Abstract
BMS-790052, a first-in-class hepatitis C virus (HCV) replication complex inhibitor, targeting nonstructural protein 5A (NS5A), displays picomolar to nanomolar potency against genotypes 1 to 5. This exceptional potency translated into robust anti-HCV activity in clinical studies with HCV genotype 1-infected subjects. To date, all BMS-790052-associated resistance mutations have mapped to the N-terminal region of NS5A. To further characterize the antiviral activity of BMS-790052, HCV replicon elimination and colony formation assays were performed. Replicon was cleared from genotype 1a and 1b replicon cells in a time- and dose-dependent manner. Elimination of the genotype 1a replicon required longer treatment durations and higher concentrations of BMS-790052 than those for the genotype1b replicon. Single amino acid substitutions that conferred relatively low levels of resistance were observed at early time points and at low doses. Higher doses and longer treatment durations yielded mutations that conferred greater levels of resistance, including linked amino acid substitutions. Replicon cells that survived inhibitor treatment remained fully sensitivity to pegylated alpha interferon (pegIFN-α) and other HCV inhibitors. Moreover, genotype 1a replicon elimination was markedly enhanced when pegIFN-α and BMS-790052 were combined. Resistant variants observed in this study were very similar to those observed in a multiple ascending dose (MAD) monotherapy trial of BMS-790052, validating replicon elimination studies as a model to predict clinical resistance. Insights gained from the in vitro anti-HCV activity and resistance profiles of BMS-790052 will be used to help guide the clinical development of this novel HCV inhibitor.
INTRODUCTION
Hepatitis C virus (HCV), a member of the Flaviviridae family of RNA viruses, is a major cause of liver disease worldwide (1). The ∼9.6-kb HCV genome encodes a polyprotein that is processed into structural proteins (core, E1, and E2), a small ion channel protein (p7), and nonstructural proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B) required for polyprotein processing and RNA replication (2). Until very recently, standard-of-care therapy for HCV-infected individuals consisted of a combination of pegylated interferon (pegIFN) and ribavirin (RBV) (18). Because of complications with side effects and incomplete antiviral efficacy, only ∼50% of individuals infected with HCV genotype 1 achieved a sustained viral response upon treatment (18). Today, an increasing number of small-molecule inhibitors targeting specific viral proteins are in various stages of development, and two drugs that target the HCV NS3 protease, telaprevir and boceprevir, have been approved for clinical use for HCV genotype 1-infected patient treatment in combination with pegIFN and RBV. Collectively referred to as directly acting antiviral agents (DAA), these virus-specific inhibitors hold the promise of improving or even replacing IFN-based HCV therapy (9). Many of the DAA in development are directed against the viral enzymatic activities of NS3 (serine protease) and NS5B (RNA-dependent RNA polymerase). In contrast, BMS-790052 targets the nonenzymatic NS5A protein. With 50% effective concentrations (EC50s) in the 5 to 50 pM range against genotype 1 replicons, BMS-790052 is the most potent HCV replication inhibitor reported to date. In early clinical trials, subjects receiving BMS-790052 generally exhibited sharp declines in HCV RNA levels (10, 19). However, viral breakthrough and relapse associated with mutations in the N-terminal region of NS5A was also observed (8, 19).
High viral RNA loads, rapid turnover, and an error-prone replicase combine to produce a heterogeneous population of HCV quasispecies in infected individuals (6, 22). This genetic diversity potentially represents a significant challenge to DAA-based HCV therapies. In fact, Guedj et al. (12) have predicted that all possible viable single and double mutants that might confer drug resistance will likely preexist within a given HCV-infected patient. A thorough understanding of the potential for resistance development for different classes of DAA is therefore essential. Previous studies have mapped resistance to BMS-790052 to several residues within the N-terminal region of NS5A, most notably L31 and Y93 in genotype 1b and M28, Q30, L31, and Y93 in genotype 1a (7, 10).
The HCV replicon system provides a convenient and widely accepted means of evaluating DAA activity in tissue culture. Bicistronic HCV replicons with a Neor selectable marker in the first cistron and the NS3-NS5B nonstructural HCV genes in the second cistron allow selection of clonal cell lines that constitutively support HCV RNA replication (3, 16). The ability of specific antivirals to eliminate or “cure” replicon RNA from established replicon cell lines has been used as a means of assessing genetic barriers of resistance and the capacity of inhibitors, alone or in combination, to suppress emerging resistant variants (11, 17). In the current study, we examined the ability of BMS-790052 of different concentrations and with different treatment durations to eliminate replicon from genotype 1a and 1b replicon cells in the absence of G418 selection. We also extensively characterized emerging resistant variants. These studies provide a dynamic picture of emerging resistance to BMS-790052 over time at suboptimal concentrations of inhibitor. Importantly, adding pegylated alpha interferon (pegIFN-α) markedly enhanced the ability of BMS-790052 to cure genotype 1a replicon cells without changing the overall BMS-790052 resistance profile. Comparison of resistant variants from this in vitro study to those observed in a monotherapy multiple ascending dose (MAD) clinical trial of BMS-790052 (8, 19) reveals a strong correlation between replicon and clinical resistance development and helps to validate the replicon system as a predictive tool to assess the clinical efficacy of anti-HCV agents.
MATERIALS AND METHODS
Cell culture and HCV inhibitors.
Human hepatoma cells (Huh-7 and derivatives) were maintained in complete Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 1× nonessential amino acids, and 1 mM sodium pyruvate. Replicon-cured Huh7 cells which are highly permissive for HCV replicon replication have been described previously (15) and were used as host cells for replicon transient assays. Genotype 1a (H77c with P1496L and S2204I adaptive mutations) and 1b (Con-1 with the S2204I adaptive mutation) replicon cell lines have been described previously (7, 15). BMS-790052, BMS-650032 (NS3 protease inhibitor), and BMS-791325 (NS5B inhibitor) have been described previously (8, 10). Pegylated alpha interferon 2a (pegIFN-α2a; Pegasys) was purchased from Hoffmann-La Roche, Inc. (Nutley, NJ).
Replicon elimination and colony formation assays.
Con1 or H77c replicon cells (∼1.5 × 106) were plated in 10-cm dishes in complete DMEM containing 0.5 mg/ml G418 and maintained at 37°C, 5% CO2. After 24 h, medium was replaced with complete DMEM without G418 and containing the desired concentration of BMS-790052 or dimethyl sulfoxide (DMSO; control). After 72 h (day 3), cells were split into three 10-cm dishes. One tissue culture dish was plated with 12.5% of the culture and was maintained without G418 and with the same concentration of BMS-790052. These cells were processed again on days 7, 10, and 14. Two additional tissue culture dishes, each plated with 25% of the cells, were maintained without inhibitor for 2 weeks in complete medium supplemented with 0.5 mg/ml G418. One of these dishes was maintained without further splitting to allow replicon-retaining cells to form colonies, which were stained with Coomassie brilliant blue R-250 staining solution (Bio-Rad, Hercules, CA). In the second dish, G418-resistant cells were amplified for phenotypic and genotypic analysis. This process was repeated on days 7, 10, and 14, except that on day 10, dishes were not prepared for colony formation or genotypic and phenotypic analysis.
Genotypic analysis.
Sequence analysis of NS5A cDNA was performed as previously described (7). Briefly, total cellular RNA was isolated from replicon cells with TRIzol reagent (Invitrogen, Carlsbad, CA) and treated with DNase (Promega, Madison, WI) prior to being used as a template for reverse transcription-PCR (RT-PCR). First-strand cDNA was synthesized from random hexamers with a Superscript first-strand cDNA synthesis kit (Invitrogen, Carlsbad, CA). Platinum Taq high-fidelity DNA polymerase (Invitrogen, Carlsbad, CA) was used for PCR amplification of NS5A cDNA. For genotype 1b, the primers were 5′-TCTATCACCAGCCCGCTCACCACCCAACA-3′ (forward) and 5′-GCCTTAACTGTGGACGCCTTCGCCTTCATCTC-3′ (reverse). For genotype 1a, the primers were 5′-CCTGGAGCCCTTGTAGTCGGTGTGGTCTG-3′ (forward) and 5′-CCAGGTCGGGGAACACGATGAGACGAGCTGG-3′ (reverse). The resulting amplicons were used for direct sequencing analysis (population sequencing) and to generate NS5A cDNA clones via ligation into a TOPO TA cloning vector (Invitrogen, Carlsbad, CA).
Phenotypic analysis.
Mutations were introduced into the NS5A coding region of Con1 and H77c replicons by recombinant PCR as previously described (7). All mutations were verified by sequence analysis. Transient replicon assays were performed as previously described (7). Stable cell lines bearing G418-resistant replicons were selected as described previously (14). Renilla luciferase assays were performed with a Renilla luciferase assay system (Promega, Madison, WI). The half maximal effective concentrations (EC50s) of the inhibitor and replication capacities of replicon variants were determined as described previously (7, 20).
Quantitative reverse transcription-PCR.
Equal amounts of total cellular RNA, isolated from inhibitor or DMSO-treated HCV replicon cells with TRIzol reagent (Invitrogen, Carlsbad, CA), was used as a template for quantitative real-time RT-PCR, which was performed with a 7900HT Fast real-time PCR system (Applied Biosystems, Foster City, CA) and using a Platinum quantitative RT-PCR ThermoScript one-step kit (Invitrogen, Carlsbad, CA). HCV-specific primers were 5′-GGGAGAGCCATAGTGGTCTGC-3′ and 5′-CCCAAATCTCCAGGCATTGA-3′, and the HCV-specific probe was 5′-6-FAM-CGGAATTGCCAGGACGACCGG-BHQ-1-3′, where FAM is 6-carboxyfluorescein and BHQ-1 is Black Hole Quencher 1 dye. HCV RNA amounts were derived from a standard curve generated with serially diluted in vitro-transcribed Con1 replicon RNA. The total cell number was counted before the RNA harvest in order to calculate the HCV RNA copy number per cell.
RESULTS
Replicon elimination and inhibitor resistance in genotype 1b replicon cells.
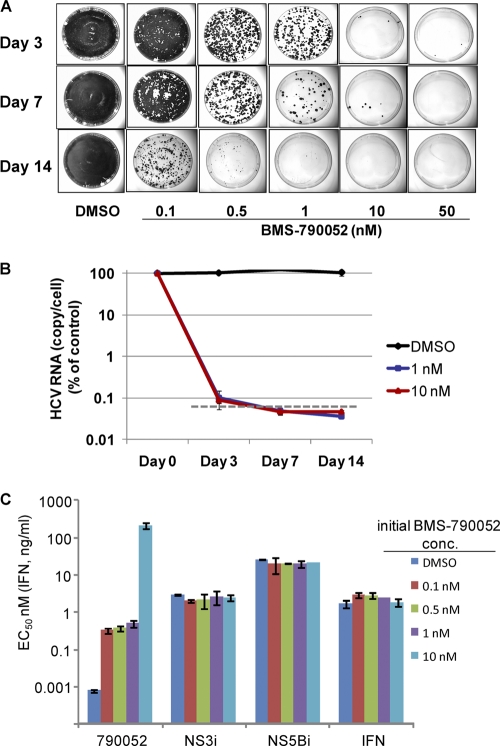
Replicon cells harboring a bicistronic Con1 replicon with a G418-selectable marker were treated with 0.1 to 50 nM BMS-790052 (∼10- to 5,000-fold over the EC50) without G418 for up to 14 days. The replicon copy number of the parental Con1 cells was determined by quantitative RT-PCR to be 6,224 ± 1,600 copies/cell; the doubling time of the cells was 40 ± 6 h. Cells were maintained as subconfluent cultures and were split on days 3, 7, 10, and 14. At each split (except on day 14), one culture dish was replenished with the original concentration of inhibitor. On days 3, 7, and 14, two additional cell culture dishes were prepared, and these were maintained in the absence of BMS-790052 but in the presence of G418 (0.5 mg/ml) so that only cells that retained viable replicon would survive. After approximately 2 weeks, cell colonies on one of the dishes were stained and photographed, while cells from the second dish were collected en masse and amplified for phenotypic and genotypic analysis. As shown in Fig. 1A, BMS-790052 eliminated replicon from the cells in a time- and dose-dependent fashion. With 7 days of treatment, 50 nM BMS-790052 was sufficient to eliminate the replicon from all of the cells, and when treatment was extended to 14 days, a 10 nM concentration of inhibitor was sufficient to completely eliminate the replicon. Lower concentrations of inhibitor (0.1 to 1 nM) also resulted in substantial decreases in the number of replicon-retaining cells, although the cells were not cured at these concentrations during the 14 days of inhibitor treatment.
Fig 1.
(A) Replicon elimination and colony formation assays with Con1 replicon cells. Colonies formed from replicon-retaining cells following treatment with BMS-790052 for the indicated durations and concentrations were stained and photographed. (B) Decline in replicon RNA over time in Con1 replicon cells treated with BMS-790052 or DMSO. The level of replicon RNA in cells treated with BMS-790052 or DMSO was measured by quantitative RT-PCR and is plotted on a log scale as the percentage relative to that in untreated control cells. Data points are the means and standard deviations from three replicate experiments. The dashed line represents the lower level of detection as determined from assays performed with non-replicon-containing Huh7 cells. (C) Inhibitor sensitivities of replicon cells recovered after 7 days of treatment with BMS-790052. Replicon cells recovered from a parallel set of tissue culture dishes to those described in Fig. 1A were used to determine the inhibitory activity of the indicated HCV inhibitors. EC50s are plotted as the means ± standard deviations from two independent assays. The initial BMS-790052 concentration (conc.) is the concentration at which the cells were treated for 7 days prior to removing inhibitor and adding G418. NS3i, BMS-650032; NS5Bi, BMS-791325; IFN, pegIFN-α2a.
To validate the replicon elimination assays, and to directly examine the effect of BMS-790052 on replicon RNA, a parallel set of experiments was performed in which replicon RNA levels in Con1 replicon cells were measured by quantitative RT-PCR after 3, 7, and 14 days of treatment with 1 or 10 nM BMS-790052. A rapid decline in replicon RNA in the inhibitor-treated cells was observed such that, on day 3, replicon RNA was slightly above the background levels, but by day 7, replicon RNA levels were below the limit of quantification in cells treated with either 1 or 10 nM BMS-790052 (Fig. 1B). The replicon-clearing experiments (Fig. 1A) reveal that some replicon remains in cells treated for 7 days with 1 nM BMS-790052, suggesting that the replicon elimination and colony formation assay was, at least under these experimental conditions, a more sensitive way to measure the effectiveness of inhibitor on replicon elimination.
The inhibitor sensitivity phenotypes of replicon cells that survived BMS-790052-treatment for 7 days are shown in Fig. 1C. As expected, the surviving replicon cells were resistant to BMS-790052. Replicon cells treated with BMS-790052 at concentrations of ≤1 nM displayed similar sensitivities to BMS-790052, while cells treated with 10 nM BMS-790052 were substantially more resistant (Fig. 1C). Replicon replication in all of the BMS-790052-treated cells remained sensitive to pegIFN-α and to control HCV NS3 and NS5B inhibitors (Fig. 1C).
Mutations associated with reduced sensitivity to BMS-790052 were identified by sequence analysis of NS5A cDNA clones isolated from inhibitor-treated cells (Table 1). Mutations at NS5A codons 31 and 93 yielding L31V and Y93H amino acid substitutions were the primary mutations identified. Several of the clones recovered from cells treated with <50 nM BMS-790052 for 3 days had no mutations in NS5A, indicating that 3 days of treatment was insufficient to completely suppress the wild-type replicon. By day 7, the incidence of clones without NS5A mutations was substantially reduced. At all time points, individual L31V and Y93H were the predominant amino acid substitutions identified following treatment with ≤1 nM BMS-790052, although linked amino acid replacements were also observed with a minority of the clones. When the concentration of BMS-790052 was increased to 10 nM and cells were treated for 7 days, linked L31V and Y93H substitutions were present in all of the clones (Table 1).
Table 1.
Number of clones with the indicated amino acid substitutions in NS5A genotype 1ba
| Variantb | No. of clones for indicated concn of BMS-790052 (nM) and treatment duration (days) |
MAD dose cohorte (mg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 |
0.5 |
1.0c |
10c |
50d |
||||||||
| 3 | 7 | 14 | 3 | 7 | 14 | 3 | 7 | 3 | 7 | 3 | ||
| WT | 20 | 3 | 3 | 6 | 1 | 2 | 10 | 2 | 22 | |||
| L31V | 5 | 12 | 14 | 13 | 18 | 10 | 5 | 6 | 1 | 27 | ||
| L31W | 1 | 1 | 5 | |||||||||
| P32L | 1 | |||||||||||
| P58S | 1 | |||||||||||
| Y93C | 1 | |||||||||||
| Y93H | 2 | 10 | 7 | 4 | 6 | 7 | 1 | 17 | ||||
| Y93N | 1 | |||||||||||
| L31V+Y93H | 5 | 2 | 5 | 2 | 1 | 3 | 3 | 27 | 10, 100 | |||
| Total no. of clones | 29 | 25 | 29 | 26 | 32 | 26 | 18 | 28 | 26 | 27 | 27 | |
NS5A cDNA clones were isolated from Con1 replicon cells following exposure to BMS-790052 at the indicated doses and treatment durations.
None of these mutant variants was observed for clones from DMSO-treated control cells at any of the time points. WT, wild type.
No replicon cells survived 14 days of treatment.
No replicon cells survived 7 or 14 days of treatment.
Monotherapy multiple ascending dose study in which subjects received once-daily doses of BMS-790052 for 14 days; mutations were identified in virus from one or more subjects at the indicated doses.
To assess the inhibitor sensitivity phenotypes of replicon variants harboring L31V, Y93H, and L31V+Y93H amino acid substitutions, these changes were introduced into the Con1 replicon and the mutant replicons were used for transient replication assays and also to derive stable replicon cell lines. Consistent with previous results (7), the L31V and Y93H substitutions by themselves yielded relatively low levels of resistance to BMS-790052, while the linked substitutions (L31V+Y93H) yielded a much greater level of resistance (Table 2). EC50s calculated for BMS-790052 with the engineered cell lines were 0.25 and 0.45 nM for L31V and Y93H, respectively, and 250 nM for L31V+Y93H (Table 2). These values correlate well with the BMS-790052 EC50s derived from cells recovered following inhibitor treatment (Fig. 1C). For example, cells treated with 0.1 nM, 0.5 nM, and 1 nM BMS-790052 for 7 days harbored replicons that predominantly carried either the L31V or Y93H mutations (Table 1). The EC50 of BMS-790052 in replicon assays performed with these cells ranged from 0.3 to 0.5 nM (Fig. 1C), similar to the potencies of BMS-790052 on the engineered L31V and Y93H replicon cell lines (Table 2). Likewise, linked L31V+Y93H substitutions were the only changes detected from NS5A cDNA recovered from cells treated with 10 nM BMS-790052 for 7 days. The EC50 of BMS-790052 on these cells was 210 nM, similar to the EC50 of 250 nM on the L31V+Y93H engineered cell line (Table 2). These results indicate that the L31V and Y93H amino acid substitutions are sufficient to explain the reduced inhibitor susceptibilities of the BMS-790052-treated cells.
Table 2.
Susceptibility of 1b replicon variants to BMS-790052a
| Replicon variant | Replication level | Transient assay |
Cell line |
||
|---|---|---|---|---|---|
| EC50 (nM) | FR | EC50 (nM) | FR | ||
| WT | 100 | 0.0026 ± 0.0009 | 1.0 | 0.0031 ± 0.0016 | 1.0 |
| L31V | 157.9 ± 53.6 | 0.072 ± 0.021 | 28 | 0.25 ± 0.016 | 81 |
| L31W | 190.7 ± 9.1 | 0.210 ± 0.002 | 81 | ND | ND |
| P32L | 17.5 ± 5.6 | 0.042 ± 0.021 | 16 | ND | ND |
| P58S | 120.5 ± 17.9 | 0.0023 ± 0.0004 | 0.9 | ND | ND |
| Y93C | 61.9 ± 4.4 | 0.009 ± 0.002 | 3.5 | ND | ND |
| Y93H | 26.9 ± 15.5 | 0.062 ± 0.025 | 24 | 0.45 ± 0.047 | 145 |
| Y93N | 18.8 ± 5.2 | 0.13 ± 0.088 | 50 | ND | ND |
| L31V+Y93H | 49.9 ± 38.0 | 38.0 ± 33.4 | 14615 | 250 ± 100 | 80645 |
Replication level, mean ± standard deviation relative to the wild type as determined from transient assays with the indicated replicons (n ≥ 3). EC50, mean ± standard deviation (n ≥ 3); WT, wild type; FR, fold resistance; ND, not determined.
In addition to L31V and Y93H, other amino acid substitutions, including L31W, P32L, P58S, Y93C, and Y93N (Table 1), were detected at low frequencies in BMS-790052-treated cells. The effects of these amino acid replacements on replicon replication and BMS-790052 potency in transient replicon assays are shown in Table 2. Of these, only L31W was not previously identified in BMS-790052 resistance studies (7). This amino acid substitution, which requires two nucleotide changes (CTG to TGG), reduced the potency of BMS-790052 approximately 80-fold in transient assays (EC50, 0.21 nM) (Table 2).
For Con1 replicons bearing linked L31V+Y93H substitutions, BMS-790052 was on average ∼7-fold more potent in transient assays than in assays performed with the engineered replicon cell line (Table 2). Similar discrepancies were also observed with Y93H and, to a lesser extent, with L31V (Table 2). In contrast, the EC50s of a control NS3 protease inhibitor were similar for the two assays (data not shown). To determine if additional replicon mutations that might have arisen during cell line selection could explain the decrease in BMS-790052 potency, HCV cDNA was recovered from the L31V+Y93H replicon cell line and the NS3-NS5B coding region was sequenced. Other than the L31V and Y93H substitutions, no additional changes were identified (data not shown). We next assessed whether differences in cellular backgrounds might account for the assay discrepancies. For this purpose, the L31V+Y93H replicon cell line was cured (4) and subsequently used as a host for transient replicon assays. In side-by-side assays with the Con1 L31V+Y93H replicon, the BMS-790052 EC50 was 21 ± 8 nM with the newly cured cells and 25 ± 7 nM with the original Huh7-cured cells, indicating that the decrease in BMS-790052 potency was not due to a stable change in the cellular environment. At this time, the reason for the observed discrepancy in BMS-790052 potency between transient and replicon cell line assays remains unclear.
Replicon elimination and inhibitor resistance in genotype 1a replicon cells.
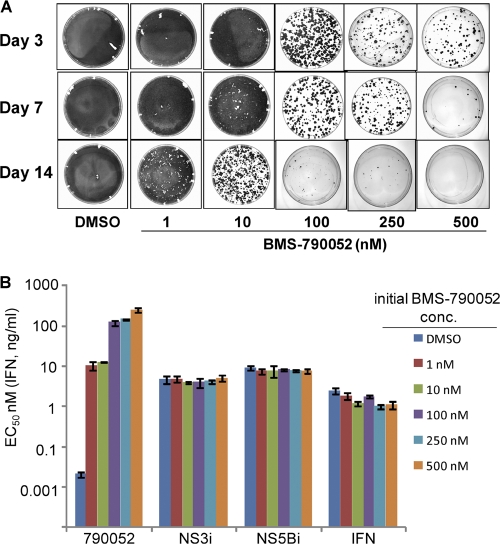
Similar replicon clearing experiments were performed with Huh-7 cells harboring a genotype 1a H77c replicon by treating the cells with BMS-790052 at concentrations ranging from 1 to 500 nM (∼20 to 10,000 times the EC50) (Fig. 2A). The replicon copy number (5,269 ± 1,122 copies/cell) and cell doubling rate (37 ± 3 h) of the H77c replicon cells were very similar to those observed for the Con1 replicon cells (see above). With the H77c replicon cells, treatment with 500 nM BMS-790052 for 14 days was sufficient to completely eliminate replicon from the cells, while treatment with 100 nM or 250 nM BMS-790052 for 14 days eliminated replicon from the majority of cells. The inhibitor sensitivity phenotypes of replicon cells that survived 7 days of treatment with BMS-790052 at concentrations ranging from 1 nM to 500 nM are shown in Fig. 2B. All of the replicon cells treated with the NS5A inhibitor were resistant to BMS-790052 but remained fully sensitive to IFN and other HCV inhibitors (Fig. 2B). The EC50 of BMS-790052 was ∼10 nM for cells treated with either 1 nM or 10 nM inhibitor. Cells treated with higher concentrations of BMS-790052 exhibited correspondingly higher levels of resistance (Fig. 2B).
Fig 2.
(A) Replicon elimination and colony formation assays with H77c replicon cells. Colonies formed from replicon-retaining cells following treatment with BMS-790052 for the indicated durations and concentrations were stained and photographed. (B) Inhibitor sensitivities of replicon cells recovered after 7 days of treatment with BMS-790052. Replicon cells recovered from a parallel set of tissue culture dishes to those described in Fig. 2A were used to determine the inhibitory activity of the indicated HCV inhibitors. EC50s are plotted as the means ± standard deviations from two independent assays. The initial BMS-790052 concentration (conc.) is the concentration at which the cells were treated for 7 days prior to removing inhibitor and adding G418. NS3i, BMS-650032; NS5Bi, BMS-791325; IFN, pegIFN-α2a.
Amino acid substitutions deduced from sequence analysis of bulk NS5A cDNA isolated from BMS-790052-treated cells are summarized in Table 3. In cells treated with 1 nM or 10 nM BMS-790052 for 3 to 14 days, the most common mutations observed were those that yielded M28T, Q30H, Q30R, L31M, or Y93H amino acid substitutions. The estimated frequencies of these mutations indicated that they were present mostly as unlinked mutations (Table 3). In cells treated with 100 nM BMS-790052, Q30E, Q30K, and Y93H were the principal amino acid substitutions observed, while a Q30E substitution was the predominant change observed with treatment for 3 or 7 days with 250 nM BMS-790052. At the 250 nM dose, Y93N replaced Y93H as the major amino acid substitution at NS5A residue 93 (Table 3). Although small colonies were observed following 14 days of treatment with 250 nM BMS-790052, efforts to amplify these colonies for further analysis were unsuccessful. In cells treated with 500 nM BMS-790052 for 3 days, Q30E was again the predominant amino acid substitution observed. Very few replicon cells survived treatment with 500 nM BMS-790052 for 7 days (Fig. 2A). NS5A cDNA recovered from these cells had Q30E (∼50%) and additional substitutions (M28T, Q30R, H58D, Y93H) at frequencies suggesting linked mutations (M28T+Y93H or Q30R+H58D).
Table 3.
Amino acid substitutions predicted from bulk NS5A cDNA recovered from genotype 1a replicon cells surviving BMS-790052 treatmenta
| Amino acid substitution | % amino acid substitutions predicted for indicated concn of BMS-790052 (nM) and treatment duration (days) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
10 |
100 |
250b |
500c |
|||||||||
| 3 | 7 | 14 | 3 | 7 | 14 | 3 | 7 | 14 | 3 | 7 | 3 | 7 | |
| M28T | 20 | 25 | 20 | 25 | 30 | 30 | 5 | 40 | |||||
| Q30D | 5 | ||||||||||||
| Q30E | 5 | 40 | 50 | 50 | 65 | 100 | 70 | 50 | |||||
| Q30H | 25 | 35 | 40 | 40 | 35 | 50 | 15 | ||||||
| Q30K | 20 | 10 | 30 | ||||||||||
| Q30R | 10 | 15 | 20 | 10 | 5 | 5 | |||||||
| L31M | 5 | 5 | 5 | 5 | 5 | ||||||||
| H58D | 5 | ||||||||||||
| Y93H | 5 | 10 | 10 | 10 | 20 | 25 | 30 | 25 | 40 | ||||
| Y93N | 5 | 5 | 10 | ||||||||||
Values in the table are estimated percentages based on visual inspection of the population cDNA sequence chromatogram.
A few colonies were observed following 14 days of treatment, but these were not successfully amplified.
No replicon cells survived 14 days of treatment.
To validate the population sequencing results, sequences of individual NS5A cDNA clones were obtained. Analysis of ∼50 clones from cells treated with 1, 10, 100, 250, or 500 nM BMS-790052 for 7 days are summarized in Table 4. The results of this analysis largely confirmed the population sequencing results. For example, Q30E was present in 51 out of 55 NS5A cDNA clones that were obtained from cells treated for 7 days with 250 nM BMS-790052. In clones from cells treated with 500 nM BMS-790052 for 7 days, 22 of 46 had Q30E and 21 of 46 had linked amino acid substitutions, mostly M28T+Y93H. In addition to the amino acid substitutions identified by population sequencing and reported in Table 3, several less-abundant mutations were identified by analysis of NS5A cDNA clones. These included additional amino acid replacements at residues 30 (Q30D/G/L/P), 31 (L31P/Q/R), and 93 (Y93C), an H58D mutation, and a collection of linked mutations (Table 4). Linked mutations were prevalent only in clones isolated from cells treated for 7 days with 500 nM BMS-790052 (Table 4).
Table 4.
Number of clones with the indicated amino acid substitutions in NS5A genotype 1aa
| Variant | No. of clones for indicated concn of BMS-790052 (nM) |
MAD dose cohort (mg)c | |||||
|---|---|---|---|---|---|---|---|
| 0b | 1 | 10 | 100 | 250 | 500 | ||
| WT | 58 | 4 | 5 | 3 | 1 | ||
| M28T | 15 | 8 | 1 | 1, 30, 60, 100 | |||
| Q30D | 1 | 1 | |||||
| Q30E | 1 | 2 | 23 | 51 | 22 | 10, 30, 60, 100 | |
| Q30H | 17 | 12 | 1, 10, 30, 60, 100 | ||||
| Q30K | 1 | 10 | 30, 60 | ||||
| Q30R | 5 | 6 | 1, 10, 30, 60, 100 | ||||
| Q30G/L/P | 1/1/1 | ||||||
| L31M | 6 | 3 | 1, 10, 30, 100 | ||||
| L31V | 2 | 10, 30, 60 | |||||
| L31P/Q/R | 1/1/1 | ||||||
| P32L | 1 | ||||||
| H58D | 1 | 100 | |||||
| Y93C | 1 | 2 | 1, 30, 60, 100 | ||||
| Y93H | 5 | 6 | 12 | 10, 30, 60, 100 | |||
| Y93N | 5 | 30, 60, 100 | |||||
| M28T/Y93H | 15 | ||||||
| Q30E/Y93H | 1 | 1 | 3 | ||||
| Q30H/Y93C | 1 | ||||||
| Q30H/Y93H | 1 | 60 | |||||
| Q30K/Y93H | 1 | ||||||
| Q30R/H58D | 3 | 100 | |||||
| Q30R/Y93H | 1 | ||||||
| Total clones | 58 | 60 | 47 | 58 | 55 | 46 | |
NS5A cDNA clones were isolated from H77c replicon cells following 7 days of exposure to BMS-790052 at the indicated doses. WT, wild type.
DMSO-treated control cells.
Monotherapy multiple ascending dose study in which subjects received once-daily doses of BMS-790052 for 14 days; mutations were identified in virus from one or more subjects at the indicated doses.
Phenotypic analysis of genotype 1a variants.
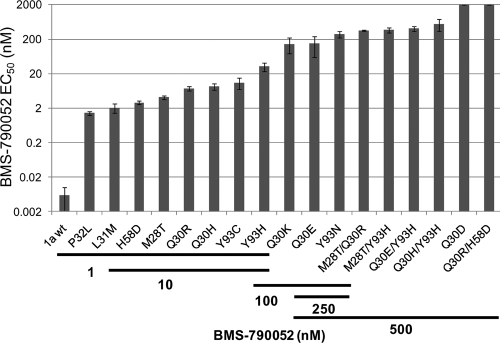
Many of the inhibitor-induced mutations identified in the H77c replicon in this study have been previously characterized (7). Novel amino acid substitutions included Q30D/G/L/P, H58D, L31P/Q/R, and several combinations of linked substitutions, such as M28T+Y93H, Q30R+H58D, Q30E+Y93H, and M28T+Q30R. The effects of several of these changes on BMS-790052 potency and replicon fitness in replicon transient assays are summarized in Table 5. For a subset of the variants, the antireplicon activity of BMS-790052 was also evaluated with stable replicon cell lines. For a given mutant replicon, the potencies of BMS-790052 were very similar for the two assays (Table 5). Residue 30 was a hot spot for resistance development in the H77c replicon and Q30E, Q30K, and Q30D, in particular, conferred high levels of resistance to BMS-790052 (Table 5). Multiple amino acid substitutions were also identified at residue 31. Replicons with L31M or L31V amino acid replacements replicated well, while replicons with L31P, L31Q, or L31R failed to replicate in transient replicon assays. As illustrated in Fig. 3, mutations that emerged in response to treatment with low doses of BMS-790052 generally gave rise to lower levels of resistance than those selected at higher doses. In part, this can be explained by the increased occurrence of linked mutations observed from cells treated with 500 nM BMS-790052. The linked mutations conferred high levels of resistance to BMS-790052, but they also generally displayed poor replication abilities (Table 5 and Fig. 3).
Table 5.
Susceptibility of 1a replicon variants to BMS-790052a
| Replicon variant | Replication level | Transient assay |
Cell line |
||
|---|---|---|---|---|---|
| EC50 (nM) | FR | EC50 (nM) | FR | ||
| 1a wt | 100 | 0.006 ± 0.004 | 1.0 | 0.022 ± 0.002 | 1.0 |
| M28T | 31 ± 23 | 4.1 ± 0.4 | 683 | 12.4 ± 5.2 | 564 |
| Q30D | 171 ± 134 | >2,000 | >333,333 | >2,000 | >90,909 |
| Q30E | 130 ± 56 | 149.6 ± 89.1 | 24,933 | 259.1 ± 38.0 | 11,777 |
| Q30G | 54 ± 22 | 51.0 ± 14.9 | 8,500 | 45.2 ± 22.1 | 2,055 |
| Q30H | 75 ± 31 | 8.7 ± 1.9 | 1,450 | 8.8 ± 0.0 | 400 |
| Q30K | 19 ± 9 | 145.9 ± 70.5 | 24,317 | 82.1 ± 9.7 | 3,732 |
| Q30L | 37 ± 11 | 0.022 ± 0.004 | 3.7 | 0.079 ± 0.0 | 3.6 |
| Q30P | 114 ± 19 | 0.009 ± 0.0009 | 1.5 | ND | ND |
| Q30R | 41 ± 16 | 7.4 ± 1.1 | 1,233 | ND | ND |
| L31M | 55 ± 15 | 2.1 ± 0.6 | 350 | ND | ND |
| L31V | 117 ± 29 | 20.1 ± 6.0 | 3,350 | 55.1 ± 19.0 | 2,505 |
| L31P/Q/R | No replication | ND | N,D | ND | ND |
| P32L | 18 ± 5 | 1.4 ± 0.2 | 233 | ND | ND |
| H58D | 92 ± 9 | 2.9 ± 0.4 | 483 | ND | ND |
| Y93C | 11 ± 7 | 11.1 ± 4.1 | 1,850 | ND | ND |
| Y93H | 18 ± 11 | 32.2 ± 9.4 | 5,367 | 52.3 ± 20.0 | 2,377 |
| Y93N | 13 ± 8 | 282.1 ± 64.7 | 47,017 | 354.2 ± 119.1 | 16,100 |
| M28T/Q30Rb | 76 ± 23 | 356.4 ± 12.0 | 59,400 | 301.0 ± 94.3 | 13,681 |
| M28T/Y93H | Poor replication | ND | ND | 365.7 ± 55.0 | 16,623 |
| Q30E/Y93H | 6 ± 1 | 403.6 ± 61.6 | 67,267 | 499.9 ± NA | 22,723 |
| Q30H/Y93C | ND | ND | ND | ND | ND |
| Q30H/Y93H | 20 ± 6 | 553.3 ± 207.4 | 92,217 | 393.6 ± NA | 17,891 |
| Q30K/Y93H | ND | ND | ND | ND | ND |
| Q30R/H58D | 60 ± 12 | 2,515.6 ± 61.7 | 419,267 | >2,000 | >90,909 |
| Q30R/Y93H | 6 ± 1 | 340.9 ± 40.8 | 56,817 | 280.5 ± 76.0 | 12,750 |
Replication level, mean ± standard deviation relative to the wild type determined from transient assays with the indicated replicons (n ≥ 3). EC50, mean ± standard deviation (n ≥ 3); 1a wt, genotype 1a wild-type replicon; FR, fold resistance; ND, not determined.
Detected in cells treated for 3 days at 500 nM BMS-790052.
Fig 3.
The resistance of emerging variants increases with increasing BMS-790052-treatment concentrations. EC50s of BMS-790052 on H77c replicon variants with the indicated amino acid substitutions are plotted. The concentrations of BMS-790052 at which variants were detected in replicon elimination assays are indicated by the bars below the plot. 1a wt, genotype 1a wild-type replicon. Values are the means ± standard deviations (n ≥ 3) from transient replicon assays, except for M28T/Y93H, which is from stable replicon cell assays since this variant did not replicate in transient assays.
Correlation between in vitro and in vivo BMS-790052-resistant variants.
For comparative purposes, we have indicated, in Tables 1 and 4, amino acid substitutions from the current study that were also identified in viral RNA recovered from subjects who received 1, 10, 30, 60, or 100 mg BMS-790052 once daily for 14 days as part of a multiple ascending dose (MAD) trial (8, 19). Linked L31V and Y93H were the dominant amino acid substitutions associated with resistance to BMS-790052 in genotype 1b replicon cells treated with 10 nM BMS-790052 for 7 days (Table 1). These linked substitutions were also identified by analysis of rebounding viral RNA recovered from HCV genotype 1b-infected subjects who received 10- or 100-mg doses of BMS-790052 (8). The majority of the genotype 1a variants identified in this study were also observed by analysis of viral RNA isolated on day 14 from one or more HCV genotype 1a-infected subjects in the MAD clinical study. For example, Q30E was a prominent resistant substitution identified in replicon cells treated with ≥100 nM BMS-790052 (Table 4) and also in rebounding virus from MAD trial subjects receiving BMS-790052 at doses ranging from 30 to 100 mg, for whom the average minimum concentration of drug in serum (Cmin) was ∼75 to 350 nM (8, 19).
pegIFN-α enhances the antireplicon activity of BMS-790052.
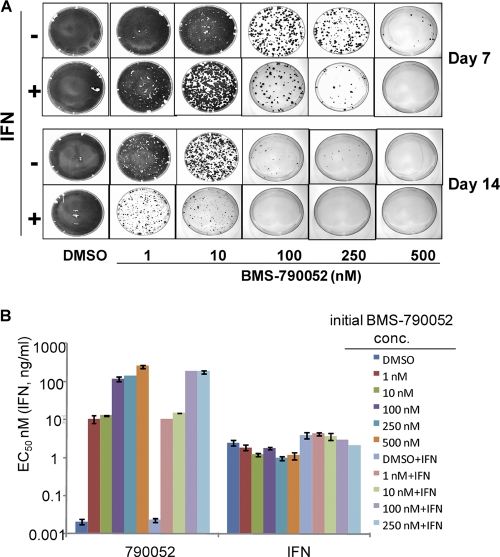
Since replicon-clearing experiments demonstrated that genotype 1a replicon cells were more difficult to “cure” than the genotype 1b replicon cells, we assessed the effect of combining BMS-790052 and pegIFN-α on genotype 1a replicon cells. Cells were treated with pegIFN-α (4 ng/ml at ∼4× EC50) either alone or in combination with increasing concentrations of BMS-790052. As shown in Fig. 4A, this concentration of pegIFN-α had little effect on the replicon elimination by itself; however, it markedly enhanced the ability of BMS-790052 to eliminate replicon from the cells. The enhanced antireplicon effect of pegIFN-α was apparent at all concentrations of BMS-790052 and was particularly evident when the cells were treated with the inhibitors for 14 days. Under the experimental conditions used, the replicon was completely cleared from the cells with a combination of 4 ng/ml pegIFN-α and ≥100 nM BMS-790052, whereas without pegIFN-α, 500 nM BMS-790052 was required to cure the cells.
Fig 4.
(A) IFN enhances the antireplicon ability of BMS-790052. Replicon elimination assays were performed with H77c replicon cells at the indicated concentrations of BMS-790052 with or without pegIFN-α (4 ng/ml) for 7 or 14 days. Colonies formed from replicon-retaining cells following treatment were stained and photographed. (B) Inhibitor sensitivities of replicon cells recovered after 7 days of treatment with BMS-790052 in the presence or absence of IFN. Replicon cells recovered from a parallel set of tissue culture dishes to those described in Fig. 4A were used to determine the inhibitory activity of the indicated HCV inhibitors. EC50s are plotted as the means ± standard deviations from two independent assays. The initial BMS-790052 concentration (conc.) is the concentration at which the cells were treated for 7 days prior to removing inhibitor and adding G418. IFN, pegIFN-α2a.
The inhibitor sensitivity phenotypes of replicon cells recovered following treatment with pegIFN-α and BMS-790052 were compared to those of cells that had been treated only with BMS-790052. As shown in Fig. 4B, the potencies of BMS-790052 were very similar for cells treated for 7 days with BMS-790052 with and without pegIFN-α. Congruent with these results, the patterns of NS5A mutations observed by analysis of NS5A cDNA from cells treated with BMS-790052 with and without pegIFN-α were very similar (compare Tables 3 and 6), suggesting that while pegIFN-α works cooperatively with the NS5A inhibitor, it does not grossly alter the BMS-790052 resistance profile. Other than the N-terminal mutations associated with BMS-790052 resistance, no additional consensus mutations were identified within NS5A cDNA from inhibitor-treated cells, including within the interferon sensitivity-determining region (ISDR; NS5A amino acids 237 to 276) (data not shown).
Table 6.
Amino acid substitutions predicted from bulk NS5A cDNA recovered from cells treated with BMS-790052 and pegIFNα-2aa
| Variant | % amino acid substitutions predicted for indicated BMS-790052 treatment concn (nM) and treatment duration (days) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
10 |
100b |
250b |
500c |
|||||||
| 3 | 7 | 14 | 3 | 7 | 14 | 3 | 7 | 3 | 7 | 3 | |
| M28T | 20 | 35 | 20 | 25 | 30 | 50 | 15 | ||||
| Q30D | 5 | ||||||||||
| Q30E | 5 | 5 | 50 | 65 | 100 | 100 | 50 | ||||
| Q30H | 35 | 50 | 20 | 55 | 60 | 50 | 50 | ||||
| Q30K | 5 | ||||||||||
| Q30R | 10 | 5 | 10 | 5 | 5 | ||||||
| L31M | 5 | 5 | 5 | ||||||||
| H58D | |||||||||||
| Y93C | 25 | ||||||||||
| Y93H | 25 | 10 | |||||||||
| Y93N | 35 | ||||||||||
pegIFNα-2a dose was 4 ng/ml. Values in the table are estimated percentages based on visual inspection of the population cDNA sequence chromatogram.
No replicon cells survived 14 days of treatment.
No replicon-retaining cells survived 7 or 14 days of treatment with BMS-790052.
DISCUSSION
In the present study, we have extended our analysis of the in vitro anti-HCV activity of BMS-790052 by examining the development of resistance and the ability of the inhibitor, either alone or in combination with pegIFN-α, to eliminate replicon from tissue culture cells. When replicon cells were treated with BMS-790052 for 14 days, a 10 nM concentration of inhibitor was sufficient to cure genotype 1b replicon, whereas 500 nM BMS-790052 was required to completely cure genotype 1a replicon cells (Fig. 1a and 2a). The parental 1a and 1b replicon cell lines had similar replicon copy numbers (∼5,000 to 6,000 copies/cell) and similar growth rates (37- to 40-h doubling times), indicating that these factors did not substantially contribute to the observed differences in replicon clearance. While the difference in BMS-790052 potency on the parental replicon cells (EC50s of 22 pM and 3.1 pM for 1a and 1b cells, respectively) probably contribute to the difference in effectiveness, these results also reflect a higher genetic barrier to resistance in genotype 1b than that for genotype 1a, such that, at the same BMS-790052 concentration (e.g., 10 nM), multiple mutations are required for replicon resistance in genotype 1b, while a single mutation is sufficient in genotype 1a. For example, L31V and Y93H were the predominant amino acid substitutions identified in NS5A cDNA recovered from genotype 1b replicon cells treated with BMS-790052. In stable replicon cells, BMS-790052 inhibited replication of these variants with EC50s of 250 and 450 pM, respectively. In contrast, cells harboring the equivalent mutations in the H77c replicon yielded BMS-790052 EC50s of 55 (L31V) and 52 (Y93H) nM. Moreover, a large number of additional substitutions (M28T, L31M, P32L, Q30H, Q30R, Q30K, Q30E, H58D, Y93C, and Y93N) were identified by analysis of NS5A cDNA recovered from genotype 1a replicon cells treated with BMS-790052, and each of these substitutions conferred substantial resistance to BMS-790052 (EC50s ranging from ∼1 nM to ∼280 nM). These findings suggest that preexisting or selected mutations that confer relatively high levels of resistance are much more likely to be encountered in a genotype 1a background than in a genotype 1b background.
Many of the NS5A replicon variants that were observed following BMS-790052 treatment in this study have been previously reported (7). Moreover, novel amino acid replacements at positions previously implicated in resistance development accounted for most of the newly identified mutations. These included L31W in the Con1 replicon and Q30D, Q30G, Q30L, Q30P, L31P, L31Q, and L31R in the H77c replicon. Of the resistant substitutions observed for the first time in this study, only H58D in the H77c replicon represented a novel site for resistance development. On its own, the H58D substitution conferred only moderate resistance to BMS-790072 (EC50, 2.9 nM); however, the combination of Q30R+H58D was very resistant (EC50, >2 μM). The paucity of new mutations identified in this study suggests that most of the major sites for resistance development to BMS-790052 in the Con1 and H77c replicons have been identified.
NS5A residue 30 was previously identified as a key site for BMS-790052 resistance development in the H77c replicon (7). In the current study, a total of eight amino acids, in addition to the parental glutamine, were identified at this position in NS5A, highlighting the importance of this residue to BMS-790052 resistance. In cells treated with 1 to 10 nM BMS-790052, single Q30H and Q30R amino acid substitutions were commonly observed (Tables 3 and 4). These substitutions conferred relatively modest levels of resistance to BMS-790052 (EC50, 7 to 9 nM) (Table 5), and consequently, these replicon variants were not observed at ≥100 nM BMS-790052 (Table 3). In contrast, amino acid replacements at residue 30 that conferred greater levels of resistance to BMS-790052 (Q30E, Q30K, and Q30D) were observed only when replicon cells were treated with ≥100 nM BMS-790052 (Table 4). These results suggest that replicon variants with Q30R and Q30H substitutions were more prevalent, either as preexisting or rapidly selected variants, than those with Q30D, Q30E, and Q30K substitutions. The relatively low abundance of the Q30K and Q30D replicon variants can possibly be explained in that Q30K negatively impacted replication fitness (Table 5), while two nucleotide changes were required for Q30D (CAA to GAT or GAC). In contrast, the Q30E variant replicated well and arose from a single nucleotide change (CAA to GAA). A low abundance of Q30E variants, however, might be explained if there is a bias against C-to-G transversion mutations by the HCV replicase as suggested in a study that examined genetic diversity and mutation rates in HCV replicons (13). This study found that C-to-G transversions occurred infrequently, accounting for only 1.8% of the total number of observed mutations. Supporting these observations, glutamic acid, aspartic acid, and lysine were not observed at residue 30 in any of the 1,497 genotype 1a NS5A sequences in the European HCV database (5), while histidine and arginine were present in 0.67 and 0.53% of the sequences, respectively.
A strong similarity was observed between replicon variants observed in this in vitro study and viral variants recently reported in a phase I MAD trial in which BMS-790052 was administered to subjects once daily for 14 days at doses ranging from 1 to 100 mg (Tables 1 and 4). Linked L31V and Y93H amino acid substitutions were identified as major resistant determinants in HCV genotype 1b both in vivo and in vitro (Table 1). In the current study, a shift from moderately resistant variants (M28T, Q30H/R, L31M) to highly resistant variants (Q30E, Y93N, Q30R+H58D, M28T+Y93H) was observed for genotype 1a replicon cells with increasing treatment concentrations of BMS-790052 (Fig. 3). In the MAD monotherapy trial, an increase in the abundance of viral variants with a Q30E substitution was also observed in subjects receiving ≥30 mg BMS-790052 (8). Since the genotypic analysis of the MAD clinical samples was derived from population sequencing, linkage relationships of the mutations were not always discernible (8). However, variants with linked amino acid substitutions (e.g., Q30H+Y93H or Q30R+H58D) were clearly the dominant species in at least two subjects treated with 60 or 100 mg BMS-790052 (8). These similarities in emerging resistant variants in vitro and in vivo validate the replicon system as a predictive tool to help guide the clinical development of BMS-790052.
The genetic diversity of HCV is expected to be very high in infected individuals, and viral variants with double and triple resistance-associated mutations may preexist in some patients (12). An effective combination therapy that prevents the emergence of multidrug-resistant strains is therefore essential. In this study, we observed that pegIFN-α substantially increased the ability of BMS-790052 to cure genotype 1a replicon cells without altering the identity of BMS-790052-induced mutations. Early results from a clinical trial in which subjects received BMS-790052 in combination with pegIFN and RBV suggest that this combination is effective in suppressing the majority of BMS-790052-resistant variants (21). In this trial, sustained viral response rates of up to 83% were achieved in subjects receiving triple therapy (BMS-790052 at once-daily doses of 20 or 60 mg plus pegIFN and RBV) compared to 25% in subjects receiving pegIFN and RBV alone. Moreover, with its distinct target and resistance profile, BMS-790052 also has the potential to be a valuable component of IFN-sparing regimens.
ACKNOWLEDGMENTS
We thank Charles Tilford and Mingyi Liu for assistance with NS5A sequence analysis and Makonen Belema and Nicholas Meanwell for helpful discussions. We thank Mark Cockett for support and leadership.
Footnotes
Published ahead of print 3 January 2012
REFERENCES
- 1. Alter MJ. 2007. Epidemiology of hepatitis C virus infection. World J. Gastroenterol. 13:2436–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartenschlager R, Cosset F-L, Lohmann V. 2010. Hepatitis C virus replication cycle. J. Hepatol. 53:583–585 [DOI] [PubMed] [Google Scholar]
- 3. Blight KJ, Kolykhalov AA, Rice CM. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972–1974 [DOI] [PubMed] [Google Scholar]
- 4. Blight KJ, McKeating JA, Rice CM. 2002. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J. Virol. 76:13001–13014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Combet C, et al. 2007. euHCVdb: the European hepatitis C virus database. Nucleic Acids Res. 35:D363–D366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cuevas JM, Gonzalez-Candelas F, Moya A, Sanjuan R. 2009. Effect of ribavirin on the mutation rate and spectrum of hepatitis C virus in vivo. J. Virol. 83:5760–5764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fridell RA, Qiu D, Wang C, Valera L, Gao M. 2010. Resistance analysis of the hepatitis C virus NS5A inhibitor BMS-790052 in an in vitro replicon system. Antimicrob. Agents Chemother. 54:3641–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fridell RA, et al. 2011. Genotypic and phenotypic analysis of variants resistant to hepatitis C virus nonstructural protein 5A replication complex inhibitor BMS-790052 in humans: in vitro and in vivo correlations. Hepatology 54:1924–1935 [DOI] [PubMed] [Google Scholar]
- 9. Gane E. 2011. Future hepatitis C virus treatment: interferon-sparing combinations. Liver Int. 31:62–67 [DOI] [PubMed] [Google Scholar]
- 10. Gao M, et al. 2010. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature 465:96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Graham EJ, et al. 2011. Colony-forming assays reveal enhanced suppression of hepatitis C virus replication using combinations of direct-acting antivirals. J. Virol. Methods 174:153–157 [DOI] [PubMed] [Google Scholar]
- 12. Guedj J, Rong L, Dahari H, Perelson AS. 2010. A perspective on modelling hepatitis C virus infection. J. Viral Hepat. 17:825–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kato N, et al. 2005. Genetic variation and dynamics of hepatitis C virus replicons in long-term cell culture. J. Gen. Virol. 86:645–656 [DOI] [PubMed] [Google Scholar]
- 14. Lemm JA, et al. 2005. Replication-competent chimeric hepatitis C virus subgenomic replicons. Intervirology 48:183–191 [DOI] [PubMed] [Google Scholar]
- 15. Lemm JA, et al. 2010. Identification of hepatitis C virus NS5A inhibitors. J. Virol. 84:482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lohmann V, et al. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110–113 [DOI] [PubMed] [Google Scholar]
- 17. McCown MF, et al. 2008. The hepatitis C virus replicon presents a higher barrier to resistance to nucleoside analogs than to nonnucleoside polymerase or protease inhibitors. Antimicrob. Agents Chemother. 52:1604–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Munir S, et al. 2010. Hepatitis C treatment: current and future perspectives. Virol. J. 7:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nettles RE, et al. 2011. Multiple ascending dose study of BMS-790052, a nonstructural protein 5A replication complex inhibitor, in patients infected with hepatitis C virus genotype 1. Hepatology 54:1956–1965 [DOI] [PubMed] [Google Scholar]
- 20. O'Boyle DR, II, et al. 2005. Development of a cell-based high-throughput specificity screen using a hepatitis C virus-bovine viral diarrhea virus dual replicon assay. Antimicrob. Agents Chemother. 49:1346–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pol S, et al. 2011. High rates of SVR24 for BMS-790052, a NS5A replication complex inhibitor, in combination with peg-IFNα-2A and ribavirin: phase 2A trial in treatment-naive HCV-genotype 1 Subjects, abstr. H1-376. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL, 16 to 20 September 2011 http://www.icaac.org/ [Google Scholar]
- 22. Simmonds P. 2004. Genetic diversity and evolution of hepatitis C virus—15 years on. J. Gen. Virol. 85:3173–3188 [DOI] [PubMed] [Google Scholar]