Abstract
A total of 299 nares and 194 blood isolates of methicillin-resistant Staphylococcus aureus (MRSA), each recovered from a unique patient, were collected from 23 U.S. hospitals from May 2009 to March 2010. All isolates underwent spa and staphylococcal cassette chromosome mec element (SCCmec) typing and antimicrobial susceptibility testing; a subset of 84 isolates was typed by pulsed-field gel electrophoresis (PFGE) using SmaI. Seventy-six spa types were observed among the isolates. Overall, for nasal isolates, spa type t002-SCCmec type II (USA100) was the most common strain type (37% of isolates), while among blood isolates, spa type t008-SCCmec type IV (USA300) was the most common (39%). However, the proportion of all USA100 and USA300 isolates varied by United States census region. Nasal isolates were more resistant to tobramycin and clindamycin than blood isolates (55.9% and 48.8% of isolates versus 36.6% and 39.7%, respectively; for both, P < 0.05). The USA300 isolates were largely resistant to fluoroquinolones. High-level mupirocin resistance was low among all spa types (<5%). SCCmec types III and VIII, which are rare in the United States, were observed along with several unusual PFGE types, including CMRSA9, EMRSA15, and the PFGE profile associated with sequence type 239 (ST239) isolates. Typing data from this convenience sample suggest that in U.S. hospitalized patients, USA100 isolates of multiple spa types, while still common in the nares, have been replaced by USA300 isolates as the predominant MRSA strain type in positive blood cultures.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) isolates continue to be a global cause of both health care- and community-associated infections (12, 20, 24). Multiple clones of MRSA have been recognized and are circulating worldwide, although strains of clonal complex 5 (CC5) and CC8 are the largest and most diverse (16, 39). Population-based studies of invasive MRSA infections in the United States by using the Centers for Disease Control and Prevention's Active Bacterial Core Surveillance (ABCs) system indicate that pulsed-field gel electrophoresis (PFGE) type USA100 (multilocus sequence type 5 [ST5], CC5) is the predominant strain type observed, although these and other studies indicate that USA300 isolates (ST8, CC8) are increasing in frequency as a cause of health care-associated bacteremia (29, 30, 32, 42). Similarly, a population-based study of nasal colonization of noninstitutionalized persons in the United States (i.e., those not in hospitals, other health care facilities, prisons, or other institutions) also showed that USA100 was the most common MRSA strain type observed (22, 47). However, over the 4-year study period (2001 to 2004), there was a significant increase in the proportion of individuals harboring USA300 isolates in their nares (22). In addition, more recent data indicate that USA300 isolates are becoming a common cause of nasal carriage among U.S. hospitalized patients, especially in the southern United States (17), and continue to be a significant cause of community-acquired skin infections (35) and skin disease in long-term care facilities (45). A model of invasive MRSA infections reported by D'Agata et al. indicated that MRSA isolates of PFGE type (PFT) USA300 were likely to increase in frequency in the United States and replace USA100 isolates as the most common cause of invasive MRSA infections in the future (11). Such isolates were hypothesized in the model, based on published literature, to show increased virulence relative to USA100 strains and required both enhanced hand washing and active surveillance for control.
Although PFGE has been the gold standard for typing of MRSA in the United States for many years (34) and a protocol for PFGE typing was established in 2003 for use in European laboratories (36), strain typing based on DNA sequence analysis of the repeated units of the staphylococcal protein A gene (i.e., spa typing) is now used much more commonly around the world for epidemiologic studies of MRSA (21, 24, 26). However, there are relatively few studies of the distribution of MRSA spa types among U.S. isolates, which makes comparisons of MRSA strain types in the United States to those in other countries, such as the most recent European MRSA surveillance data (24), difficult.
Accordingly, the goals of this study were to (i) determine the spa types of MRSA strains that colonize the nares and invade the bloodstream of patients in U.S. hospitals, (ii) translate the spa types of the MRSA strains into PFGE types, either by parallel testing of the isolates or by prediction of the PFGE types using information available both from the Ridom Spaserver website and published studies, (iii) compare the MRSA strain typing data from this survey to previous U.S. data from the National Health and Nutrition Examination Survey (NHANES) (22, 47), the ABCs data for invasive MRSA isolates (30, 32), and recent European surveillance data (24), and (iv) determine if there are differences in the antimicrobial susceptibility patterns of the MRSA strains isolated from nasal and blood cultures.
(These results were presented in part at the 50th Interscience Conference on Antimicrobial Agents and Chemotherapy 20 to 25 September, 2010, Boston, MA.)
MATERIALS AND METHODS
Bacterial isolates.
A total of 493 MRSA isolates (299 nares isolates and 194 blood isolates), collected from 10 to 20 unique patients (but without specific selection criteria) from 23 U.S. laboratories from May 2009 to March 2010 were studied. All isolates were deidentified prior to shipment so that they could not be linked to specific patients. The isolates were confirmed at Cepheid as MRSA by broth microdilution susceptibility testing using oxacillin (see below), Gram stain, catalase, and coagulase testing and by additional biochemical testing when necessary (3).
Antimicrobial susceptibility testing.
Antimicrobial susceptibility testing was performed using the Clinical and Laboratory Standards Institute (CLSI) broth microdilution method (9). Mueller-Hinton broth was obtained from Becton Dickinson (Sparks, MD), and cation concentrations were adjusted following CLSI guidelines (9). After inoculation, MIC trays were incubated at 35°C in ambient air for 18 to 24 h. Antimicrobial agent powders were obtained from Sigma-Aldrich (St. Louis, MO) or directly from the antimicrobial agent manufacturers. Microdilution wells containing daptomycin were supplemented with calcium to a final concentration of 50 μg/ml as described by CLSI, and wells containing oxacillin were supplemented with NaCl to a final concentration of 2% (9, 48). Selected isolates with borderline-susceptible results to linezolid and daptomycin were tested using Etest strips (bioMérieux, Durham, NC) as described by the manufacturer. Testing was performed on Mueller-Hinton agar plates obtained from Remel (Lenexa, KS) and interpreted as previously described (49). Tigecycline interpretive criteria were those established for staphylococci by the U.S. Food and Drug Administration, i.e., ≤0.5 μg/ml indicating susceptibility (no intermediate or resistant breakpoints were defined) (http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021821lbl.pdf, accessed on 8 September 2011). Quality control strains included S. aureus ATCC 29213 and Enterococcus faecalis ATCC 29212 (10). Mueller-Hinton plates with Etest strips were incubated in ambient air at 35°C for 24 h. A subset of the combined MIC results for nasal and blood isolates, along with data on mechanisms of aminoglycoside resistance (not discussed here), have been published previously (48).
Strain typing methods.
Isolates underwent spa (44) and SCCmec typing (7) and were tested for genes encoding Panton-Valentine leucocidin (PVL) (41), arginine catabolic metabolic element (ACME) (15), and the high-level mupirocin resistance gene, mupA (1). A subset of 84 isolates representing common spa types and all isolates with unusual or rare spa types, were typed by pulsed-field gel electrophoresis using SmaI as previously described (20). spa types were assigned to PFGE types (PFTs) based on parallel testing of isolates with both methods, data from the Ridom SpaServer website (http://spaserver2.ridom.de/spaserver/query, accessed 1 June, 2011), and published literature (20, 21). Strains with the same spa type and antibiograms, particularly those that were from the same laboratory, were not typed by PFGE. Simpson's index of diversity was calculated using programs available at http://darwin.phyloviz.net/ComparingPartitions/index.php?link=Tool.
Minimum spanning tree analysis.
Cluster analysis of spa sequences was performed using the spa typing plug-in tool of the BioNumerics software program (version 6.5; Applied Maths, Ghent, Belgium). The analysis compares and aligns sequences via an algorithm based on potential tandem spa repeat duplications, substitutions, and indels (the DSI model) (4). A minimum spanning tree (MST) was generated from the similarity matrix with the root node assigned to the spa sequence type with the greatest number of related types. The default software parameters were used for analysis with a bin distance of 1.0%. Thus, the distance between spa types of 99% to 100% similarity was 0, 98% to 99% similarity was assigned a distance of 1, etc., on the MST. For cluster analysis, only spa types separated by an MST distance of ≤2 (i.e., if they were ≥97% similar) were considered closely related and assigned to the same cluster.
Statistical methods.
Fisher's exact test was used to compare the rates of resistance between nasal and blood isolates.
RESULTS
Typing results.
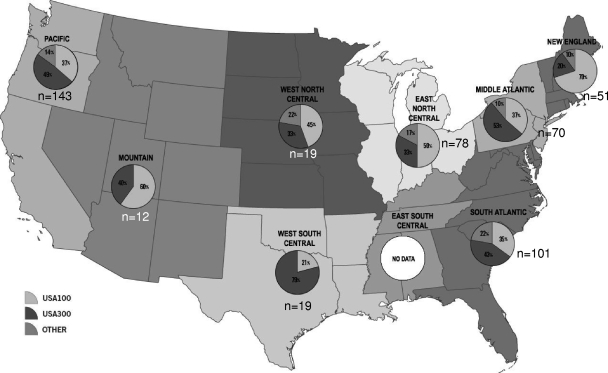
The spa types (using Ridom nomenclature) and associated SCCmec types for the 299 nares isolates and 194 blood isolates are shown in Table 1. (The eGenomics spa types corresponding to the Ridom spa types are listed in Table S1 in the supplemental material.) Among blood isolates, spa type t008-SCCmec IV (which showed PFT USA300 patterns) was the most common strain type observed (n = 76), followed by t002-SCCmec II (which showed PFT USA100 patterns) (n = 43). Overall, the spa types of blood isolates associated with USA300 (n = 93) encompassed 48% of the isolates and were more common than those associated with USA100 (n = 69, 36% of isolates). In addition, there were eight spa type t064-SCCmec IV isolates of PFT USA500 and two isolates (spa types t008 and t197) that were PFT CMRSA9 (Canadian nomenclature [21]), both of which carried SCCmec VIII. These two isolates were from two different cities in Florida. Two isolates of spa type t037-SCCmec III demonstrating PFTs consistent with ST239 were isolated from California and Indiana, and one spa type t020-SCCmec IV isolate of PFT EMRSA15 was detected from California. Among blood isolates, the proportion of spa types corresponding to USA100 and USA300 varied by U.S. census region (Fig. 1). USA300 was the most common PFT in the Middle Atlantic (data from 4 laboratories), South Atlantic (data from 5 laboratories), Pacific (data from 7 laboratories), and West South Central (data from 1 laboratory) regions, while USA100 strains were the predominant type in New England (data from 2 laboratories), East North Central (data from 2 laboratories), West North Central (data from 1 laboratory), and Mountain (data from 1 laboratory) regions. No data from the East South Central region were available.
Table 1.
spa, SCCmec, and pulsed-field gel electrophoresis types of study isolatesa
| PFGE type | Blood |
Nares |
||
|---|---|---|---|---|
| spa type-SCCmec (n)b | Total no. of isolates (194) | spa type-SCCmec (n) | Total no. of isolates (299) | |
| USA100 | t002-II (43), t002-NT (1), t045-II (4), t062-II (1), t062-IV (1), t067-II (1), t088-II (1), t214-II (1), t242-II (9), t242-NT (1), t688-II (1), t985-II (1), t1062-II (1), t2358-II (1), t2666-II (1), t6778-II (1) | 69 | t002-II (110), t002-NT (5), t010-II (1), t045-II (5), t062-II (1), t067-II (4), t071-II (1), t088-II (3), t105-II (3), t105-NT (4), t242-II (15), t242-NT (5), t509-II (2), t535-II (1), t539-II (2), t548-II (1), t548-NT (1), t570-II (1), t586-II (1), t837-II (1), t895-II (1), t1220-II (1), t1567-II (1), t2666-II (1), t3557-II (1), t3558-II (1), t4371-II (1), t4916-II (1), t4963-II (1), t5081-II (1), t-6778-II (1) | 178 |
| USA200 | None observed | 0 | t021-IV (1) | 1 |
| USA300 | t008-IV (76), t008-NT (2), t024-IV (1), t051-IV (1), t304-IV (2), t334-IV (1), t622-IV (1), t648-IV (1), t1617-IV (1), t2743-IV (2), t3908-IV (1), t4069-IV (2), t4229-IV (1), t6774-IV (1) | 93 | t008-IV (60), t008-NT (3), t024-IV (3), t190-IV (1), t211-IV (1), t334-II (1), t622-IV (1), t681-IV (1), t951-IV (1), t1779-IV (1), t6127-IV (1), t6774-IV (1) | 75 |
| USA400 | t127-IV (1) | 1 | t1178-IV (1) | 1 |
| USA500 | t064-IV (8) | 8 | t064-IV (8); t451-IV (1) | 9 |
| USA700 | None observed | 0 | t148-IV (1), t148-NT (1) | 2 |
| USA800 | t002-IV (3), t062-IV (1), t1265-IV (1) | 5 | t002-IV (12), t088-IV (3), t111-IV (1), t306-IV (1), t1265-IV (2), t5213-IV (1) | 20 |
| USA1000 | t216-IV (1) | 1 | t216-IV (1), t316-IV (1), t437-IV (1), t976-NT (1) | 4 |
| CMRSA9 | t008-VIII (1), t197-VIII (1) | 2 | t008-VIII (1) | 1 |
| EMRSA15 | t020-IV (1) | 1 | t032-IV (1) | 1 |
| ST239-associated pattern | t037-III (2) | 2 | t037-III (2) | 2 |
| Unnamedc | Unnamed-II (1), unnamed-IV (1), t189-IV (1), t668-II (5), t6770-II (2), t6771-IV (1), t6775-II (1) | 12 | Unnamed-II (1), unnamed-IV (2), t450-II (1), t579-II (1) | 5 |
PFGE types were determined directly for 84 isolates, including all unusual spa types, and on representative isolates of clusters of similar isolates (same spa type and antibiograms) from the same laboratory.
NT, not typeable.
These PFGE profiles have not been described previously.
Fig 1.
Proportions of USA100, USA300, and other pulsed-field types from blood culture isolates delineated by U.S. census region. The percentages of USA100 isolates are indicated in light gray, the percentages of USA300 isolates are indicated in dark gray, and other pulsed-field types are indicated in the intermediate gray color. The numbers of isolates obtained from each region are also shown.
Among nasal isolates, spa type t002-SCCmec II (USA100) was the most common strain type (n = 110), followed by t008-SCCmec IV (USA300) (n = 60), t002-SCCmec IV (n = 12) (USA800), and t064-SCCmec IV (n = 8) (USA500). Overall, strains with the PFT USA100, which encompassed 29 unique spa types, were most common (n = 178), encompassing 60% of the isolates. The second most common PFT among nasal isolates was USA300 (n = 75, 25% of isolates). One t008-SCCmec VIII isolate from California (PFT CMRSA9) and two t037-SCCmec III isolates (with PFT ST239 from Washington and California), as well as a t032-SCCmec IV (EMRSA15) isolate from Illinois, were also observed among nasal isolates. For nasal isolates, the USA100 PFT was predominant in each of the census regions (data not shown).
Three spa types—t002, t062, and t088—were shared among USA100 and USA800 isolates. The isolates were assigned to PFT USA100 or USA800 (both of which are typically in multilocus sequence typing [MLST] type CC5) primarily based on the PFGE banding pattern but also with consideration of the SCCmec type (SCCmec II is usually present in USA100 and SCCmec IV is usually present in USA800). The antibiograms of the isolate were also considered and in each case were consistent with either USA800 (i.e., susceptible to tobramycin, clindamycin, and ciprofloxacin) or USA100 (i.e., resistant to those antimicrobial agents) (34). Most of the spa t008-SCCmec IV strains (whether from blood or nasal cultures) were positive by PCR for the genes encoding PVL and ACME, consistent with USA300 strains (14, 34, 46). Only three non-USA300 isolates, including one spa t064-SCCmec IV-USA500 blood isolate and two nasal isolates with SCCmec IV but novel spa and PFGE types, were positive for both PVL and ACME. ACME (but not PVL) was also present in six spa t242-USA100 nasal isolates.
Index of diversity and minimum spanning tree.
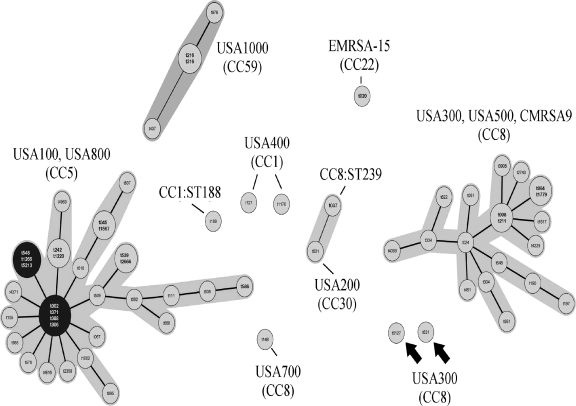
Simpson's index of diversity was 0.792 for spa typing and 0.637 for PFGE typing, confirming the greater diversity recognized by spa typing. A minimum spanning tree was constructed for all 493 isolates in order to visualize the relationships among the spa types (Fig. 2). Two major clusters were observed. The first is a cluster of CC5-related spa types corresponding primarily to PFTs USA100 and USA800. There are seven potential spa type founders listed in the two black circles on the left of the figure. The program could not identify a unique founder strain. This large cluster contains strains of SCCmec types II and IV. The CC8 cluster on the right contains multiple PFTs, including USA300, USA500, and CMRSA9, most of which contain SCCmec IV; however, the CMRSA9 strains (spa type t008) contain SCCmec VIII, as noted previously. Two isolates of spa types t6127 and t631 (indicated by arrows in the center of the figure) demonstrated PFGE profiles similar to USA300 but did not cluster with the other USA300 isolates among the CC8 strains. Other spa types showed minimal clustering. The overall clustering of the spa types is consistent with previously published data (21).
Fig 2.
Minimum spanning tree of spa typing data. PFTs and MLST clonal complexes are listed based on the Ridom SpaServer website (http://spaserver2.ridom.de/spaserver/query, accessed 1 June 2011). There is no PFT defined for the ST188 strain. Founder spa types are indicated in black circles. No founder could be assigned for the CC8 cluster. Arrows indicate two unique USA300 isolates (spa types t6127 and t631) which fell outside the CC8 cluster.
Antimicrobial susceptibility testing.
The results of antimicrobial susceptibility testing are shown in Table 2. Nasal MRSA isolates were more resistant to levofloxacin (81.3% versus 76.8%; P < 0.01) and tobramycin (55.9% versus 36.6%; P < 0.001) and showed more inducible clindamycin resistance (i.e., were D-zone test positive) than blood isolates (67.6% versus 48.5%; P < 0.001). Constitutive clindamycin resistance, although higher among nasal isolates, was of borderline significance (P = 0.052). These figures likely reflect the predominance of USA100 strains among the nasal isolates. However, 65.7% of t008-IV isolates (USA300-like) were ciprofloxacin resistant. High-level mupirocin resistance was low among MRSA isolates from both specimen types (3.3% for nasal isolates and 5.2% for blood isolates); all mupirocin-resistant isolates were positive for mupA. spa types associated with USA100 strains displayed antimicrobial resistance patterns typical of USA100, including resistance to erythromycin (98%), tobramycin (87%), clindamycin (both constitutive and inducible, 97%), and fluoroquinolones (both ciprofloxacin and levofloxacin resistance proportions were >98% resistant, whereas moxifloxacin resistance was 93%). USA300 strains, traditionally resistant only to β-lactam and macrolide agents (34), showed increased resistance to tobramycin (36.6%) and fluoroquinolones (64% resistant to ciprofloxacin and 58% resistant to levofloxacin, although only 21.5% were resistant to moxifloxacin). There were no significant differences between the antibiograms of the same spa types from nares and blood sources, with the exception of clindamycin resistance, which was significantly different for USA100 isolates from the nares (12%) versus those from blood cultures (8%, P = 0.003) (data not shown). No daptomycin resistance or linezolid nonsusceptible phenotypes were observed among the isolates.
Table 2.
Activity of selected antimicrobial agents against recent isolates of MRSA from the U.S. hospitals
| Drug | Nasal isolates |
Blood isolates |
P valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC |
% resistant | MIC |
% resistant | ||||||
| 50% | 90% | Range | 50% | 90% | Range | ||||
| Amikacin | 8 | 32 | ≤1–>64 | 5.4 | 8 | 32 | ≤1–>64 | 2.1 | >0.05 |
| Ciprofloxacin | >8 | >8 | ≤0.12–>8 | 83.6 | >8 | >8 | ≤0.12–>8 | 81.4 | >0.05 |
| Clindamycin (not induced) | 0.25 | >8 | ≤0.06–>8 | 48.8 | 0.125 | >8 | ≤0.06–>8 | 39.7 | 0.052 |
| Clindamycin (after induction) | 67.6 | 48.5 | <0.001 | ||||||
| Daptomycin | 1 | 1 | 0.25–1 | 0.0 | 1 | 1 | 0.5–2 | 1.5 | >0.05 |
| Erythromycin | >16 | >16 | 0.25–>16 | 89.3 | >16 | >16 | 0.25–>16 | 88.1 | >0.05 |
| Gentamicin | 0.5 | 1 | ≤0.12–>16 | 3.0 | 0.5 | 1 | ≤0.12–>16 | 4.1 | >0.05 |
| Levofloxacin | >8 | >8 | ≤0.06–>8 | 81.3 | 4 | >8 | ≤0.06–>8 | 76.8 | <0.01 |
| Linezolid | 2 | 4 | 1–4 | 0.0 | 2 | 4 | 1–4 | 0.0 | >0.05 |
| Moxifloxacin | 4 | >4 | ≤0.03–>4 | 69.9 | 2 | >4 | ≤0.03–>4 | 56.2 | >0.05 |
| Mupirocin | ≤4 | ≤4 | ≤4–>512 | 3.3 | ≤4 | 8 | ≤4–>512 | 5.2 | >0.05 |
| Nitrofurantoin | 16 | 16 | 4–>128 | 0.3 | 16 | 16 | 8–64 | 0.0 | >0.05 |
| Oxacillin | >32 | >32 | 4–>32 | 100 | 16 | >32 | 4–>32 | 100 | >0.05 |
| Tetracycline | ≤0.25 | 0.5 | ≤0.25–>32 | 4.3 | ≤0.25 | 0.5 | ≤0.25–>32 | 4.6 | >0.05 |
| Tigecycline | 0.125 | 0.25 | 0.016–0.5 | 0.0 | 0.125 | 0.25 | 0.06–0.5 | 0.0 | >0.05 |
| Tobramycin | >16 | >16 | 0.25–≥16 | 55.9 | 1 | >16 | ≤0.12–>16 | 36.6 | <0.001 |
| Trimethoprim-sulfamethoxazole | ≤0.125/2.375 | 0.25/4.75 | ≤0.125/2.375–>16/304 | 5.4 | <0.125/2.375 | 0.25/4.75 | ≤0.125/2.375–>16/304 | 6.2 | >0.05 |
| Vancomycin | 0.5 | 1 | 0.25–1 | 0.0 | 0.5 | 1 | 0.5–2 | 0.0 | >0.05 |
P values determined using Fisher's exact test.
DISCUSSION
In 2003, McDougal et al. described a system of nomenclature for PFGE typing of S. aureus strains (the USA types) to help clarify the epidemiologic spread of MRSA strains in the United States (34). Subsequently, most reports describing the MRSA strains that either colonized or caused invasive infections among individuals in the United States have used the USA nomenclature to report strain types (29, 30, 32). The MRSA strain type USA300, which initially was seen primarily as a cause of community-associated skin and soft tissue infections (18), has now been reported from invasive infections in the United States and in many countries around the world (2, 19, 23, 29, 31, 40, 43, 46, 50).
Although PFGE typing continues to be of value for epidemiologic studies of MRSA strains (32), spa typing data, often reported in conjunction with SCCmec typing data, are used with increasing frequency around the world for MRSA strain identification (20, 24). In this study, we used spa typing as our primary method of strain identification and then translated the spa types into PFGE types to allow comparisons with prior U.S. data sets.
Data from NHANES surveys of nasal colonization in 2001 through 2004 indicated that MRSA isolates of PFT USA100 were the most common colonizers of individuals in community settings (i.e., persons not in health care centers, prisons, or other institutions) (22), although the proportion of USA300 isolates increased in frequency during the 2003-2004 time period compared to 2001-2002 (22, 47). The spa typing data from our convenience sample when translated to PFGE types, suggest that USA100 is still the predominant strain type in the nares of U.S. patients. However, in our study, USA100 isolates, which represented 53.2% of the 1,984 isolates in a CDC ABCs study of invasive isolates from 2005-2006, (32), have been replaced by USA300 isolates as the predominant type among MRSA strains isolated from positive blood cultures. In contrast, USA300 isolates represented only 31.4% of the isolates in the ABCs study, which included isolates collected from 48 hospitals in eight metropolitan areas across the United States. The change in strain type from USA100 to USA300 among invasive isolates in the present study was predicted by the mathematical models developed by D'Agata et al. (11) but was not apparent in all census regions across the United States (although data from three regions were limited to a single laboratory). The shift was observed in the West South Central region, where USA300 has been recognized for many years, particularly in children (6, 28), and in the Middle Atlantic and South Atlantic regions, where increasing rates of USA300 colonization have been observed (Fig. 1) (17). A recent report on MRSA strain types from 43 U.S. medical centers by Richter et al. (38) shows a larger proportion of USA100 than USA300 isolates among blood isolates nationwide, even though, overall, there were over 3 times as many USA300 isolates recovered across all specimen types compared to USA100 isolates (1,137 versus 377, respectively). Given that five of nine U.S. census regions showed similar data in our convenience sample (where USA100 predominates), we do not believe that our data are necessarily in conflict with those reported by Richter et al. (38). Unfortunately, the data in the latter study were not reported by geographic region.
Recent data from European surveillance studies indicate that five spa types accounted for 48.1% of the invasive MRSA isolates collected from 450 hospitals in 26 countries (24). Epidemiologic data suggest that local spread of MRSA strains is accompanied by local evolution. The most common spa type in Europe, t032, corresponds to PFT EMRSA15 (ST22) SCCmec IV; however, only two such isolates were observed in our study. The next most common spa type, t008, is a conglomeration of multiple STs, including ST8 (which includes the PFT USA300), ST247, ST250, and ST254. Although isolates of PFT USA300 remain uncommon in European countries, there are other spa types with PFGE profiles that correspond to USA300 that were recognized (see below). The third and fourth most common spa types, t041 and t003, were not observed in our convenience sample. The fifth type, t002 (6.4% of isolates), including ST5 and ST231, encompasses the USA100 PFT (which is typically ST5). Other spa types that were among the top 20 in Europe (although all had <3% prevalence) that were also recognized among our U.S. isolates (and their most common predicted PFTs) include t067 (USA100), t037 (ST239), t024 (USA300), t190 (USA300), t045 (USA100), and t127 (USA400). The fact that there is considerable diversity among the major spa types and PFTs in Europe and the United States supports the concept of local spread and microevolution of strains (24, 37).
USA300 isolates, as originally described by McDougal et al., were typically susceptible to fluoroquinolones, clindamycin, and tobramycin (34). In our more recent survey, isolates with spa types corresponding to USA300 (supported by the fact that they were positive for the presence of PVL and ACME genes) are now largely resistant to fluoroquinolones and showed emerging resistance to clindamycin and tobramycin. Resistance to fluoroquinolones, clindamycin, and tetracycline among USA300 isolates was reported both by Han et al. (25) and Diep et al. (13), while in a follow-up study, McDougal et al. reported resistance to fluoroquinolones, clindamycin, and gentamicin (33). The strains in the McDougal study reflected both the accumulation of chromosomal mutations (for fluoroquinolones) and plasmid-mediated resistance genes [including ermA and aph(2″)-Ia/aac(6′)-Ie]. The rise in tobramycin resistance observed among isolates from this study has been attributed to the widespread dissemination of the ant(4′)-Ia determinant in the United States (48). Taken together, these changes in susceptibility may have implications for the management of MRSA cases, especially in areas where USA300 isolates are common. Fortunately, high-level mupirocin resistance among all isolates has remained low among strains from U.S. hospitals despite greater emphasis on decolonization of MRSA-positive patients (5).
An interesting aspect of this study is the detection of several SCCmec types and PFTs which have been infrequently reported in the United States, including SCCmec type III and SCCmec type VIII and PFTs CMRSA9, EMRSA15, and the pattern associated with ST239. SCCmec III (in ST239 isolates of spa type t037) has been common in Europe for over a decade, but SCCmec type VIII is a more recent SCCmec type, which has emerged primarily in Canada in CMRSA9 isolates (27, 51). It is particularly interesting that one SCCmec VIII isolate was from California while the other was from Florida, suggesting that these are randomly imported cases that have not yet spread geographically. ST239 strains, although rare, have been reported previously from Florida (8).
In summary, the diversity of MRSA strain types in the United States continues to expand but varies by geographic region. The notable increase in the proportion of USA300 isolates causing invasive infections is of concern, since such strains have been predicted to be more virulent (11). spa types associated with USA300 strains have also become much more resistant to antimicrobial agents, particularly fluoroquinolones, although the reasons for this are unknown. In this study, spa typing demonstrated greater strain diversity than did PFGE typing (as reflected in the higher index of diversity for spa typing) and especially when used in conjunction with SCCmec data, facilitated the comparison of U.S. data to data sets from other countries. Knowledge of the spa types for USA100 and USA300 strains should facilitate the tracking of their spread internationally.
Supplementary Material
ACKNOWLEDGMENTS
We thank Joseph Whitmore for assistance with statistical analysis. We also thank Eliana Armstrong, George Miller, Aya Kubo, and Sara Lopez for assistance with antimicrobial susceptibility testing.
Conflicts of interests are as follows: F.C.T., I.A.T., and D.H.P. are employees and shareholders of Cepheid, and B.N.K. and R.V.G. have received research grants from Cepheid.
This study was funded by Cepheid.
MRSA Consortium members are as follows: Matthew Bankowski, Stephen Brecher, Susan Cohen, Phyllis Della-Latta, Ferric Fang, Miller Go, Paul Granato, Diane Halstead, Nell Jernigan, Robert Jerris, James Jorgensen, Margie Morgan, Sandra Richter, Paul Schreckenberger, Susan Sharp, Mary Stepney, David Tison, Allan Truant, Mike Voeller, and Richard Wong.
Footnotes
Published ahead of print 12 December 2011
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Anthony RM, Connor AM, Power EG, French GL. 1999. Use of the polymerase chain reaction for rapid detection of high-level mupirocin resistance in staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. 18:30–34 [DOI] [PubMed] [Google Scholar]
- 2. Arias CA, et al. 2008. MRSA USA300 clone and VREF—a U.S.-Colombian connection? N. Engl. J. Med. 359:2177–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bannerman TL, Peacock SJ. 2007. Staphylococcus, Micrococcus, and other catalase-positive cocci, p 390–411 In Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA. (ed), Manual of clinical microbiology, 9th ed ASM Press, Washington, DC [Google Scholar]
- 4. Benson G. 1997. Sequence alignment with tandem duplication. J. Comput. Biol. 4:351–367 [DOI] [PubMed] [Google Scholar]
- 5. Calfee DP, et al. 2008. Strategies to prevent transmission of methicillin-resistant Staphylococcus aureus in acute care hospitals. Infect. Control Hosp. Epidemiol. 29(Suppl. 1):S62–S80 [DOI] [PubMed] [Google Scholar]
- 6. Chavez-Bueno S, et al. 2005. Inducible clindamycin resistance and molecular epidemiologic trends of pediatric community-acquired methicillin-resistant Staphylococcus aureus in Dallas, Texas. Antimicrob. Agents Chemother. 49:2283–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen L, et al. 2009. Multiplex real-time PCR for rapid Staphylococcal cassette chromosome mec typing. J. Clin. Microbiol. 47:3692–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung M, Dickinson G, De Lencastre H, Tomasz A. 2004. International clones of methicillin-resistant Staphylococcus aureus in two hospitals in Miami, Florida. J. Clin. Microbiol. 42:542–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clinical Laboratory Standards Institute 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 8th ed., M7–A8 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 10. Clinical Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing, 21st informational supplement, M100–S21 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 11. D'Agata EM, Webb GF, Horn MA, Moellering RC, Jr, Ruan S. 2009. Modeling the invasion of community-acquired methicillin-resistant Staphylococcus aureus into hospitals. Clin. Infect. Dis. 48:274–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deleo FR, Otto M, Kreiswirth BN, Chambers HF. 2010. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375:1557–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diep BA, et al. 2008. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann. Intern. Med. 148:249–257 [DOI] [PubMed] [Google Scholar]
- 14. Diep BA, et al. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367:731–739 [DOI] [PubMed] [Google Scholar]
- 15. Diep BA, et al. 2008. The arginine catabolic mobile element and staphylococcal chromosomal cassette mec linkage: convergence of virulence and resistance in the USA300 clone of methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 197:1523–1530 [DOI] [PubMed] [Google Scholar]
- 16. Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Freitas EA, Harris RM, Blake RK, Salgado CD. 2010. Prevalence of USA300 strain type of methicillin-resistant Staphylococcus aureus among patients with nasal colonization identified with active surveillance. Infect. Control Hosp. Epidemiol. 31:469–475 [DOI] [PubMed] [Google Scholar]
- 18. Fridkin SK, et al. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352:1436–1444 [DOI] [PubMed] [Google Scholar]
- 19. Gilbert M, et al. 2007. Prevalence of USA300 colonization or infection and associated variables during an outbreak of community-associated methicillin-resistant Staphylococcus aureus in a marginalized urban population. Can. J. Infect. Dis. Med. Microbiol. 18:357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goering RV, et al. 2008. Molecular epidemiology of methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolates from global clinical trials. J. Clin. Microbiol. 46:2842–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Golding GR, et al. 2008. A preliminary guideline for the assignment of methicillin-resistant Staphylococcus aureus to a Canadian pulsed-field gel electrophoresis epidemic type using spa typing. Can. J. Infect. Dis. Med. Microbiol. 19:273–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gorwitz RJ, et al. 2008. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J. Infect. Dis. 197:1226–1234 [DOI] [PubMed] [Google Scholar]
- 23. Gottlieb T, Su WY, Merlino J, Cheong EY. 2008. Recognition of USA300 isolates of community-acquired methicillin-resistant Staphylococcus aureus in Australia. Med. J. Aust. 189:179–180 [DOI] [PubMed] [Google Scholar]
- 24. Grundmann H, et al. 2010. Geographic distribution of Staphylococcus aureus causing invasive infections in Europe: a molecular-epidemiological analysis. PLoS Med. 7:e1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Han LL, et al. 2007. High frequencies of clindamycin and tetracycline resistance in methicillin-resistant Staphylococcus aureus pulsed-field type USA300 isolates collected at a Boston ambulatory health center. J. Clin. Microbiol. 45:1350–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harmsen D, et al. 2003. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 41:5442–5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC) 2009. Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements. Antimicrob. Agents Chemother. 53:4961–4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaplan SL. 2005. Osteomyelitis in children. Infect. Dis. Clin. North Am. 19:787–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klevens RM, et al. 2006. Community-associated methicillin-resistant Staphylococcus aureus and healthcare risk factors. Emerg. Infect. Dis. 12:1991–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klevens RM, et al. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771 [DOI] [PubMed] [Google Scholar]
- 31. Larsen A, Stegger M, Goering R, Sorum M, Skov R. 2007. Emergence and dissemination of the methicillin resistant Staphylococcus aureus USA300 clone in Denmark (2000–2005). Euro Surveill. 12:2 [Google Scholar]
- 32. Limbago B, et al. 2009. Characterization of methicillin-resistant Staphylococcus aureus isolates collected in 2005 and 2006 from patients with invasive disease: a population-based analysis. J. Clin. Microbiol. 47:1344–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McDougal LK, et al. 2010. Emergence of resistance among USA300 methicillin-resistant Staphylococcus aureus isolates causing invasive disease in the United States. Antimicrob. Agents Chemother. 54:3804–3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McDougal LK, et al. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moran GJ, et al. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666–674 [DOI] [PubMed] [Google Scholar]
- 36. Murchan S, et al. 2003. Harmonization of pulsed-field gel electrophoresis protocols for epidemiological typing of strains of methicillin-resistant Staphylococcus aureus: a single approach developed by consensus in 10 European laboratories and its application for tracing the spread of related strains. J. Clin. Microbiol. 41:1574–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nübel U, et al. 2008. Frequent emergence and limited geographic dispersal of methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 105:14130–14135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Richter SS, et al. 2011. Activity of ceftaroline and epidemiologic trends in Staphylococcus aureus isolates collected from 43 medical centers in the United States in 2009. Antimicrob. Agents Chemother. 55:4154–4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Robinson DA, Enright MC. 2004. Multilocus sequence typing and the evolution of methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 10:92–97 [DOI] [PubMed] [Google Scholar]
- 40. Ruppitsch W, et al. 2007. Occurrence of the USA300 community-acquired Staphylococcus aureus clone in Austria. Euro Surveill. 12:E071025.1. [DOI] [PubMed] [Google Scholar]
- 41. Saïd-Salim B, et al. 2005. Differential distribution and expression of Panton-Valentine leucocidin among community-acquired methicillin-resistant Staphylococcus aureus strains. J. Clin. Microbiol. 43:3373–3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Seybold U, et al. 2006. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin. Infect. Dis. 42:647–656 [DOI] [PubMed] [Google Scholar]
- 43. Shibuya Y, et al. 2008. Emergence of the community-acquired methicillin-resistant Staphylococcus aureus USA300 clone in Japan. J. Infect. Chemother. 14:439–441 [DOI] [PubMed] [Google Scholar]
- 44. Shopsin B, et al. 1999. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J. Clin. Microbiol. 37:3556–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tattevin P, Diep BA, Jula M, Perdreau-Remington F. 2009. Methicillin-resistant Staphylococcus aureus USA300 clone in long-term care facility. Emerg. Infect. Dis. 15:953–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tenover FC, Goering RV. 2009. Methicillin-resistant Staphylococcus aureus strain USA300: origin and epidemiology. J. Antimicrob. Chemother. 64:441–446 [DOI] [PubMed] [Google Scholar]
- 47. Tenover FC, et al. 2008. Characterization of Staphylococcus aureus isolates from nasal cultures collected from individuals in the United States in 2001 to 2004. J. Clin. Microbiol. 46:2837–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tenover FC, et al. 2011. Activity of ACHN-490 against methicillin-resistant Staphylococcus aureus isolates from patients in U.S. hospitals. International J. Antimicrob. Agents 38:352–354 [DOI] [PubMed] [Google Scholar]
- 49. Tenover FC, et al. 2007. Accuracy of six antimicrobial susceptibility methods for testing linezolid against staphylococci and enterococci. J. Clin. Microbiol. 45:2917–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Valentini P, et al. 2008. An uncommon presentation for a severe invasive infection due to methicillin-resistant Staphylococcus aureus clone USA300 in Italy: a case report. Ann. Clin. Microbiol. Antimicrob. 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang K, McClure JA, Elsayed S, Conly JM. 2009. Novel staphylococcal cassette chromosome mec type, tentatively designated type VIII, harboring class A mec and type 4 ccr gene complexes in a Canadian epidemic strain of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:531–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.