient regarding antibiotic prescription was classified into the following three categories by the treating physician, i.e., the physician's perception of a patient's attitude: (i) patients favoring antibiotic treatment, (ii) patients adopting a neutral position regarding antibiotics, and (iii) patients being reluctant to undergo antibiotic therapy. For analysis we grouped “neutral” and “reluctant” attitudes into patients “not favoring” antibiotic treatment, since the “reluctant” group was a small proportion of the total (2%) and did not show significant differences in univariate analysis (data not shown).
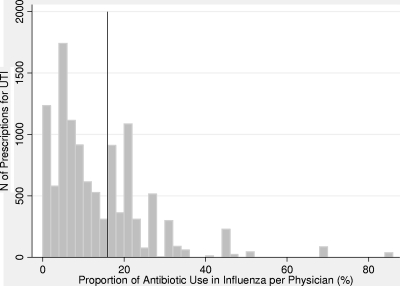
A physician's prescribing practice of antibiotics was classified based on data collected for activity of influenza-like illnesses between 2006 and 2008. For each physician, the proportion of influenza-like illness that resulted in antibiotic prescription was calculated. Of a total of 5,250 reported influenza-like illness visits, 606 (12%) patients were prescribed an antibiotic. According to the proportion of antibiotic prescriptions for presumed influenza physicians were classified into two categories. A rate of ≤16% was defined as “low prescriber”; a rate of >16% was considered “high prescriber.” The cutoff was chosen according to the bimodal distribution of the prescriptions for UTI showing two peaks (Fig. 1). Physicians were not classified for their prescribing practice if they did not provide data on the use of antibiotics in influenza (n = 93 prescriptions) or if they did not see more than 20 influenza patients between 2006 and 2008 (n = 528 prescriptions).
Fig 1.
Frequency (%) of antibiotic treatment in outpatients presenting with influenza-like illness by physician. Data on influenza-like illness visits are from the Sentinel Surveillance Network System between 2006 and 2008. Prescriptions of 150 physicians described by their prescribing rate in influenza are displayed. The cutoff point for classification of physicians' prescribing rates is indicated by the vertical line. A rate of ≤16% stands for a low prescriber, and a rate of >16% indicates a high prescriber.
Two geographic regions were defined according to language borders: the Latin part, comprising the French- and the Italian-speaking parts (the latter accounted for only 451 prescriptions), and the German part, which represents the German-speaking area. It is well recognized that there are sociocultural, socioeconomic, and political differences between the language regions. Repeated visits by the same patient could not be accounted for because reports of patients were anonymous.
Statistical analyses.
All analyses were performed using STATA statistical software, version 10.0 (StatCorp, Texas). A P value of less than 0.05 (two-tailed) was considered statistically significant. A chi-square test was used to compare proportions. Predictors of antibiotic treatment with quinolone were evaluated by univariate analysis. Factors significantly contributing to the choice of quinolone were identified and incorporated into a multivariate logistic regression model. In the analyses we used logistic regression models based on robust standard errors that allowed the correlation of multiple episodes within a practice cluster. We checked for potential interactions, none of which added significantly to the model.
RESULTS
Prescription of antibiotics.
A total of 11,799 antibiotic prescriptions for urinary tract infection in adult outpatients between January 2006 and December 2008 were analyzed. Female patients predominated (82%). The average age was 57.3 (standard deviation [SD], 21.7) years, and patients aged 65 and older accounted for 43% of the prescriptions. TMP-SMX was prescribed to 2,537 patients (22%), whereas a quinolone was chosen in 78% of the cases. The frequency of TMP-SMX prescription did not change over time (odds ratio [OR], 1.14; 95% confidence interval [CI], 0.95 to 1.37 for 2007; OR, 0.91; 95% CI, 0.73 to 1.12 for 2008). Cystitis was the indication for an antibiotic in 10,674 prescriptions (90%). The German region accounted for 9,411 prescriptions (80%).
Attitude of patients.
In the majority of cases, patients adopted a neutral position regarding antibiotic treatment (86%). Patients in favor of antibiotic treatment (87%) were more often women compared to neutral patients (81%) (P < 0.001); 61% of “favoring” patients were younger than 65 years in contrast to 56% in nondemanding patients (P < 0.001).
Characteristics of physicians.
Physicians were predominantly general practitioners (70%). The proportions of influenza-like illness visits that resulted in antibiotic prescriptions by physician are shown in Fig. 1. Low prescribers accounted for 63% of all prescriptions, and 37% were prescribed by high prescribers.
Attitudes of physicians and their patients.
The physician's prescribing practice was significantly associated with a patient's attitude favoring antibiotic treatment. The percentage of patients favoring antibiotic treatment increased with increasing prescribing rate of the treating physician (Table 1).
Table 1.
Association between attitude of patient and physician's prescribing practice (n = 11,178)
| Physician's prescribing practicea | Attitude of patientb |
|||
|---|---|---|---|---|
| Neutral |
Favoring |
|||
| n | % | n | % | |
| Low | 6,113 | 63.77 | 922 | 57.87 |
| High | 3,472 | 36.33 | 671 | 42.13 |
Categories are based on frequency of antibiotic treatment by physician among patients presenting with influenza-like illness.
Attitude of patient regarding antibiotic prescription. Pearson chi-square = 20.37; P < 0.001.
Factors favoring quinolones.
Several factors were associated with the choice of quinolones rather than TMP-SMX (Table 2). According to unadjusted analysis, quinolones were more often prescribed to patients diagnosed with pyelonephritis (OR, 3.51; 95% CI, 2.67 to 4.61). Physicians with high prescribing rates were more likely to choose a quinolone (OR, 1.74; 95% CI, 1.10 to 2.74). In contrast, quinolones were less often prescribed to female patients (OR, 0.69; 95% CI, 0.54 to 0.89). In the multivariable model (Table 2) independent predictors of quinolone choice included indication for antibiotic treatment and physician's prescribing behavior. Female gender was predictive for the use of TMP-SMX.
Table 2.
Predictors of quinolone versus trimethoprim-sulfamethoxazole (TMP-SMX) choice among patients prescribed an antibiotic for urinary tract infectiona
| Characteristic | Result (no. [%]) for: |
OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|---|
| TMP-SMX (n = 2,537) | Quinolone (n = 9,262) | |||
| Indication | ||||
| Cystitis | 2,449 (96.53) | 8,225 (88.80) | Referent | Referent |
| Pyelonephritis | 88 (3.47) | 1,037 (11.20) | 3.51 (2.67–4.61) | 3.71 (2.76–4.98) |
| Age, yr | ||||
| ≥17 to <65 | 1,452 (57.23) | 5,228 (56.45) | Referent | |
| ≥65 | 1,085 (42.77) | 4,034 (43.55) | 1.03 (0.84–1.28) | |
| Sex | ||||
| Male | 366 (14.43) | 1,810 (19.54) | Referent | Referent |
| Female | 2,171 (85.57) | 7,452 (80.46) | 0.69 (0.54–0.89) | 0.71 (0.55–0.90) |
| Region | ||||
| German | 2,142 (84.43) | 7,269 (78.48) | Referent | |
| Latin | 395 (15.57) | 1,993 (21.52) | 1.49 (0.93–2.37) | |
| Attitude of patient | ||||
| Neutral | 2,104 (82.93) | 8,053 (86.95) | Referent | |
| Favoring | 433 (17.07) | 1,209 (13.05) | 0.73 (0.46–1.16) | |
| Yr | ||||
| 2006 | 797 (31.42) | 2,888 (31.18) | Referent | |
| 2007 | 780 (30.74) | 3,221(34.78) | 1.14 (0.95–1.37) | |
| 2008 | 960 (37.84) | 3,153 (34.04) | 0.91 (0.73–1.12) | |
| Physician specialty | ||||
| General practitioner | 1,937 (76.35) | 6,414 (69.25) | Referent | |
| Internal medicine | 600 (23.65) | 2,848 (30.75) | 1.43 (0.88–2.32) | |
| Prescribing rateb | ||||
| Low | 1,763 (69.49) | 5,272 (56.92) | Referent | Referent |
| High | 669 (26.37) | 3,474 (37.51) | 1.74 (1.10–2.74) | 1.75 (1.10–2.77) |
Abbreviations: CI, confidence interval; OR, odds ratio; TMP-SMX, trimethoprim-sulfamethoxazole.
Total number of prescriptions analyzed for prescribing rate is 11,178. Categories are based on frequency of antibiotic treatment by physician among patients presenting with influenza-like illness.
Factors without effect on antibiotic choice.
There was no substantial difference in antibiotic prescription between age groups (OR, 1.03; 95% CI, 0.84 to 1.28) or between physician specialties (OR, 1.43; 95% CI, 0.88 to 2.32). The German and Latin regions did not show variance in antibiotic prescribing patterns (OR, 1.49; 95% CI, 0.93 to 2.37).
DISCUSSION
Our study has two main findings. First, physicians favored quinolones in 78.5% over TMP-SMX as antibiotic therapy for UTI. Second, we found that physician and patient determinants significantly contributed to the choice between TMP-SMX and quinolones. According to recommendations, quinolones were more often chosen in patients diagnosed with pyelonephritis than in those diagnosed with cystitis (41, 44).
We used a physician's antibiotic prescribing habits for influenza-like illness as a surrogate for a liberal attitude regarding the use of antibiotics. Interestingly, high prescribers also more often chose quinolone as the primary therapy in UTI. Thus, both for influenza-like illness and for UTI, high-prescribing physicians do not closely follow recommendations. Although the validity of the classification of different prescribing levels has not been independently evaluated, a Scandinavian study found that being a high prescriber was associated with issuing a broad-spectrum antibiotic prescription for respiratory infection (7).
Various factors may influence a physician's antibiotic prescribing practices. Among them are patient pressure and practice location (43). A financial incentive could also explain why a physician would possibly prescribe more antibiotics. Physicians' self-dispensing was shown to be associated with higher levels of antibiotic prescription rates for respiratory infection (17).
Our study also found that female patients were more likely to be treated with TMP-SMX. This preference of TMP-SMX probably reflects the higher frequency of acute uncomplicated cystitis in younger women (16). The primary choice of TMP-SMX is in accordance with guideline recommendations for uncomplicated cystitis (16, 44). The difference between men and women may reflect the trend toward more complicated UTIs among men, including UTIs associated with prostatitis, which would favor the use of a quinolone in these cases. Old age, however, did not influence the choice of the antibiotics in women or men, despite the frequency of comorbidities in elderly patients (13, 39).
Our study showed no difference between the Latin and German regions regarding prescribing patterns. Numerous studies have documented that the geographic setting where a physician practices can influence the choice of an antibiotic (5, 14, 21, 40). Filippini et al. (6) demonstrated that Southwestern Switzerland, corresponding to the Latin part of Switzerland, had the highest outpatient consumption of antibiotics within Switzerland. But, when analyzing different antibiotic classes, they did not find a significant geographic pattern for quinolones, which agrees with our observation. The physician's specialty was also not found to be predictive for the use of different antibiotic classes in the treatment of UTI. In contrast, in the treatment of respiratory infections, physician specialty was described as a predictor for different prescribing habits (24, 40).
There was also no association between the attitude of patients regarding antibiotic prescription and the choice of one antibiotic over the other. However, patients favoring antibiotics were more frequently seen by physicians with high prescribing practice. A number of studies have demonstrated that a physician's perception of patient expectations positively correlated with prescription rates (2, 4, 30). Furthermore, the physician's perception of the patient's expectation more strongly predicted antibiotic prescribing than the patient's actual preferences, suggesting that physicians tend to overestimate patients' expectations (26).
Patients in favor of antibiotic treatment were mainly females aged between 17 and 65 years. Young women may feel a pressure to get well as soon as possible. On the other hand, young women may seek medical attention with a greater level of suffering after unsuccessful attempts at self-managing their infection (29). An attitude in favor of antibiotics has also been associated with decreased knowledge about antibiotics (25).
Our study has a number of limitations. Selection of physicians for the Sentinel Surveillance Network ensures representativeness for geographic region and urban area as well as physician specialty, but participation of physicians is voluntary. A comparison between Sentinel physicians and all physicians practicing in Switzerland did not show significant differences. Nevertheless, we cannot exclude that antibiotic prescribing patterns differ between Sentinel and non-Sentinel physicians. The fact that the study considered only regularly reporting physicians may have affected the results, but the number of excluded physicians was small and most likely had no significant effect on the results. As all the data were gathered from the Sentinel physicians, we also studied the physician's perception of a patient's attitude favoring antibiotics. A more direct method would be to ask the patients themselves with a patient survey.
There were no data available on comorbidity and whether the patient presented with a complicated or uncomplicated cystitis. Age as a surrogate for complexity of infection was not associated with prescribing behavior. The survey also did not provide data on whether treatment was empirical or based on urine culture. Finally, we analyzed antibiotic prescriptions being aware that there may be a discrepancy in prescribed medication and consumed medication (15).
Because we analyzed a large data set, small differences reached statistical significance. While one can question the clinical relevance of these differences, even small differences translate into a considerable consumption of antibiotics and should be considered relevant.
In conclusion, quinolones accounted for the major part of antibiotic prescriptions in outpatient UTI. We identified several physician and patient determinants contributing to the choice between TMP-SMX and quinolones. The preference of quinolones despite a guideline recommending TMP-SMX implies the need of interventions in order to improve quality of care. Several studies indicate that antibiotic prescribing behavior can be influenced by the implementation of such interventions (1, 8, 18, 19, 27, 31, 37).
ACKNOWLEDGMENTS
We acknowledge the support of the Swiss Sentinel Work Group.
The Sentinel Surveillance Network is supported by the Swiss Federal Office of Health.
The authors declare no conflict of interest.
Footnotes
Published ahead of print 9 January 2012
REFERENCES
- 1. Arnold SR, Straus SE. 2005. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst. Rev. 4:CD003539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Britten N, Ukoumunne O. 1997. The influence of patients' hopes of receiving a prescription on doctors' perceptions and the decision to prescribe: a questionnaire survey. BMJ 315:1506–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown PD, Freeman A, Foxman B. 2002. Prevalence and predictors of trimethoprim-sulfamethoxazole resistance among uropathogenic Escherichia coli isolates in Michigan. Clin. Infect. Dis. 34:1061–1066 [DOI] [PubMed] [Google Scholar]
- 4. Cockburn J, Pit S. 1997. Prescribing behaviour in clinical practice: patients' expectations and doctors' perceptions of patients' expectations—a questionnaire study. BMJ 315:520–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coco AS, Horst MA, Gambler AS. 2009. Trends in broad-spectrum antibiotic prescribing for children with acute otitis media in the United States, 1998-2004. BMC Pediatr. 9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Filippini M, Masiero G, Moschetti K. 2006. Socioeconomic determinants of regional differences in outpatient antibiotic consumption: evidence from Switzerland. Health Policy 78:77–92 [DOI] [PubMed] [Google Scholar]
- 7. Gjelstad S, Dalen I, Lindbaek M. 2009. GPs' antibiotic prescription patterns for respiratory tract infections—still room for improvement. Scand. J. Prim. Health Care 27:208–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gjelstad S, et al. 2006. Can. antibiotic prescriptions in respiratory tract infections be improved? A cluster-randomized educational intervention in general practice—the Prescription Peer Academic Detailing (Rx-PAD) Study [NCT00272155]. BMC Health Serv. Res. 6:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goossens H, Ferech M, Vander Stichele R, Elseviers M, Project Group ESAC 2005. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 365:579–587 [DOI] [PubMed] [Google Scholar]
- 10. Grijalva CG, Nuorti JP, Griffin MR. 2009. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA 302:758–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guneysel O, Onur O, Erdede M, Denizbasi A. 2009. Trimethoprim/sulfamethoxazole resistance in urinary tract infections. J. Emerg. Med. 36:338–341 [DOI] [PubMed] [Google Scholar]
- 12. Gupta K, Hooton TM, Stamm WE. 2001. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann. Intern. Med. 135:41–50 [DOI] [PubMed] [Google Scholar]
- 13. Gupta K, Sahm DF, Mayfield D, Stamm WE. 2001. Antimicrobial resistance among uropathogens that cause community-acquired urinary tract infections in women: a nationwide analysis. Clin. Infect. Dis. 33:89–94 [DOI] [PubMed] [Google Scholar]
- 14. Harbarth S, Albrich W, Brun-Buisson C. 2002. Outpatient antibiotic use and prevalence of antibiotic-resistant pneumococci in France and Germany: a sociocultural perspective. Emerg. Infect. Dis. 8:1460–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hersberger KE, et al. 2009. Prescribed medications and pharmacy interventions for acute respiratory tract infections in Swiss primary care. J. Clin. Pharm. Ther. 34:387–395 [DOI] [PubMed] [Google Scholar]
- 16. Hooton TM, Besser R, Foxman B, Fritsche TR, Nicolle LE. 2004. Acute uncomplicated cystitis in an era of increasing antibiotic resistance: a proposed approach to empirical therapy. Clin. Infect. Dis. 39:75–80 [DOI] [PubMed] [Google Scholar]
- 17. Huang N, Chou YJ, Chang HJ, Ho M, Morlock L. 2005. Antibiotic prescribing by ambulatory care physicians for adults with nasopharyngitis, URIs, and acute bronchitis in Taiwan: a multi-level modeling approach. Fam. Pract. 22:160–167 [DOI] [PubMed] [Google Scholar]
- 18. Huttner B, Goossens H, Verheij T, Harbarth S, on behalf of the CHAMP consortium 2010. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect. Dis. 10:17–31 [DOI] [PubMed] [Google Scholar]
- 19. Hux JE, Melady MP, DeBoer D. 1999. Confidential prescriber feedback and education to improve antibiotic use in primary care: a controlled trial. CMAJ 161:388–392 [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson L, et al. 2008. Emergence of fluoroquinolone resistance in outpatient urinary Escherichia coli isolates. Am. J. Med. 121:876–884 [DOI] [PubMed] [Google Scholar]
- 21. Kahan E, Kahan NR, Chinitz DP. 2003. Urinary tract infection in women—physician's preferences for treatment and adherence to guidelines: a national drug utilization study in a managed care setting. Eur. J. Clin. Pharmacol. 59:663–668 [DOI] [PubMed] [Google Scholar]
- 22. Kallen AJ, Welch HG, Sirovich BE. 2006. Current antibiotic therapy for isolated urinary tract infections in women. Arch. Intern. Med. 166:635–639 [DOI] [PubMed] [Google Scholar]
- 23. Katsarolis I, et al. 2010. Acute uncomplicated cystitis: from surveillance data to a rationale for empirical treatment. Int. J. Antimicrob. Agents. 35:62–67 [DOI] [PubMed] [Google Scholar]
- 24. Kozyrskyj AL, et al. 2004. Evidence-based prescribing of antibiotics for children: role of socioeconomics status and physician characteristics. CMAJ 171:139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuzujanakis M, Kleinman K, Rifas-Shiman S, Finkelstein JA. 2003. Correlates of parental antibiotic knowledge, demand, and reported use. Ambul. Pediatr. 3:203–210 [DOI] [PubMed] [Google Scholar]
- 26. Lado E, Vacariza M, Fernández-González C, Gestal-Otero JJ, Figueiras A. 2008. Influence exerted on drug prescribing by patients' attitudes and expectations and by doctors' perception of such expectations: a cohort and nested case-control study. J. Eval. Clin. Pract. 14:453–459 [DOI] [PubMed] [Google Scholar]
- 27. Lagerløv P, Loeb M, Andrew M, Hjortdahl P. 2000. Improving doctors' prescribing behaviour through reflection on guidelines and prescription feedback: a randomised controlled study. Qual. Health Care 9:159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Le TP, Miller LG. 2001. Empirical therapy for uncomplicated urinary tract infections in an era of increasing antimicrobial resistance: a decision and cost analysis. Clin. Infect. Dis. 33:615–621 [DOI] [PubMed] [Google Scholar]
- 29. Leydon GM, Turner S, Smith H, Little P, UTIS team 2009. The journey from self-care to GP care: a qualitative interview study of women presenting with symptoms of urinary tract infection. Br. J. Gen. Pract. 59:e219–e225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Little P, et al. 2004. Importance of patient pressure and perceived pressure and perceived medical need for investigations, referral, and prescribing in primary care: nested observational study. BMJ 328:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lundborg CS, Wahlström R, Oke T, Tomson G, Diwan VK. 1999. Influencing prescribing for urinary tract infection and asthma in primary care in Sweden: a randomized controlled trial of an interactive educational intervention. J. Clin. Epidemiol. 52:801–812 [DOI] [PubMed] [Google Scholar]
- 32. McCaig LF, Besser RE, Hughes JM. 2002. Trends in antimicrobial prescribing rates for children and adolescents. JAMA 287:3096–3102 [DOI] [PubMed] [Google Scholar]
- 33. McEwen LN, Farjo R, Foxman B. 2003. Antibiotic prescribing for cystitis: how well does it match published guidelines? Ann. Epidemiol. 13:479–483 [DOI] [PubMed] [Google Scholar]
- 34. McNulty CA, et al. 2006. Clinical relevance of laboratory-reported antibiotic resistance in acute uncomplicated urinary tract infection in primary care. J. Antimicrob. Chemother. 58:1000–1008 [DOI] [PubMed] [Google Scholar]
- 35. Metz-Gercek S, et al. 2009. Ten years of antibiotic consumption in ambulatory care: trends in prescribing practice and antibiotic resistance in Austria. BMC Infect. Dis. 9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Naber KG, et al. 2001. EAU guidelines for the management of urinary and male genital tract infections. Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU). Eur. Urol. 40:576–588 [DOI] [PubMed] [Google Scholar]
- 37. Samore MH, et al. 2005. Clinical decision support and appropriateness of antimicrobial prescribing: a randomized trial. JAMA 294:2305–2314 [DOI] [PubMed] [Google Scholar]
- 38. Schito GC, et al. 2009. The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int. J. Antimicrob. Agents. 34:407–413 [DOI] [PubMed] [Google Scholar]
- 39. Shortliffe LM, McCue JD. 2002. Urinary tract infection at the age extremes: pediatrics and geriatrics. Am. J. Med. 113:55S–66S [DOI] [PubMed] [Google Scholar]
- 40. Steinman MA, Landefeld CS, Gonzales R. 2003. Predictors of broad-spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA 289:719–725 [DOI] [PubMed] [Google Scholar]
- 41. Talan DA, et al. 2008. Prevalence and risk factor analysis of trimethoprim-sulfamethoxazole- and fluoroquinolone-resistant Escherichia coli infection among emergency department patients with pyelonephritis. Clin. Infect. Dis. 47:1150–1158 [DOI] [PubMed] [Google Scholar]
- 42. Taur Y, Smith MA. 2007. Adherence to the Infectious Diseases Society of America guidelines in the treatment of uncomplicated urinary tract infection. Clin. Infect. Dis. 44:769–774 [DOI] [PubMed] [Google Scholar]
- 43. Wang KY, Seed P, Schofield P, Ibrahim S, Ashworth M. 2009. Which practices are high antibiotic prescribers? A cross-sectional analysis. Br. J. Gen. Pract. 59:e315–e320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Warren JW, et al. 1999. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clin. Infect. Dis. 29:745–758 [DOI] [PubMed] [Google Scholar]