Abstract
Intermittent preventive treatment (IPT) is increasingly used to reduce malaria morbidity and mortality in children and pregnant women. The efficacy of IPT depends on the pharmacokinetic and pharmacodynamic properties of the antimalarial drugs used. Healthy adult male volunteers whose occupation put them at high risk of malaria on the Northwest border of Thailand were randomized to receive a 3-day-treatment dose of dihydroartemisinin-piperaquine monthly (DPm) or every 2 months (DPalt) or an identical placebo with or without fat (6.4g/dose) over a 9-month period. All volunteers were monitored weekly. One thousand adults were recruited. Dihydroartemisinin-piperaquine was well tolerated. There were 114 episodes of malaria (49 Plasmodium falciparum, 63 P. vivax, and 2 P. ovale). The protective efficacy against all malaria at 36 weeks was 98% (95% confidence interval [CI], 96% to 99%) in the DPm group and 86% (95% CI, 81% to 90%) in the DPalt group (for both, P < 0.0001 compared to the placebo group). As a result, the placebo group also had lower hematocrits during the study (P < 0.0001). Trough plasma piperaquine concentrations were the main determinant of efficacy; no malaria occurred in participants with a trough concentration above 31 ng/ml. Neither plasma piperaquine concentration nor efficacy was influenced by the coadministration of fat. DPm is safe to use and is effective in the prevention of malaria in adult males living in an area where P. vivax and multidrug-resistant P. falciparum malaria are endemic.
INTRODUCTION
Progress has been made in reducing malaria morbidity and mortality in recent years. This can be attributed to several factors, including economic development, increased availability of insecticide-impregnated bed nets, and more effective antimalarial treatments, in particular those combining an artemisinin derivative with a partner drug (ACTs). Despite these factors, malaria remains one of the most important febrile illnesses in tropical countries. In areas of a low, unstable intensity of transmission, people of all ages are affected, but malaria is a particular problem in those who are exposed to the disease because of their occupations. Interventions that have been proposed to reduce the risk of malaria in these high-risk groups include impregnated nets (7), hammocks (13), and other materials (2, 3, 8); insect repellents (9); and continuous antimalarial chemoprophylaxis. An alternative approach, developed originally for pregnant women in areas of high intensity of stable transmission, is the administration of an antimalarial treatment dose at regular intervals to exposed individuals, irrespective of parasitemia. Intermittent preventive treatment (IPT) is essentially intermittent chemoprophylaxis for which the duration of protection (often referred to as the posttreatment prophylactic effect) is critically dependent on the antimalarial drug efficacy and elimination kinetics. The antimalarial most studied for IPT is sulfadoxine-pyrimethamine (SP), which can be administered in a single dose and provides approximately 35 days of protection against malaria if the prevalent parasites are fully sensitive (14). SP is recommended in pregnancy and has been advocated for infants and children, but controversy remains over its utility because of widespread resistance and uncertainty regarding the levels of resistance or transmission intensities at which it should no longer be used. In addition, the timing of the administration (two to three times during pregnancy and at vaccination dates in infancy) does not provide complete protection against malaria, as there are long interdose intervals with subtherapeutic drug levels. Clearly, new drugs and new regimens are needed. Piperaquine has a long terminal elimination half-life (approximately 30 days), and it is currently used in a fixed combination with dihydroartemisinin (12). This ACT has shown excellent efficacy and tolerability, with a substantial posttreatment prophylactic effect against Plasmodium falciparum and P. vivax in studies in Asia and Africa (11, 15, 16). In areas of low, unstable intensities of transmission, young adult males tend to bear the brunt of malaria, as their occupations often involve travel and exposure to foci of transmission. We report here the results of a randomized, double-blind, placebo-controlled trial of two IPT regimens with dihydroartemisinin-piperaquine (DP) in men at high risk for malaria living on the Thai-Myanmar border.
MATERIALS AND METHODS
The northwestern border of Thailand with Myanmar is a hilly, forested area where transmission of both falciparum and vivax malaria is unstable, low, and seasonal. Adult males are more at risk of malaria than any other population group (1). P. falciparum in this area is multidrug resistant. Participants were recruited from the clinics of the Shoklo Malaria Research Unit (SMRU) in Tak Province, where Karen and Burmese migrant workers cross the border to seek medical care. All volunteers were eligible for enrollment in the study provided they met the following criteria: male, at least 18 years of age, willing to attend follow-up for 36 weeks, and providing written informed consent. Exclusion criteria included having malaria (any species) asexual-stage parasitemia, having had dihydroartemisinin-piperaquine treatment within the past 6 months, having had mefloquine treatment within the past 2 months, and having known hypersensitivity to artemisinins or piperaquine.
Drugs and dosages.
Dihydroartemisinin-piperaquine (Duo-Cotecxin; Holley-Cotec Pharmaceuticals, Beijing, China) was used. Each adult dose (3 tablets) contained 120 mg of dihydroartemisinin (DHQ) and 960 mg of piperaquine (PQ) (i.e., a 1:8 ratio). Identical DP and placebo tablets (Holley-Cotec Pharmaceuticals, Beijing, China) were made for the trial. The volunteers were randomized into 3 groups: (i) those receiving a monthly regimen (DPm), three tablets of DP daily for 3 days once a month; (ii) those receiving an alternate regimen (DPalt), three tablets of DP daily for 3 days for the first month followed by 3 tablets of placebo daily for 3 days for the following month (active drug and an identical placebo were given alternately for the duration of the study); and (iii) those receiving a placebo, three tablets of a placebo daily for 3 days once a month.
Patients and study design.
Meetings were held with the community leaders in the study area to explain the trial objectives and the role of participants. The annual incidence of falciparum malaria in the adult male community was estimated to be 25%. In order to detect a 40% reduction and assuming a 2:2:1 ratio with a two-sided alpha of 0.05 and 80% power, 400 volunteers were needed in each DP group and 200 in the placebo group. Randomization was computer generated in blocks of 20, and in each treatment group half of the patients were rerandomized to receive either water or a 200-ml carton of chocolate milk containing 6.4 g of fat to be taken with each dose. This was done to compare drug exposures of orally administered piperaquine with and without fat. The treatment regimens and placebo tablets were kept in sealed plastic bottles containing silica prepared in advance and were labeled on site with the randomized list of drug codes. Therefore, the study teams and participants were blind to treatment allocation. Patients were seen daily for 3 days during each monthly visit to the clinic for 36 weeks or until they had a malaria episode or left the study area. Patients were censored at the first episode of malaria for analysis so that one subject counted for one episode. Each month, the drug administration was supervised, and a venous blood sample was taken for measurement of the piperaquine plasma concentration and capillary blood was taken for hematocrit and a malaria blood smear. A symptom questionnaire was completed. The same procedure was repeated at each monthly visit. All concomitant medications taken in the intervening period were recorded. In between the monthly visits, trained workers visited each volunteer at home weekly. At each home visit, a short questionnaire was completed. Every 2 weeks, an additional blood smear and hematocrit were obtained. A dose was repeated in full if vomiting occurred within 30 min of administration, and a half dose was repeated if vomiting occurred between 30 min and 1 h postdosing. All events were documented on the case record form. Patients presenting to the clinic within the follow-up period with microscopically confirmed malaria had an additional venous blood sample taken for plasma piperaquine concentration. Patients with falciparum malaria were treated with the standard 3-day regimen of mefloquine (25 mg/kg total dose) and artesunate (12 mg/kg total dose). Patients with P. vivax, P. ovale, or P. malariae monoinfections were treated for 3 days with chloroquine (25 mg/kg base total dose; GPO, Thailand).
Translated written study information was provided in the languages of the participants. It was explained clearly that a decision not to participate in the trial would not affect availability of routine care at SMRU clinics. Ethical approval for the study was obtained from the Ethics Review Board of the Faculty of Tropical Medicine, Mahidol University, Bangkok, and the Oxford Tropical Research Ethics Committee (OXTREC), Oxford University. An independent Data Safety Monitoring Board monitored the trial.
Piperaquine drug measurements.
Plasma piperaquine concentrations were measured by the Clinical Pharmacology Laboratory, MORU, at the Faculty of Tropical Medicine, Mahidol University, Bangkok. The plasma samples were assayed using solid phase extraction (SPE) followed by liquid chromatography with detection by tandem mass spectrometry (MS/MS) (5). A stable isotope-labeled internal standard, D6-PQ, was used to compensate for any method variations. Quantification was performed using selected reaction monitoring (SRM) for the transitions m/z 535 to 288 for PQ and 541 to 294 for D6-PQ. The assay uses 50 μl of plasma and covers a range from 1.5 to 500 ng/ml. Performance during analysis was evaluated through analysis of three replicate quality control samples at three concentrations for each batch of samples. The total precision (relative standard error) for the quality controls (n = 237 at each concentration) were 3.77, 2.52, and 2.14% at 4.50, 20, and 400 ng/ml, respectively, for piperaquine.
Statistical analysis.
The primary end point was protective efficacy against all malaria at 36 weeks of follow-up. All malaria occurrences (any species) within 36 weeks of follow-up in the DPm, DPalt, and placebo groups were compared using the Kaplan-Meier method for survival analysis. Time to event was defined as the day of malaria occurrence; otherwise, participants were censored at the day last seen. Groups were compared using the log rank test or the Wilcoxon-Breslow test if the survival lines crossed. Attack rates were calculated for each treatment group as the number of malaria infections per person-year. In a subgroup analysis, efficacies in participants who received treatment with fat and those who received treatment without fat were compared. The risk of malaria was quantified using Cox regression, adjusted for age, and stratified by site. Proportional hazards were tested using Schoenfeld residuals. The overall model fit was visually confirmed by plotting the cumulative hazard versus the Cox-Snell residuals.
The number needed to treat (NNT) estimates the number of participants that would need to be given treatment for one to avoid malaria. This was calculated as 1/(difference in efficacy at week 36). Adverse events were summarized as frequencies (%) of patients reporting that event at least once during the follow-up period. For gametocytemia and adverse events, incidence rates were estimated. Gametocyte densities were calculated based on a count of gametocytes per 500 white blood cells in a thick film. Gametocytemia was defined as having any blood slide positive for gametocytes and was analyzed as a binary variable. Incidence was calculated as the number of participants with gametocytes (or an adverse event) over person-years at risk, which was defined as the total follow-up time for each treatment group. Due to small numbers, confidence intervals (CIs) for incidence were calculated using exact methods. To assess whether IPT with DP had an effect on hematocrit, the area under the hematocrit curve was calculated for each participant using the composite trapezoid rule (where each subinterval was defined by the time points at which hematocrit was measured). The area under each hematocrit curve was divided by the number of days enrolled in the study for each patient to adjust for differences in follow-up duration. Plasma piperaquine concentrations were compared in relation to efficacy outcome between the two drug-containing regimens and between subgroups dosed with or without fat (6.4 g/dose). All data were analyzed using Stata v11 (StataCorp).
RESULTS
One thousand adult males aged 18 to 70 years (median age, 32 years) were enrolled from October 2006 to June 2008. On a repeat check of enrollment blood smears, 39 participants (3.9%) were found to have low parasitemias and were excluded, leaving 961 participants for analysis (387 in DPm, 381 in DPalt, and 193 in the placebo group). Baseline characteristics were similar among groups (Table 1). A higher proportion of patients in the DPm group were lost during follow-up than were those in the DPalt and placebo groups (35% versus 24% and 25%, respectively; P = 0.003), although this was not related to adverse effects. Participants in the placebo group had a shorter median follow-up time (P = 0.0001) related to the higher number of breakthrough malaria infections in this group. Adherence to the study protocol was satisfactory throughout the trial; there were 47 (4.9%) participants lost by week 4 of follow-up and 276 (28.7%) by week 36 (Table 2). Among the cases lost during follow-up, 166 left the study area for work-related reasons, 34 because of political instability, and 76 for other reasons unrelated to the trial. Another 50 volunteers withdrew their consent, including 4 (2 from the DPalt group and 2 from the placebo group) who stopped their participation because of adverse effects. The mean daily dose of piperaquine was very similar in the DPm and the DPalt groups: 18.1 mg/kg (12.2 to 24.0 mg/kg; 95% CI, 17.9 to 18.3) and 18.1 mg/kg (12.8 to 24.0 mg/kg; 95% CI, 17.9 to 18.3), respectively. The proportion of patients that received a low dose of DP (<15 mg/kg/day of piperaquine [PIP] and <1.85 mg/kg/day of dihydroartemisinin [DHA]) was 5.21% (40/768), the proportion that received a dose within the expected range (15 to 20 and 1.85 to 2.5 mg/kg/day) was 75.7% (581/768), and the proportion that received a dose in the high range (≥20 and ≥2.5 mg/kg/day of PIP and DHA, respectively) was 19.1% (147/768).
Table 1.
Characteristics at baseline of volunteers allocated to different treatment regimens
| Characteristica | Treatment |
Total value for all groups | P valueb | ||
|---|---|---|---|---|---|
| DPm | DPalt | Placebo | |||
| No. of participants | |||||
| Per group | 387 | 381 | 193 | 961 | |
| Per site | |||||
| Mawker Thai | 105 | 103 | 51 | 259 | |
| Mun Ru Chai | 40 | 39 | 19 | 98 | |
| Mae Khon Ken | 210 | 207 | 108 | 525 | |
| Wang Pha | 32 | 32 | 15 | 79 | |
| Age (yr) | 0.15 | ||||
| Median | 30 | 34 | 33 | 32 | |
| Range | 18–70 | 18–68 | 18–70 | 18–70 | |
| Wt (kg) | 0.22 | ||||
| Mean | 53.8 | 53.7 | 52.9 | 53.6 | |
| SD | 6.09 | 6.16 | 6.81 | 6.27 | |
| Hematocrit (%) | 0.22 | ||||
| Mean | 42.7 | 42.9 | 43.4 | 42.9 | |
| 95% CI | 42.2–43.1 | 42.4–43.4 | 42.8–44.0 | 42.6–43.2 | |
| No. with anemia (%) | 2 (0.52) | 5 (1.32) | 1 (0.53) | 8 (0.84) | 0.53c |
| Temp (°C) | 0.97 | ||||
| Median | 36.2 | 36.2 | 36.2 | 36.2 | |
| IQR | 36.0–36.5 | 36.0–36.5 | 36.0–36.5 | 36.0–36.5 | |
| Systolic BP (mmHg) | 0.93 | ||||
| Median | 110 | 110 | 110 | 110 | |
| IQR | 100–120 | 100–120 | 100–120 | 100–120 | |
| Respiratory rate (breaths/min) | 20 | 20 | 20 | 20 | 0.90 |
| IQR | 20–22 | 20–22 | 20–22 | 20–22 | |
| No. with enlarged liver (%) | 5 (1.29) | 5 (1.31) | 4 (2.07) | 14 (1.46) | 0.73 |
| No. with enlarged spleen (%) | 8 (2.07) | 3 (0.79) | 2 (1.04) | 13 (1.35) | 0.33 |
Anemia was defined as having a hematocrit of <30%. BP, blood pressure.
Medians were determined by the Kruskal-Wallis test; categorical variables were determined by the chi-square or Fisher's exact test, as appropriate. Analysis of variance (ANOVA) was used for comparison of means.
There were 10 missing values for volunteers with anemia.
Table 2.
Characteristics at follow-up of volunteers allocated to different treatment regimens
| Treatment parameter | Treatment |
Total | P valuea | ||
|---|---|---|---|---|---|
| DPm | DPalt | Placebo | |||
| No. of times seen | 0.0001 | ||||
| Median | 15 | 15 | 8 | 14 | |
| IQR | 6–19 | 8–19 | 3–17 | 6–19 | |
| No. (%) lost at follow-up | 135 (34.9) | 93 (24.4) | 48 (24.8) | 276 (28.7) | 0.003 |
| Follow-up time (wk) | 0.0001 | ||||
| Median | 35.6 | 35.9 | 14.3 | 32 | |
| IQR | 12.6–36.0 | 16.0–36.0 | 6.00–36 | 10–36 | |
Determined by Kruskal-Wallis test.
Malaria.
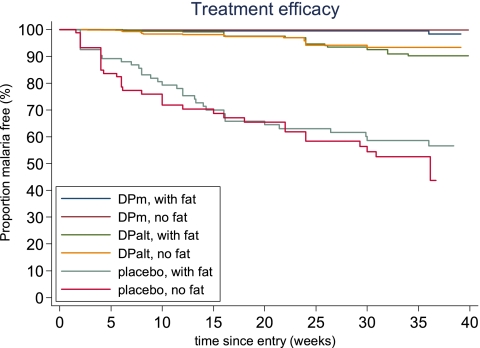
There were a total of 114 episodes of malaria (49 P. falciparum, 63 P. vivax, and 2 P. ovale). Placebo recipients had 69 episodes (including 13 symptomatic), and those receiving DP every 2 months (DPalt) had 40 episodes (11 symptomatic), giving a yearly incidence of 0.98 and 0.21 infections per person-year, respectively (P < 0.0001). The DPm recipients experienced significantly fewer episodes of malaria (n = 5 cases, 1 symptomatic; i.e., a yearly incidence of 0.03 infections per person-year; P < 0.0001) (Fig. 1 and Table 3). The protective efficacy against all malaria at 36 weeks was 98% (95% CI, 96 to 99%) in the DPm group and 86% (95% CI, 81% to 90%) in the DPalt group (P < 0.0001 for both compared to the placebo group). The corresponding figures for P. falciparum and P. vivax were 99% (96% to 99%) and 99.7% (98% to 100%) in the DPm group and 93% (89% to 96%) and 93% (89% to 95%) in the DPalt group, respectively. The protective efficacy of DP was not dependent on whether the treatment was taken with fat (comparison within each group, P ≥ 0.20 for all; Table 2). Thus, compared with participants receiving monthly DP dosing, participants who received dosing every 2 months were 8 times more likely to get malaria (adjusted hazards ratio [AHR], 8.24; 95% CI, 3.25 to 20.9), and participants in the placebo group were 41 times more likely to get malaria within 36 weeks (AHR, 41.3; 95% CI, 16.6 to 102.8) when results were adjusted for age and stratified by site (Table 3). The number needed to treat (95% CI) to prevent an additional case of malaria using the DPm regimen was 3 (1.87 to 3.0) compared to that for the placebo group, and 9 (5.68 to 14.3) compared to that for the DPalt group (Table 3). The hematocrit areas under the concentration-time curve (AUCs) during the study period were similar for participants in the DPm and DPalt groups (P = 0.48) but were significantly lower for those in the placebo group (for both P = 0.0001).
Fig 1.
Kaplan-Meier survival curves for malaria-preventive efficacy in the three groups. Dihydroartemisinin-piperaquine treatment doses (over 3 days) monthly (DPm) or every 2 months (DPalt) or an identical placebo were given with or without 6.4 g of fat for each dose administered.
Table 3.
Efficacies of antimalarial protection of DPm and DPalta
| Parameterb | Value(s) for indicated treatment regimen |
P value | ||
|---|---|---|---|---|
| DPm | DPalt | Placebo | ||
| Proportion (range) of participants without any malariac | 0.98 (0.96–0.99) | 0.86 (0.81–0.90) | 0.55 (0.46–0.63) | <0.0001 |
| No. of failures (no. who took the drug with fat) | 5 (4) | 40 (20) | 69 (34) | |
| AHR (95% CI) | Reference value | 8.24 (3.25–20.9) | 41.3 (16.6–102.8) | |
| NNT (95% CI) | 3 (1.87–3.0) | 9 (5.68–14.3) | Reference value | |
| Proportion (range) of participants without P. falciparum malariad | 0.99 (0.96–0.99) | 0.93 (0.89–0.96) | 0.79 (0.70–0.85) | <0.0001 |
| No. of failures (no. who took the drug with fat) | 4 (3) | 19 (8) | 26 (14) | |
| AHR (95% CI) | Reference value | 4.91 (1.67–14.5) | 20.6 (7.11–59.5) | |
| NNT (95% CI) | 5 (3.23–8.98) | 17 (9.9–74.1) | Reference value | |
| Proportion (range) of participants without P. vivax malariad | 0.99 (0.98–1.0) | 0.93 (0.89–0.95) | 0.71 (0.62–0.78) | <0.0001 |
| No. of failures (no. who took the drug with fat) | 1 (1) | 21 (12) | 41 (20) | |
| AHR (95% CI) | Reference value | 21.7 (2.92–161.3) | 119.3 (16.4–870.2) | |
| NNT (95% CI) | 4 (2.57–5.37) | 15 (8.55–29.7) | Reference value | |
Efficacies were assessed at the end of the trial (at 36 weeks) by Kaplan-Meier analysis.
For comparison of fat versus no fat received with treatment, P = 0.20 for DPm, P = 0.81 for DPalt, and P = 0.41 for the placebo group. Hazard ratios were adjusted for age and stratified by site. For AHRs and NNTs, DPm was compared with placebo and DPm with DPalt.
Includes P. falciparum, P. vivax, and P. ovale malaria. There were two failures, at weeks 10 and 18; both were in the placebo group and both had P. ovale.
Participants infected with all other malaria were censored at the time of infection.
Piperaquine plasma concentrations.
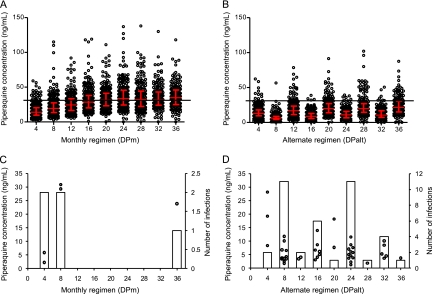
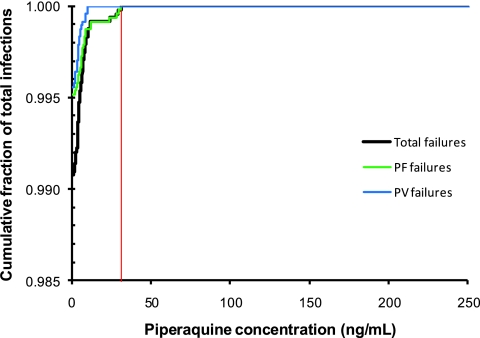
Plasma piperaquine trough concentrations increased throughout the study, with a mean (95% CI) accumulation from week 4 (first dose) to week 36 (end of study) of 336% (271 to 402) and 267% (146 to 381) for DPm and DPalt, respectively (Fig. 2A and B). Steady-state piperaquine plasma concentrations were reached after 4.5 months of treatment, at week 20. The piperaquine mean terminal elimination half-life was calculated as 40 days using the individual estimates from the DPalt group. Individual estimates were calculated using the slope of a line drawn between logarithmic trough concentrations the month when no drug was given and the subsequent month. There was no difference at any time point in piperaquine trough concentrations between the group that received DP with fat and the group that received DP without fat (data not shown). As expected, piperaquine trough concentrations were significantly (P < 0.0001) lower in the DPalt group than in the DPm group (Fig. 2A and B). Low plasma piperaquine concentrations were a significant predictor of the risk of malaria infection by logistic regression (P < 0.0001). All subjects who developed malaria had plasma piperaquine concentrations below 31 ng/ml (<11 ng/ml for vivax malaria and <31 ng/ml for falciparum malaria) (Fig. 3). Median trough values (interquartile range [IQR]) at the last appointment before malaria were significantly lower than all other trough values (5.6 [3.71 to 8.46] versus 18.8 [11.2 to 29.9] ng/ml; P = 0.0001). There was a clear pattern, with more infections appearing during the month without drug administration in the DPalt group (Fig. 2C and D). These accounted for 87% (34/39) of all infections in the DPalt arm. A higher proportion of all subjects in the DPalt group had piperaquine concentrations below 31 ng/ml during the month without drug administration (98.1%) than that in the month after drug administration (87.3%).
Fig 2.
Plasma piperaquine trough concentrations and incidences of malaria infections. Graphs show individual piperaquine trough concentrations (open circles) with median values and interquartile range (red bars) for DPm (A) and DPalt (B). The solid black horizontal line indicates the piperaquine cutoff concentration of 31 ng/ml above which there were no malaria infections. Five data points in the DPm group (167 ng/ml at week 12, 204 ng/ml at week 24, 167 ng/ml and 180 ng/ml at week 32, 251 ng/ml at week 36) are outside the axis limits. (C and D) Individual piperaquine trough concentrations for subjects that developed a new malaria infection (open circles) are shown on the primary y axis, and the total number of malaria infections for that period (open bars) are shown on the secondary y axis.
Fig 3.
Cumulative fraction of total malaria infections versus piperaquine trough concentrations. The solid red line indicates the trough plasma piperaquine cut-off concentration of 31 ng/ml, above which there were no malaria infections.
Gametocytemia.
Gametocytemia was detected during follow-up in 24 participants. One participant from the DPm group presented with P. vivax and gametocytemia at day 28. Seven gametocytemic participants (5 with P. vivax and 2 with P. falciparum) were in the DPalt group, and 16 were in the placebo group (2 with P. ovale, 12 with P. vivax, and 2 with P. falciparum). The incidences of gametocytemia were not significantly different in the DPm and DPalt groups (0.005 versus 0.04 per person-year, respectively; incidence rate ratio [IRR], 6.96; 95% CI, 0.89 to 314). Gametocytemia was 43 times greater in the placebo group than in the DPm group (0.23 per person-year; IRR, 43.5; 95% CI, 6.76 to 1823).
Adverse events.
Of the 18,210 occasions the volunteers took their doses of three tablets, only one patient vomited on one occasion. Overall, 300 participants (31.2%) reported no adverse events during the entire course of the study. The proportions of participants reporting at least one adverse event at any time during the study were similar for participants in the DPm group and the DPalt group (IRR, 1.02; 95% CI, 0.86 to 1.22) and for participants in the placebo group (IRR, 1.16; 95% CI, 0.93 to 1.45). When the 20 most frequently reported adverse events were adjusted for follow-up time, the only difference observed was an increased risk of joint pain reported by participants in the placebo group (Table 4). For all groups, the median number of different adverse events (IQR) reported (from those listed in Table 4) was 1 (0 to 3). One volunteer died of a land mine injury. There was no other serious adverse event reported.
Table 4.
Frequency, incidence, and risk of the 20 most frequently reported adverse events
| Adverse event | No. of participants in indicated treatment group who reported adverse event (%)a |
Incidence rate for indicated groupb |
IRR (95% CI) for DPm and DPalt | IRR (95% CI) for DPm and placebo | ||||
|---|---|---|---|---|---|---|---|---|
| DPm | DPalt | Placebo | DPm | DPalt | Placebo | |||
| Dizziness | 127 (32.8) | 119 (31.2) | 49 (25.4) | 0.66 | 0.62 | 0.69 | 0.93 (0.72–1.21) | 1.05 (0.74–1.47) |
| Headache | 115 (29.7) | 108 (28.4) | 49 (25.4) | 0.60 | 0.56 | 0.69 | 0.93 (0.71–1.23) | 1.16 (0.81–1.63) |
| Soft stool | 99 (25.6) | 82 (21.5) | 29 (15.0) | 0.52 | 0.42 | 0.41 | 0.82 (0.61–1.12) | 0.80 (0.51–1.22) |
| Abdominal pain | 67 (17.3) | 57 (15.0) | 28 (14.5) | 0.35 | 0.30 | 0.40 | 0.85 (0.58–1.22) | 1.14 (0.70–1.79) |
| Muscle pain | 65 (16.8) | 63 (16.5) | 35 (18.1) | 0.34 | 0.33 | 0.50 | 0.96 (0.67–1.38) | 1.46 (0.94–2.24) |
| Fever | 47 (12.1) | 50 (13.1) | 27 (14.0) | 0.24 | 0.26 | 0.38 | 1.06 (0.70–1.61) | 1.56 (0.94–2.56) |
| Cough | 44 (11.4) | 42 (11.9) | 17 (8.81) | 0.23 | 0.22 | 0.24 | 0.95 (0.61–1.48) | 1.05 (0.56–1.88) |
| Joint pain | 40 (10.3) | 50 (13.1) | 30 (15.5) | 0.21 | 0.26 | 0.42 | 1.24 (0.80–1.93) | 2.04 (1.23–3.36) |
| Dry mouth | 33 (8.53) | 33 (8.66) | 11 (5.70) | 0.17 | 0.17 | 0.16 | 0.99 (0.60–1.66) | 0.91 (0.41–1.84) |
| Insomnia | 34 (8.79) | 36 (9.45) | 17 (8.81) | 0.18 | 0.19 | 0.24 | 1.05 (0.64–1.73) | 1.36 (0.71–2.50) |
| Sleep disturbance. | 32 (8.27) | 33 (8.66) | 14 (7.25) | 0.17 | 0.17 | 0.20 | 1.03 (0.61–1.72) | 1.19 (0.59–2.29) |
| Anorexia | 24 (6.20) | 26 (6.82) | 10 (5.18) | 0.13 | 0.13 | 0.14 | 1.08 (0.59–1.96) | 1.13 (0.48–2.46) |
| Nausea | 22 (5.68) | 24 (6.30) | 11 (5.70) | 0.11 | 0.12 | 0.16 | 1.09 (0.58–2.03) | 1.36 (0.60–2.92) |
| Diarrhea | 20 (5.17) | 24 (6.30) | 5 (2.59) | 0.10 | 0.12 | 0.07 | 1.19 (0.63–2.28) | 0.68 (0.20–1.87) |
| Itching | 15 (3.88) | 8 (2.10) | 7 (3.63) | 0.08 | 0.04 | 0.10 | 0.53 (0.19–1.33) | 1.27 (0.44–3.31) |
| Vomiting | 15 (3.88) | 10 (2.62) | 3 (1.55) | 0.08 | 0.05 | 0.04 | 0.66 (0.27–1.58) | 0.54 (0.10–1.92) |
| Fatigue | 15 (3.88) | 17 (4.46) | 11 (5.70) | 0.08 | 0.09 | 0.16 | 1.13 (0.53–2.42) | 1.99 (0.83–4.65) |
| Skin rash | 4 (1.03) | 4 (1.05) | 2 (1.04) | 0.02 | 0.02 | 0.03 | 0.99 (0.19–5.34) | 1.36 (0.12–9.48) |
| Palpitation | 3 (0.78) | 5 (1.31) | 3 (1.55) | 0.02 | 0.03 | 0.04 | 1.66 (0.32–10.7) | 2.72 (0.36–20.3) |
| Back pain | 2 (0.52) | 4 (1.05) | 2 (1.04) | 0.01 | 0.02 | 0.03 | 1.99 (0.29–22.0) | 2.72 (0.20–37.5) |
Number of participants who reported an adverse event at least once.
Per person-year at risk.
DISCUSSION
Intermittent preventive treatment or seasonal malaria chemoprevention has become an important component of the control of malaria in countries in which malaria is endemic where transmission intensities are continuously or seasonally high but has not been evaluated previously in settings where transmission is low. To prevent malaria, IPT must first eliminate any parasite in the host (presumptive treatment) and then provide circulating drug levels above the MICs for prevalent parasites to prevent the multiplication of newly acquired parasites during the time period before the next dosing (preventive or posttreatment prophylactic effect [PTP]). Obviously, the efficacy of a drug used in IPT will depend both on the sensitivity of the prevalent parasites to this drug and on its pharmacokinetic properties. A rapidly eliminated drug provides a brief PTP and so must be given more frequently, while a drug with a long terminal elimination half-life can be administered less frequently. Other factors, such as absorption and tolerability, are also important. The majority of published studies on IPT, whether in pregnancy or in children, have been conducted in Africa using SP. The protective effect of SP reported has been variable, depending on the primary end point used (clinical malaria, parasitemia, low birth weight in pregnancy), the frequency of administration, and the degree of resistance of the local parasites (mainly P. falciparum). In many areas, such as Southeast Asia, high levels of resistance to SP preclude its use. In this trial, conducted in an area of high-level drug resistance, we set out to determine the optimum regimen of dihydroartemisinin-piperaquine for use in IPT in order to achieve protection against malaria (any species). In this area, the target population (adult males) is particularly exposed to malaria by their occupation. The men that received monthly DP experienced slightly more gastrointestinal side effects, but these were mild, did not warrant interruption or specific treatment, and were not significantly different among the treatment groups. The monthly administration of a treatment dose of dihydroartemisinin-piperaquine was highly effective against multidrug-resistant P. falciparum and against increasingly chloroquine-resistant P. vivax malaria. In the DPm group, four out of five episodes of parasitemia occurred in the first 2 months, before drug levels had reached steady state. These parasitological failures were all in volunteers who had plasma piperaquine concentrations below 31 ng/ml for P. falciparum failures and below 11 ng/ml for P. vivax failures (Fig. 2A and B). In the DPalt group, there was a clear relationship between piperaquine concentration, parasitemic episode, and the second month between active drug dosing (Fig. 2C and D). This was to be expected, since the estimated terminal elimination half-life of piperaquine is 30 days; i.e., concentrations 2 months after dosing are approximately 50% lower than those 1 month after dosing. The estimated mean terminal elimination half-life in subjects receiving DP every 2 months was 40 days, which is slightly longer than previously published values (4, 12). Although fat has been reported to increase the oral bioavailability of piperaquine (6, 10), fat coadministration had no effect on piperaquine concentrations and therefore on antimalarial efficacy in this study. The most important determinant of protective efficacy was the trough plasma concentration of piperaquine, and this was determined by the dosing frequency. The drug concentrations required for successful prevention of malaria infections are not maintained when the doses are given every 2 months. This suggests that for effective prevention of malaria, DP should be given monthly. There are limitations to this study. First, this was a closely supervised trial, so all doses were given under supervision, but the loss of participants during follow-up was relatively high (≈30%). It is likely that outside of the research context, a lower adherence would be obtained, since the treatment requires 3 days of self-administration. Second, the study was conducted in adult males, and it is possible that the kinetics of piperaquine would be different in children and in pregnant women. Third, the design of the study (malaria cases were censored at first occurrence) means that the differences among the three groups were probably underestimated. Nevertheless, monthly DP proved to be a well-tolerated, safe, and effective method of preventing malaria in adults at high risk. Further studies will be needed to determine the doses needed in pregnancy and in children to achieve these drug concentrations.
ACKNOWLEDGMENTS
We thank the patients who participated in the study and the staff of SMRU as well as the members of the Data Safety Monitoring Board.
Holley Cotec Pharmaceuticals (PRC) supported the study but took no part in the design or conduct of the trial or in analysis and interpretation. SMRU is part of the Mahidol Oxford University Research Unit, Mahidol University, Bangkok, Thailand, supported by the Wellcome Trust of Great Britain.
This study has been registered in the ISRCTN Register under controlled trial no. ISRCTN65524939.
Footnotes
Published ahead of print 17 January 2012
REFERENCES
- 1. Carrara VI, et al. 2009. Changes in the treatment responses to artesunate-mefloquine on the northwestern border of Thailand during 13 years of continuous deployment. PLoS One 4:e4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Graham K, et al. 2002. Comparison of three pyrethroid treatments of top-sheets for malaria control in emergencies: entomological and user acceptance studies in an Afghan refugee camp in Pakistan. Med. Vet. Entomol. 16:199–206 [DOI] [PubMed] [Google Scholar]
- 3. Graham K, et al. 2002. Insecticide-treated plastic tarpaulins for control of malaria vectors in refugee camps. Med. Vet. Entomol. 16:404–408 [DOI] [PubMed] [Google Scholar]
- 4. Hung TY, et al. 2004. Population pharmacokinetics of piperaquine in adults and children with uncomplicated falciparum or vivax malaria. Br. J. Clin. Pharmacol. 57:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lindegardh N, Annerberg A, White NJ, Day NP. 2008. Development and validation of a liquid chromatographic-tandem mass spectrometric method for determination of piperaquine in plasma stable isotope labeled internal standard does not always compensate for matrix effects. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 862:227–236 [DOI] [PubMed] [Google Scholar]
- 6. Nguyen TC, et al. 2008. Pharmacokinetics of the antimalarial drug piperaquine in healthy Vietnamese subjects. Am. J. Trop. Med. Hyg. 79:620–623 [PubMed] [Google Scholar]
- 7. Phillips-Howard PA, et al. 2003. The efficacy of permethrin-treated bed nets on child mortality and morbidity in western Kenya I. Development of infrastructure and description of study site. Am. J. Trop. Med. Hyg. 68(Suppl. 4):3–9 [PubMed] [Google Scholar]
- 8. Rowland M, et al. 1999. Permethrin-treated chaddars and top-sheets: appropriate technology for protection against malaria in Afghanistan and other complex emergencies. Trans. R. Soc. Trop. Med. Hyg. 93:465–472 [DOI] [PubMed] [Google Scholar]
- 9. Rowland M, et al. 2004. DEET mosquito repellent provides personal protection against malaria: a household randomized trial in an Afghan refugee camp in Pakistan. Trop. Med. Int. Health 9:335–342 [DOI] [PubMed] [Google Scholar]
- 10. Sim IK, Davis TM, Ilett KF. 2005. Effects of a high-fat meal on the relative oral bioavailability of piperaquine. Antimicrob. Agents Chemother. 49:2407–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smithuis F, et al. 2010. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect. Dis. 10:673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tarning J, et al. 2008. Population pharmacokinetics of piperaquine after two different treatment regimens with dihydroartemisinin-piperaquine in patients with Plasmodium falciparum malaria in Thailand. Antimicrob. Agents Chemother. 52:1052–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thang ND, et al. 2009. Long-lasting insecticidal hammocks for controlling forest malaria: a community-based trial in a rural area of central Vietnam. PLoS One 4:e7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White NJ. 2005. Intermittent presumptive treatment for malaria. PLoS Med. 2:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zongo I, et al. 2007. Randomized comparison of amodiaquine plus sulfadoxine-pyrimethamine, artemether-lumefantrine, and dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in Burkina Faso. Clin. Infect. Dis. 45:1453–1461 [DOI] [PubMed] [Google Scholar]
- 16. Zwang J, et al. 2009. Safety and efficacy of dihydroartemisinin-piperaquine in falciparum malaria: a prospective multi-centre individual patient data analysis. PLoS One 4:e6358. [DOI] [PMC free article] [PubMed] [Google Scholar]