Abstract
From 2006 to 2008, Neisseria gonorrhoeae isolates were identified with decreased susceptibility to the extended-spectrum cephalosporin (ESC) cefotaxime among visitors of the Amsterdam sexually transmitted infections (STI) clinic, the Netherlands. Spread, clonality, and characteristics of 202 isolates were examined using antibiograms, conventional penA mosaic gene PCR, and N. gonorrhoeae multiple-locus variable-number tandem repeat analysis (NG-MLVA). A strictly defined subset was further characterized by N. gonorrhoeae multiantigen sequence typing (NG-MAST) and sequencing of ESC resistance determinants (penA, mtrR, and porB1b). Seventy-four N. gonorrhoeae isolates with a cefotaxime MIC of >0.125 μg/ml (group A), 54 with a cefotaxime MIC of 0.125 μg/ml (group B), and a control group of 74 with a cefotaxime MIC of <0.125 μg/ml (group C) were included. Fifty-three clonally related penA mosaic-positive isolates (penicillin-binding protein 2 type XXXIV) were identified in group A (n = 47 isolates; 64%) and B (n = 6 isolates; 11%). The 53 penA mosaic-positive isolates were predominantly NG-MAST ST1407 (87%) and contained an mtrR promoter A deletion (98%) and porB1b alterations G101K/A102N. All were assigned to the same NG-MLVA cluster that comprised in total 56 isolates. A correlation was found between decreased cefotaxime susceptibility and ST1407 that was highly prevalent among visitors of the Amsterdam STI clinic. The rapid spread of this strain, which also has been identified in many other countries, might be facilitated by high-risk sexual behavior and should be monitored closely to identify potential treatment failure. Quality-assured surveillance of ESC susceptibility on the national and international levels and exploration of new drugs and/or strategies for treatment of gonorrhea are crucial.
INTRODUCTION
Neisseria gonorrhoeae is the etiological agent of the sexually transmitted infection (STI) gonorrhea. During the last decades, N. gonorrhoeae has effectively developed plasmid-mediated and/or chromosomally mediated antibiotic resistance to penicillins, tetracyclines, fluoroquinolones, and most other antibiotics used for treatment of gonorrhea (3, 16, 25). In many countries, this loss of treatment efficacy led to discontinuation of these antibiotics as standard first-line treatment regimens, posing new public health concerns (5, 25, 35).
At present, in most countries extended-spectrum cephalosporins (ESCs), such as cefixime (oral) and ceftriaxone (injectable), are the first-line treatment for gonorrhea (3, 4, 25). In the Netherlands, until 2006 the national clinical guidelines recommended cefotaxime as first-line therapy for gonorrhea. However, since 2006 ceftriaxone is available as an intramuscular injection in the Netherlands and is recommended as the first-line treatment (8). Although parenteral ESCs, such as ceftriaxone and cefotaxime, generally show good clinical efficacy for gonococcal infection, various studies have reported the emergence of N. gonorrhoeae strains with decreased susceptibility to these antibiotics (10, 17, 20, 21, 24, 25, 33, 38). Worryingly, a recent report described the first European case (in Sweden) of verified treatment failure of pharyngeal gonorrhea using ceftriaxone (29), and the first strain worldwide with high-level resistance to ceftriaxone has recently been reported from Japan (20, 29).
One important antibiotic resistance determinant that has been associated with this decreased susceptibility and resistance to ESCs in gonococci is the penA mosaic gene, which encodes a mosaic variant of penicillin binding protein 2 (PBP2), the primary lethal target of β-lactam antibiotics (2, 14). Polymorphisms in at least three important mosaic PBP2 residues (I312M, V316T, and G545S) and epistasis of the polymorphisms in mosaic PBP2 variants cause a marked decrease in susceptibility to cefixime and ceftriaxone (23, 27). However, additional polymorphisms in the penA gene, e.g., alterations in A501 in PBP2, are important, and still a lot of knowledge is lacking. Yet, with the exception of the Japanese strain (19), it has been well established that the identified alterations in the penA mosaic gene do not seem to be sufficient to attain very high levels of ESC resistance without the contribution of mtrR, porB1b, and at least one additional unknown resistance determinant (10, 17, 20, 25, 38).
Since 2006, cefotaxime has been used to monitor susceptibility to ESCs in the Netherlands. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) and Clinical and Laboratory Standards Institute (CLSI) have defined different MIC resistance breakpoints for cefotaxime of >0.125 μg/ml and >0.5 μg/ml, respectively. Although N. gonorrhoeae isolates with a MIC over the CLSI breakpoint have not yet been found in the Netherlands, isolates with a MIC between 0.125 and 0.5 μg/ml have been frequently identified (7). In 2009, we reported a notable 7.3% increase in prevalence over a 2-year period (2006 to 2008) of multidrug-resistant N. gonorrhoeae isolates with such a decreased susceptibility to cefotaxime among visitors of the STI outpatient clinic in Amsterdam (7). This sharp increase in prevalence was associated with high-risk sexual behavior and STI coinfections, which suggested rapid clonal expansion of an antibiotic-resistant N. gonorrhoeae strain, such as that described in other studies (10, 13, 14, 36).
In the present study, we investigated the transmission patterns, clonality, and phenotypic as well as genotypic characteristics of N. gonorrhoeae isolates, which were classified as intermediate susceptible/resistant, borderline susceptible, and susceptible to cefotaxime, according to EUCAST criteria. All isolates were obtained from well-defined high-risk populations in Amsterdam.
MATERIALS AND METHODS
Study setting, population, and sampling.
The STI outpatient clinic of the Amsterdam Public Health Service, the Netherlands, is a low-threshold clinic serving approximately 30,000 clients annually. During 2007 and 2008, a total of 3,791 cases of gonorrhea were diagnosed in national public health settings, of which 2,077 (55%) were diagnosed in the Amsterdam STI outpatient clinic. Clients can visit the STI clinic anonymously, free of charge, and without referral of a health care provider. Upon arrival, clients are prioritized based on a short questionnaire to estimate the risk for having an STI (11). All clients who are considered high-risk patients get a full STI checkup, including collection of swabs for N. gonorrhoeae cultivation and a detailed questionnaire concerning risk behavior. High-risk groups include all men who have sex with men (MSM), persons who are paid for having sex, persons with symptoms, persons who report that their partner has an STI, persons who have been referred to the outpatient department by another health care provider, and persons originating from Sub-Saharan Africa (increased risk for HIV; a rapid HIV test is offered). Only asymptomatic clients from low-risk groups are evaluated by nucleic acid amplification testing (NAAT) only. Patients diagnosed with an N. gonorrhoeae infection (urogenital, anorectal, and/or pharyngeal infection) are treated with 500 mg ceftriaxone intramuscularly according to the national guidelines of the Dutch Dermatological and Venereological Society (8). Azithromycin (1 g, single oral dose) as treatment for coexisting Chlamydia trachomatis infection is given to all patients with proven coinfection and as syndromic treatment in patients who are positive for gonorrhea by microscopy after Gram staining during the screening visit.
The samples and data for this study were collected as part of the routine clinical procedure; therefore, no ethical committee approval was needed.
Bacterial cultivation, antimicrobial susceptibility, and lysate preparation.
For the cultivation of N. gonorrhoeae, urethral, cervical, rectal, and/or pharyngeal swab specimens were directly inoculated onto GC-Lect agar plates (Becton Dickinson) and incubated in an aerobic, carbon dioxide-enriched environment at 37°C for 40 to 48 h. The identification of N. gonorrhoeae was based on Gram-staining, oxidase, sugar utilization, and aminopeptidase reactions, and a DNA probe test, in accordance with the instructions from the manufacturer (AccuProbe; Gen-Probe). For all N. gonorrhoeae isolates, MICs of cefotaxime, ceftriaxone, cefixime, penicillin G, tetracycline, and ciprofloxacin were measured using the Etest method, in accordance with the instructions from the manufacturer (AB bioMérieux, Sweden). The 2008 WHO N. gonorrhoeae reference strains G, K, L, M, O, and P (28) were used as control strains for all antimicrobial susceptibility testing. Since cefotaxime MICs were not available for these strains, we determined them for our conditions. WHO reference strains K and L had decreased susceptibility to extended-spectrum cephalosporins and had cefotaxime MICs of 0.19 and 0.25 μg/ml, respectively. The other strains had cefotaxime MICs varying from 0.006 to 0.047 μg/ml. Colonies of N. gonorrhoeae-positive cultures were suspended in sterile saline (0.5 McFarland standard), lysed at 95°C for 10 min, and stored at −80°C prior to amplification.
Selected N. gonorrhoeae isolates from 2006 to 2008.
To include the lower levels of decreased susceptibility and/or antimicrobial resistance, N. gonorrhoeae isolates were selected according to the EUCAST resistance breakpoint (version 1.3) for cefotaxime (MIC > 0.125 μg/ml) rather than using the higher resistance breakpoint issued by the CLSI (MIC > 0.5 μg/ml). From October 2006 to December 2008, N. gonorrhoeae isolates with MICs of cefotaxime over 0.125 μg/ml and isolates with MICs of cefotaxime of 0.125 μg/ml had been cultured from 90 and 70 patients, respectively. Group A consisted of 74 (82%) isolates with a MIC over 0.125 μg/ml which could be recultured. Group B included 54 (77%) isolates with a MIC of 0.125 μg/ml which were still viable. Only one isolate per patient was included in the present study; if several isolates had been cultured from different locations of a single patient, the isolate with the highest MIC was chosen. Control group C consisted of 74 N. gonorrhoeae isolates with a cefotaxime MIC of <0.125 μg/ml. For each group A isolate, a control isolate was included within a three-day time span from inclusion. Of the 202 isolates included in the present study, 96 (47.5%) were cultured from urethra, 87 (43.1%) from rectum, 10 (5.0%) from pharynx, and nine (4.5%) from cervix. The percentage of urethral isolates was significantly higher in group C (58%) than in group A (41%) (P = 0.048). The number of rectal isolates was significantly higher in group A (51%) than in group C (32%) (P = 0.030). Significantly more pharyngeal isolates were found in group B (9%) than in group C (0%) (P = 0.012).
Detection of the penA mosaic gene.
The detection of penA mosaic alleles in N. gonorrhoeae lysates was based on a protocol, previously described by Whiley et al. (32). PCR was performed using a 25-μl reaction volume containing 2 μl of heat-treated N. gonorrhoeae lysates, 1 U Taq DNA polymerase (Promega), 5 μl of 5× Flexi GoTaq buffer (Promega), 2.5 mM MgCl2 solution (Promega), 200 μM deoxynucleoside triphosphates (dNTPs) (Roche Diagnostics, Switzerland), and 0.36 μM forward primer penA-F, 0.04 μM (each) reverse primers penA-R1 to -R4, and 0.2 μM reverse primer penA-R5. Amplification was performed on a Bio-Rad C1000 PCR system (Bio-Rad) at 95°C for 2 min, followed by 35 amplification cycles of 30 s at 94°C, 60 s at 60°C, and 60 s at 72°C. The PCR products were electrophoresed on a 10% polyacrylamide gel (Bio-Rad). WHO strains K and L were used as a positive and negative control, respectively. WHO strains K and L (penA D345a allele) strains both display decreased susceptibility to ESCs due to a penA mosaic allele and a penA A501V mutation, respectively (28).
N. gonorrhoeae MLVA.
Amplification of the variable number of tandem repeat sequences (VNTRs) was performed on a Bio-Rad C1000 PCR system (Bio-Rad). Five VNTR loci were amplified in two different multiplex PCRs as described previously (12). The amplified samples were diluted 1:20 in water, and 2 μl of each diluted sample was mixed with 18 μl of a 1:450 dilution of GeneScan LIZ 500 size standard (Applied Biosystems) in water.
After heat denaturation for 5 min at 95°C, the fragments were separated with an ABI 3130 automated sequencer using the fragment analysis module. Sizing and calculation of the number of repeats of each VNTR were performed with GeneMarker software version 1.80 (SoftGenetics).
NG-MAST.
N. gonorrhoeae multiantigen sequence typing (NG-MAST) was performed as previously described (18, 31). NG-MAST allele numbers of porB and tbpB and sequence types (STs) were assigned using the NG-MAST database (www.ng-mast.net).
penA, mtrR, and porB1b (penB) sequencing.
The penA gene, promoter region, and the coding sequence of mtrR and the full-length porB1b gene were sequenced as previously described (17). DNA of the N. gonorrhoeae WHO F reference strain and sterile, UV-treated water were included in all runs as positive and negative controls, respectively (28).
Data analysis and statistics.
Cluster analysis of the MLVA types was performed with Bionumerics Software version 5.1 (Applied Math, Belgium). Statistical analyses were performed using SPSS version 17.0 software (SPSS Inc.). Sequence alignments were performed using BioEdit version 7.0.9 (http://www.mbio.ncsu.edu/bioedit/bioedit.html).
RESULTS
Patient characteristics.
Of the included patients, 155 (77%) were MSM, 30 (15%) heterosexual men, and 17 women (8%). The patients were predominantly of Dutch ethnicity (70%), and the median age was 35 years (range, 27 to 43 years). Chlamydia trachomatis (31%) and HIV (31%) coinfections were common. Of eight (4%) patients (four women and four MSM) identified as commercial sex workers, five were from Eastern Europe (Table 1).
Table 1.
Patient characteristics of all included patients (n = 202) and of the patients that were assigned to large (n ≥ 10 isolates) NG-MLVA clusters I (n = 56 isolates) and II (n = 39 isolates)
| Characteristic | Valuea |
|||
|---|---|---|---|---|
| Total study population | Cluster I | Cluster II | Others | |
| Total patients | 202 (100) | 56 (100) | 39 (100) | 107 (100) |
| Median (IQR)b age (yr) | 35 (27–43) | 37 (29–44) | 36 (28–43) | 34 (25–41) |
| Gender and sexual orientation | ||||
| Men who have sex with men | 155 (77) | 48 (86) | 36 (92) | 71 (66) |
| Heterosexual men | 30 (15) | 5 (9) | 2 (5) | 23 (21) |
| Women | 17 (8) | 3 (5) | 1 (3) | 13 (12) |
| Nationality | ||||
| Dutch | 142 (70) | 40 (71) | 31 (79) | 71 (66) |
| Eastern Europeanc | 14 (7) | 7 (13) | 0 | 7 (7) |
| Surinamese/Antillean | 12 (6) | 2 (4) | 1 (3) | 9 (8) |
| Other | 34 (17) | 7 (13) | 7 (18) | 20 (17) |
| Coinfections | ||||
| Chlamydia trachomatis | 63 (31) | 18 (32) | 12 (31) | 33 (31) |
| HIV positive | 63 (31) | 17 (30) | 14 (36) | 32 (30) |
| Status unknown | 22 (11) | 4 (7) | 3 (8) | |
| Sex workers | ||||
| Men | 4 (2) | 2 (4) | 0 | 2 (2) |
| Women | 4 (2) | 2 (4) | 0 | 2 (2) |
Values are the numbers (percentages) of patients unless otherwise noted.
IQR, interquartile range.
Albania, Bulgaria, Hungary, Ukraine, Romania, Russia, and Czech Republic.
Antimicrobial susceptibility of all selected N. gonorrhoeae isolates.
The MIC range of isolates with decreased susceptibility or resistance to cefotaxime was 0.125 to 0.50 μg/ml. No verified treatment failures were observed during the study period; however, these were not actively searched for. In vitro resistance to penicillin, tetracycline, and ciprofloxacin was common in groups A and B. In group B, similar proportions of the isolates had resistance to these antibiotics, while the proportions in control group C were markedly lower (Table 2). Using a MIC of 0.016 μg/ml as an arbitrary cutoff, 68% of the group A and 11% of group B isolates showed decreased susceptibility to cefixime, and 76% and 57%, respectively, showed decreased susceptibility to ceftriaxone. In contrast, 0 and 5% of control group C isolates showed decreased susceptibility to cefixime and ceftriaxone, respectively.
Table 2.
Percentage of isolates with MICs above the susceptibility breakpoints of several antibiotics in cefotaxime groups A, B, and Ca
| Cefotaxime group (MIC in μg/ml) | % of isolates with indicated MIC breakpoints |
||
|---|---|---|---|
| Penicillin G (>1 μg/ml) | Tetracycline (>1 μg/ml) | Ciprofloxacin (>0.064 μg/ml) | |
| A (>0.125) | 22 | 80 | 96 |
| B (0.125) | 24 | 74 | 94 |
| C (<0.125) | 14 | 35 | 47 |
Penicillin G, tetracycline, and ciprofloxacin resistance breakpoints are from EUCAST version 1.3. Percentages of isolates with MICs above the CLSI ciprofloxacin resistance breakpoint (MIC ≥ 1 μg/ml) in cefotaxime groups A, B, and C were 96%, 94%, and 54%, respectively.
Detection of a penA mosaic allele in N. gonorrhoeae isolates.
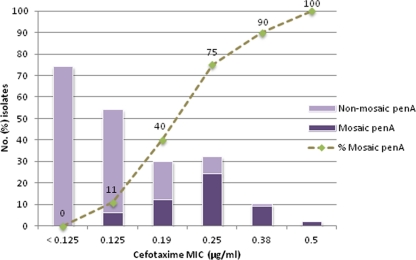
A penA mosaic allele was identified in 53 (26%) of the 202 examined N. gonorrhoeae isolates. The penA mosaic gene was exclusively found in isolates that had decreased susceptibility or resistance to cefotaxime. There was a strong positive correlation between N. gonorrhoeae isolates with higher cefotaxime MICs and the presence of a penA mosaic allele (Fig. 1). The frequency of the penA mosaic gene was significantly higher in group A (47/74, 64%) than in both group B (6/54, 11%) and group C (0/74) (P < 0.0001, chi-square test). Also the difference between group B and C was significant (P = 0.0119, chi-square test).
Fig 1.
Association between the presence of a penA mosaic allele (n = 53 isolates) and cefotaxime susceptibility.
Cluster analysis of NG-MLVA profiles.
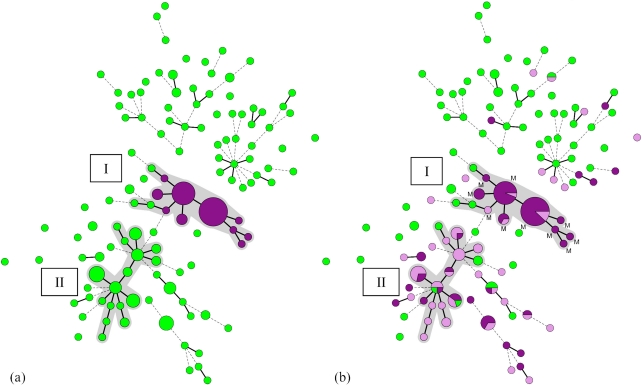
To examine whether there was a clonal expansion of a penA mosaic-positive N. gonorrhoeae strain within the Amsterdam high-risk population, all samples were genotyped with NG-MLVA. Hierarchical cluster analysis of the MLVA profiles of the 202 isolates identified two large clusters (≥10 isolates) that were assigned cluster I and II (Fig. 2a).
Fig 2.
(a) Minimum spanning tree of 202 N. gonorrhoeae isolates typed by NG-MLVA. Hierarchical cluster analysis of the MLVA DNA profiles was performed to display the genetic relationship between the MLVA types. Each circle represents an MLVA type, and the size of each circle corresponds to the number of identical MLVA types it contains. MLVA types that contain the mosaic penA gene are represented by a purple circle. MLVA types that are connected by a thick, solid line differ in 1 VNTR locus from each other, while MLVA types connected by a dotted line differ in 2 VNTR loci. A cluster was assigned when adjacent MLVA types did not differ by more than one VNTR locus and if a minimum of 10 MLVA types met this criterion. MLVA types that belong to a cluster are represented by gray shading. Two large clusters (n ≥ 10 isolates) were identified and assigned as clusters I (n = 56 total isolates) and II (n = 39 total isolates). (b) MLVA types that contain the mosaic penA gene are represented by the letter M (see also Fig. 2a). MLVA types with a cefotaxime MIC of <0.125 μg/ml, 0.125 μg/ml, or >0.125 μg/ml are represented by a green (group C), lilac (group B), or purple (group A) circle, respectively.
NG-MLVA cluster I contained 56 isolates, including all 53 (95% of the cluster) penA mosaic-containing isolates. The remaining three were nonmosaic N. gonorrhoeae isolates that were susceptible to cefotaxime. In cluster II (n = 39 isolates), no penA mosaic isolates were found.
The presence of cluster I, which included all 53 penA mosaic-positive isolates with an identical penA mosaic allele (PBP2 pattern XXXIV), see below, strongly suggests the circulation of one clonal N. gonorrhoeae strain. A marked association was observed between NG-MLVA cluster I and decreased susceptibility and/or resistance to cefotaxime (95%). Most isolates in cluster I displayed in vitro resistance to tetracycline (89%) and ciprofloxacin (98%) according to EUCAST breakpoints (version 1.3). In total, 118/128 isolates (92%) with decreased susceptibility to cefotaxime were also resistant to tetracycline and ciprofloxacin.
NG-MLVA cluster II may represent another group of clonally related strains, characterized by modestly increased MICs of cefotaxime. The proportion of isolates in the cefotaxime MIC groups A, B, and C in clusters I and II is illustrated in Fig. 2b. Twenty-six (67%) of the isolates in cluster II (n = 39 total isolates) had a cefotaxime MIC of 0.125 μg/ml. Interestingly, 22/128 isolates (17%) with a cefotaxime MIC of ≥0.125 μg/ml could not be assigned to a large cluster.
Characteristics of the patients in NG-MLVA cluster I.
The patients whose isolates comprised cluster I did not differ significantly from the total population regarding age, gender, sexual orientation, nationality, or coinfection status (Table 1). Of the patients in cluster I, 48 were MSM (86%), 5 were heterosexual men (9%), and 3 were women (5%). The patients in cluster I were predominantly Dutch (71%), and coinfections with chlamydia (32%) and HIV (30%) were common. These characteristics were similar in cluster II. The only important difference between clusters I and II was the presence of seven (13%) patients with an Eastern European nationality who were identified only in cluster I. Of these, three (5%) patients were identified as commercial sex workers: one MSM (bisexual man) and two women. In total, four (7%) commercial sex workers were identified in cluster I, while none of the patients in cluster II were commercial sex workers.
NG-MAST of the isolates in NG-MLVA cluster I.
On all 56 isolates of cluster I, N. gonorrhoeae multiantigen sequence typing (NG-MAST) was performed to confirm the clonality of the N. gonorrhoeae strain harboring a penA mosaic allele and to identify the NG-MAST sequence type(s) (ST) involved. Among these 56 isolates, 9 different NG-MAST STs were identified, two of which have not been previously described. Forty-six (87%) of the 53 penA mosaic-positive isolates in cluster I were assigned to ST1407, and the remaining seven isolates were further differentiated in 5 other STs (Table 3). Compared to ST1407, four of these five STs contained only a single nonsynonymous nucleotide substitution in porB, confirming closely related genotypes.
Table 3.
Phenotypic and genotypic characteristics of the isolates in NG-MLVA cluster I (n = 56)
| Group (cefotaxime MIC [μg/ml]) | NG-MAST ST | No. of isolates | Presence of mosaic penA allele | PBP2 pattern | Polymorphism(s) in: |
|
|---|---|---|---|---|---|---|
| mtrR | porB1b | |||||
| A (>0.125) | ST1407 | 41 | Yes | XXXIV | Promoter A deletion | K101, N102 |
| ST3378 | 2 | Yes | XXXIV | Promoter A deletion | K101, N102 | |
| ST2212 | 1 | Yes | XXXIV | Promoter A deletion | K101, N102 | |
| ST4120 | 1 | Yes | XXXIV | Promoter A deletion | K101, N102 | |
| ST3149 | 1 | Yes | XXXIV | Promoter A deletion | K101, N102 | |
| ST5013a | 1 | Yes | XXXIV | Promoter A deletion | WT, WT | |
| B (0.125) | ST1407 | 5 | Yes | XXXIV | Promoter A deletion | K101, N102 |
| ST2212 | 1 | Yes | XXXIV | Promoter A deletion | K101, N102 | |
| C (<0.125) | ST437 | 1 | No | II | Promoter A deletion, G45D | K101, D102 |
| ST1466 | 1 | No | II | Promoter A deletion, G45D | K101, D102 | |
| ST5011a | 1 | No | II | Promoter A deletion, G45D | WT, WT | |
New NG-MAST sequence type.
The remaining 3 nonmosaic penA isolates in cluster I had 3 different STs with multiple substitutions in both porB and tbpB, suggesting that these isolates contained more distantly related N. gonorrhoeae genotypes (Table 3).
penA sequences of the isolates within NG-MLVA cluster I.
penA sequencing was performed on all 56 isolates in NG-MLVA cluster I to confirm the identified penA mosaic-positive gel patterns, determine the clonal relatedness of penA, and identify the penA mosaic sequence type. Within these 56 isolates, all 53 penA mosaic-positive isolates showed an identical penA mosaic sequence (PBP2 pattern XXXIV; GenBank accession no. GU723422). In the remaining 3 nonmosaic penA isolates, the penA sequence pattern II was identified (Table 3).
Specific mutations in mtrR and porB1b (penB) in the isolates of NG-MLVA cluster I.
We examined two additional antibiotic resistance determinants that are associated with decreased ESC susceptibility. A specific nucleotide (A) deletion in the promoter region of the mtrR gene that causes overexpression of the MtrCDE efflux pump was observed in all 56 isolates of NG-MLVA cluster I. The remaining 3 nonmosaic isolates in NG-MLVA cluster I contained an additional G45D amino acid substitution in MtrR.
PorB1b amino acid substitutions G101K and A102N were detected in all but one penA mosaic-positive isolates, suggesting a decreased intake of the ESCs, while only one isolate had a wild-type porB1b sequence. One mosaic and one nonmosaic isolate had a wild-type porB1b sequence, while the other two nonmosaic isolates had amino acid substitutions G101K and A102D (Table 3).
Genotypic characterization of a subset of isolates in cluster II.
We genotypically characterized a subset of nine penA nonmosaic isolates from cluster II. Eight of the nine isolates had a cefotaxime MIC of ≥0.125 μg/ml; four of these isolates had a borderline cefotaxime MIC of 0.125 μg/ml (group B), and four isolates had a cefotaxime MIC of 0.19 to 0.25 μg/ml (group A) and were considered resistant according to EUCAST (version 1.3) resistance breakpoints. The remaining isolate that was included was susceptible to cefotaxime (group C) but had a ceftriaxone MIC of 0.023 μg/ml.
All nine nonmosaic penA isolates contained PBP2 pattern XII and the specific nucleotide (A) deletion in the promoter region of the mtrR gene. Eight of the nine isolates (including the group C isolate) contained porB1b alterations G101K/A102D and were assigned NG-MAST ST225. The remaining group A isolate had porB1b alterations G101K/A102N and was assigned NG-MAST ST5012.
DISCUSSION
Decreased in vitro susceptibility to extended-spectrum cephalosporins (ESCs), the last remaining treatment option for gonorrhea, is reported in many parts of the world. Due to the increasing number of reports concerning the loss of clinical treatment efficacy of orally administered ESCs, the parenteral ESC ceftriaxone is often considered to be the preferred first-line treatment for gonorrhea (6, 25). However, a recent report described the first strain displaying high-level resistance to ceftriaxone (most likely related to a treatment failure using ceftriaxone) and complete characterization of this strain (20). It is now a fear that gonorrhea may become untreatable during certain circumstances and especially in some settings (20, 25).
In the Netherlands, cefotaxime was used as the first treatment option before 2006 due to nonavailability of ceftriaxone in recommended dosages. From 2006 onward, recommended dosages became available and ceftriaxone was recommended as the first-line treatment for gonorrhea infections according to the national clinical guidelines which were issued by the Dutch Dermatological and Venereological Society (8). However, cefotaxime remained the drug of choice for monitoring ESC susceptibility to obtain comparable resistance data over a period of several years.
In the present study, the systematic monitoring of cefotaxime susceptibility from 2006 to 2008 allowed the detection of N. gonorrhoeae isolates with decreased susceptibility to ESCs among visitors of the STI outpatient clinic in Amsterdam (7). To assess these lower levels of antimicrobial resistance, N. gonorrhoeae isolates were classified according to the EUCAST resistance breakpoint for cefotaxime (MIC > 0.125 μg/ml) rather than using the higher resistance breakpoint issued by the CLSI (MIC > 0.5 μg/ml). Including N. gonorrhoeae isolates with borderline MICs of exactly 0.125 μg/ml enabled us to identify antimicrobial resistance determinants that would have remained undetected when the EUCAST breakpoint for cefotaxime was strictly applied, and thus only isolates with a MIC of >0.125 μg/ml were included. In order to confirm whether cefotaxime is a good marker for susceptibility to extended-spectrum cephalosporins in general, we also recultured the N. gonorrhoeae strains and assessed their sensitivity to cefixime and ceftriaxone. Using an arbitrary breakpoint, we confirmed that decreased susceptibility to these other cephalosporins was frequently found in isolates with decreased susceptibility to cefotaxime and not in control isolates.
Genotypic characterization of all of the included isolates showed the emergence of an N. gonorrhoeae strain that harbored a penA mosaic allele (PBP2 pattern XXXIV) within the Amsterdam high-risk population that has also been found in San Francisco, CA, Ontario (Canada), and Northern Taiwan (1, 13, 22). NG-MLVA revealed two large clusters (named NG-MLVA clusters I and II), and all penA mosaic-positive isolates (n = 53) were classified to cluster I (n = 56 total isolates). Additional NG-MAST analysis of the penA mosaic-positive isolates in NG-MLVA cluster I revealed that ST1407 (87%) was predominant, whereas the remaining isolates contained some evolving but, to ST1407, very closely related subtypes. Three nonmosaic penA isolates (PBP2 pattern II) that were susceptible to ESCs were also assigned to NG-MLVA cluster I, suggesting that these were more distantly related N. gonorrhoeae genotypes (12). The genotypic characterization of a limited number of isolates in cluster II predominantly revealed ST225, a worldwide-prevalent N. gonorrhoeae strain (9), that harbored a penA nonmosaic allele (PBP2 pattern XII).
The emergence and spread in many countries worldwide suggests ST1407 to be an N. gonorrhoeae strain that is successful in regard to transmission. The strain probably originated in the WHO Western Pacific Region and has acquired additional resistance determinants over time. Antimicrobial multiresistant N. gonorrhoeae strains such as ST1407 may have a survival advantage in an antibiotic-rich environment, which is common in high-frequency-transmitting populations where many individuals are treated for various STIs on a regular basis (1, 10, 13, 22, 25, 26, 30). However, proof is lacking that this strain is indeed more biologically fit. A well-designed and quality-assured study that assesses the biological fitness of such an N. gonorrhoeae strain in vitro (e.g., various culture media) and in vivo (animal models), including transformation experiments, would be very valuable and provide more insight in this matter (20). We assume that ST1407 was imported in the Dutch population, although it is difficult to determine the exact time point of introduction. This assumption might be supported by the fact that oral ESCs such as cefixime were never part of the standard treatment regimen in the Netherlands, whereas ST1407 has shown a strong association with decreased susceptibility and/or resistance to oral ESCs (10, 22).
A significant association was observed between the presence of the penA mosaic allele and the decreased susceptibility and/or resistance to cefotaxime. However, when the total number of isolates with decreased susceptibility to cefotaxime (n = 128) was taken into account, only 41% of these isolates contained the penA mosaic allele. Many of the nonmosaic isolates found in NG-MLVA cluster II (n = 39) had moderately increased MICs for cefotaxime. The decreased susceptibility to cefotaxime in NG-MLVA cluster I was associated with the penA mosaic allele in synergy with alterations in the mtrR promoter region and porB1b, whereas the isolates in NG-MLVA cluster II lacked a penA mosaic allele. Although it is too early for final conclusions since only a limited number of isolates in cluster II was characterized, the data suggested that the moderately increased cefotaxime MICs were caused by the synergistic behavior of sequential alterations in nonmosaic penA alleles, mtrR, porB1b, and possibly unknown antimicrobial resistance determinants. As recent studies showed that penicillin resistance determinants pilQ and ponA did not significantly affect susceptibility to ESCs, other unknown alterations are likely involved in the development of decreased susceptibility and resistance to ESC (10, 15, 17, 34, 38). However, accurate determination of the effect of these individual resistant determinants on cefotaxime susceptibility is hindered by the penA epistatic mutational effects, synergistic behavior of the identified antimicrobial resistance determinants, and deficient knowledge concerning the unknown resistance factors involved. Accordingly, the clinical relevance of the moderately increased MICs for cefotaxime is still not clear.
The rapid increase in prevalence of the clonally related isolates suggests a continuous transmission of this ST1407 N. gonorrhoeae strain among visitors of the Amsterdam STI clinic (7). The high number of MSM (86%) and the high frequency of coinfections that were found in NG-MLVA cluster I indicate that transmission was facilitated by high-risk sexual behavior that is characterized by numerous, often anonymous, sexual contacts over short periods of time. The identified commercial sex workers might have acted as a bridge group that could possibly explain the circulation of ST1407 among both heterosexuals and MSM. Prospective genotyping of isolates with decreased susceptibility and/or resistance to cefotaxime and other ESCs might provide further insight in gonococcal transmission patterns and the identification of potential transmission networks or core groups.
Special attention should be given to the clinical identification and treatment of pharyngeal gonorrhea, which is predominantly found among MSM and female sex workers. In the present study, only 4.5% of strains originated from the pharynx. Pharyngeal gonorrhea is often asymptomatic and more difficult to treat (19, 35). This reduced cure rate facilitates accumulation of genetic alterations and might cause the pharynx to act as a reservoir for gene transfer and recombination.
In conclusion, disturbing reports of cefixime and ceftriaxone treatment failure in well-documented cases of urogenital and pharyngeal gonorrhea, respectively, might be the beginning of an impending threat toward ESC resistance and possibly untreatable gonorrhea in certain circumstances spreading worldwide (21, 25, 29, 30, 37). The findings of the present study underline the importance of continued surveillance of ESC susceptibility on the national and international levels. However, serious exploration of alternative antimicrobial treatment options as well as strategies, such as antimicrobial combination therapy, is essential as the development of new antimicrobial agents is hindered by declining investments and resistance to ESC spreading worldwide seems to be a matter of time.
ACKNOWLEDGMENTS
We thank R.A. Coutinho and M. F. Schim van der Loeff for critical review of the paper and helpful comments. We thank the laboratory technicians of the Public Health Laboratory (Cluster of Infectious Diseases, AHS) and S. Kroon for technical support.
We do not have any commercial or other association that might pose a conflict of interest regarding the study presented in this paper.
The present work was supported by grants from the research and development fund of the Public Health Service, GGD Amsterdam, the Netherlands, and from the Örebro County Council Research Committee and the Foundation for Medical Research at Örebro University Hospital, Sweden.
The present study was performed at the Public Health Laboratory, Health Service of Amsterdam, the Netherlands, and the WHO Collaborating Centre for Gonorrhoea and other STIs, Örebro University Hospital, Örebro, Sweden.
Footnotes
Published ahead of print 3 January 2012
REFERENCES
- 1. Allen VG, et al. 2011. Molecular analysis of antimicrobial resistance mechanisms in Neisseria gonorrhoeae isolates from Ontario, Canada. Antimicrob. Agents Chemother. 55:703–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ameyama S, et al. 2002. Mosaic-like structure of penicillin-binding protein 2 Gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob. Agents Chemother. 46:3744–3749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barry PM, Klausner JD. 2009. The use of cephalosporins for gonorrhea: the impending problem of resistance. Expert. Opin. Pharmacother. 10:555–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bignell C. 2009. 2009 European (IUSTI/WHO) guideline on the diagnosis and treatment of gonorrhoea in adults. Int. J. STD AIDS 20:453–457 [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention (CDC) 2011. Cephalosporin susceptibility among Neisseria gonorrhoeae isolates—United States, 2000–2010. MMWR Morb. Mortal. Wkly. Rep. 60:873–877 [PubMed] [Google Scholar]
- 6. Deguchi T, Yasuda M, Maeda S. 2008. Lack of nationwide surveillance of antimicrobial resistance of Neisseria gonorrhoeae in Japan. Ann. Intern. Med. 149:363–364 [DOI] [PubMed] [Google Scholar]
- 7. de Vries HJ, van der Helm JJ, Schim van der Loeff MF, van Dam AP. 2009. Multidrug-resistant Neisseria gonorrhoeae with reduced cefotaxime susceptibility is increasingly common in men who have sex with men, Amsterdam, the Netherlands. Euro Surveill. 14(37):pii=19330 [DOI] [PubMed] [Google Scholar]
- 8. Domain Group for Sexually Transmitted Infections (NVDV) 2009. Diagnostics and treatment of sexually transmitted infections, version 2008/2009. SoaAids, Amsterdam, the Netherlands: http://www.soaaids-professionals.nl/medische_richtlijnen/nvdv. (In Dutch.) [Google Scholar]
- 9. Florindo C, et al. 2010. Genotypes and antimicrobial-resistant phenotypes of Neisseria gonorrhoeae in Portugal (2004–2009). Sex. Transm. Infect. 86:449–453 [DOI] [PubMed] [Google Scholar]
- 10. Golparian D, Hellmark B, Fredlund H, Unemo M. 2010. Emergence, spread and characteristics of Neisseria gonorrhoeae isolates with in vitro decreased susceptibility and resistance to extended-spectrum cephalosporins in Sweden. Sex. Transm. Infect. 86:454–460 [DOI] [PubMed] [Google Scholar]
- 11. Heijman TL, et al. 2007. Effectiveness of a risk-based visitor-prioritizing system at a sexually transmitted infection outpatient clinic. Sex. Transm. Dis. 34:508–512 [DOI] [PubMed] [Google Scholar]
- 12. Heymans R, Schouls LM, van der Heide HG, van der Loeff MF, Bruisten SM. 2011. Multiple-locus variable-number tandem repeat analysis of Neisseria gonorrhoeae. J. Clin. Microbiol. 49:354–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang CT, et al. 2010. Characteristics and dissemination of mosaic penicillin-binding protein 2-harboring multidrug-resistant Neisseria gonorrhoeae isolates with reduced cephalosporin susceptibility in northern Taiwan. Antimicrob. Agents Chemother. 54:4893–4895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ito M, et al. 2005. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in Central Japan. Antimicrob. Agents Chemother. 49:137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee SG, et al. 2010. Various penA mutations together with mtrR, porB and ponA mutations in Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime or ceftriaxone. J. Antimicrob. Chemother. 65:669–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lewis DA. 2010. The Gonococcus fights back: is this time a knock out? Sex. Transm. Infect. 86:415–421 [DOI] [PubMed] [Google Scholar]
- 17. Lindberg R, Fredlund H, Nicholas R, Unemo M. 2007. Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime and ceftriaxone: association with genetic polymorphisms in penA, mtrR, porB1b, and ponA. Antimicrob. Agents Chemother. 51:2117–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin IM, Ison CA, Aanensen DM, Fenton KA, Spratt BG. 2004. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J. Infect. Dis. 189:1497–1505 [DOI] [PubMed] [Google Scholar]
- 19. Moran JS. 1995. Treating uncomplicated Neisseria gonorrhoeae infections: is the anatomic site of infection important? Sex. Transm. Dis. 22:39–47 [DOI] [PubMed] [Google Scholar]
- 20. Ohnishi M, et al. 2011. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea? Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob. Agents Chemother. 55:3538–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohnishi M, et al. 2011. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg. Infect. Dis. 17:148–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pandori M, et al. 2009. Mosaic penicillin-binding protein 2 in Neisseria gonorrhoeae isolates collected in 2008 in San Francisco, California. Antimicrob. Agents Chemother. 53:4032–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takahata S, Senju N, Osaki Y, Yoshida T, Ida T. 2006. Amino acid substitutions in mosaic penicillin-binding protein 2 associated with reduced susceptibility to cefixime in clinical isolates of Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 50:3638–3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tapsall JW. 2009. Neisseria gonorrhoeae and emerging resistance to extended spectrum cephalosporins. Curr. Opin. Infect. Dis. 22:87–91 [DOI] [PubMed] [Google Scholar]
- 25. Tapsall JW, Ndowa F, Lewis DA, Unemo M. 2009. Meeting the public health challenge of multidrug-and extensively drug-resistant Neisseria gonorrhoeae. Expert. Rev. Anti. Infect. Ther. 7:821–834 [DOI] [PubMed] [Google Scholar]
- 26. Tapsall JW, Ray S, Limnios A. 2010. Characteristics and population dynamics of mosaic penA allele-containing Neisseria gonorrhoeae isolates collected in Sydney, Australia, in 2007–2008. Antimicrob. Agents Chemother. 54:554–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tomberg J, Unemo M, Davies C, Nicholas RA. 2010. Molecular and structural analysis of mosaic variants of penicillin-binding protein 2 conferring decreased susceptibility to expanded-spectrum cephalosporins in Neisseria gonorrhoeae: role of epistatic mutations. Biochemistry 49:8062–8070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Unemo M, Fasth O, Fredlund H, Limnios A, Tapsall J. 2009. Phenotypic and genetic characterization of the 2008 WHO Neisseria gonorrhoeae reference strain panel intended for global quality assurance and quality control of gonococcal antimicrobial resistance surveillance for public health purposes. J. Antimicrob. Chemother. 63:1142–1151 [DOI] [PubMed] [Google Scholar]
- 29. Unemo M, Golparian D, Hestner A. 2011. Ceftriaxone treatment failure of pharyngeal gonorrhoea verified by international recommendations, Sweden, July 2010. Euro Surveill. 16(6):pii=19792 [PubMed] [Google Scholar]
- 30. Unemo M, Golparian D, Syversen G, Vestrheim DF, Moi H. 2010. Two cases of verified clinical failures using internationally recommended first-line cefixime for gonorrhoea treatment, Norway, 2010. Euro Surveill. 15(47):pii=19721 [DOI] [PubMed] [Google Scholar]
- 31. Unemo M, et al. 2007. Molecular characterization of Neisseria gonorrhoeae identifies transmission and resistance of one ciprofloxacin-resistant strain. APMIS 115:231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whiley D, et al. 2007. Use of a novel screening PCR indicates presence of Neisseria gonorrhoeae isolates with a mosaic penA gene sequence in Australia. Pathology 39:445–446 [DOI] [PubMed] [Google Scholar]
- 33. Whiley DM, et al. 2010. Reduced susceptibility to ceftriaxone in Neisseria gonorrhoeae is associated with mutations G542S, P551S and P551L in the gonococcal penicillin-binding protein 2. J. Antimicrob. Chemother. 65:1615–1618 [DOI] [PubMed] [Google Scholar]
- 34. Whiley DM, et al. 2010. Alterations of the pilQ gene in Neisseria gonorrhoeae are unlikely contributors to decreased susceptibility to ceftriaxone and cefixime in clinical gonococcal strains. J. Antimicrob. Chemother. 65:2543–2547 [DOI] [PubMed] [Google Scholar]
- 35. Workowski KA, Berman SM, Douglas JM., Jr 2008. Emerging antimicrobial resistance in Neisseria gonorrhoeae: urgent need to strengthen prevention strategies. Ann. Intern. Med. 148:606–613 [DOI] [PubMed] [Google Scholar]
- 36. Xia M, Whittington WL, Shafer WM, Holmes KK. 2000. Gonorrhea among men who have sex with men: outbreak caused by a single genotype of erythromycin-resistant Neisseria gonorrhoeae with a single-base pair deletion in the mtrR promoter region. J. Infect. Dis. 181:2080–2082 [DOI] [PubMed] [Google Scholar]
- 37. Yokoi S, et al. 2007. Threat to cefixime treatment for gonorrhea. Emerg. Infect. Dis. 13:1275–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao S, et al. 2009. Genetics of chromosomally mediated intermediate resistance to ceftriaxone and cefixime in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 53:3744–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]