Abstract
The majority of human infections associated with H7 influenza viruses have resulted in ocular and not respiratory disease. While oseltamivir has been prescribed to individuals presenting with conjunctivitis following H7 virus exposure, it is unknown if oseltamivir inhibits virus replication in ocular tissue. We demonstrate that H7 viruses possess sensitivity to neuraminidase inhibitors and that administration of oseltamivir before ocular virus challenge in mice inhibits H7N7 and H7N3 virus replication in ocular and respiratory tissues.
TEXT
Neuraminidase (NA) inhibitors are the most widely used class of antiviral drug for influenza viruses and are well documented to alleviate the symptoms of respiratory disease following seasonal influenza virus infection in humans (11). However, unlike other influenza virus subtypes that cause predominant respiratory disease in humans, H7 influenza viruses frequently result in ocular rather than respiratory symptoms (3). Oseltamivir prophylaxis has nonetheless been prescribed during several H7 virus outbreaks resulting in human infection, including H7N7 virus in The Netherlands in 2003, H7N3 virus in Canada in 2004, and H7N2 in Wales in 2007 (9, 16, 25) (Table 1). Retrospective studies have examined the role of oseltamivir prophylaxis in developing conjunctivitis and/or influenza-like illness following H7 virus exposure, finding that the prophylactic use of oseltamivir resulted in a decreased risk of virus infection, including self-reported conjunctivitis (16, 17, 20, 22). While a limited number of studies have demonstrated the sensitivity of H7 viruses to currently available antiviral drugs (13, 14, 16, 21), the ability of oseltamivir to inhibit H7 virus infection has not been examined in vivo. Furthermore, it has not been shown experimentally if prophylaxis with neuraminidase inhibitors can reduce directly the presence of virus in the eye or inhibit virus spread from ocular sites to respiratory tract tissues. Previous studies from our laboratory have identified that intranasal and ocular inoculation of virus results in different kinetics of virus replication and spread in vivo, underscoring the need to examine the efficacy of antiviral treatments for multiple routes of exposure (5; J. A. Belser, K. M. Gustin, T. R. Maines, J. M. Katz, and T. M. Tumpey, unpublished data).
Table 1.
Sensitivity of H7 influenza viruses associated with disease in humans in the NA inhibition assay with MUNANAd substrate
| Virusa | Subtypeb | Patient symptom(s)a | Mean IC50 ± SD (nM)c |
||
|---|---|---|---|---|---|
| Zanamivir | Oseltamivir carboxylate | Peramivir | |||
| A/Netherlands/219/2003 | HPAI H7N7 | Respiratory, fatal | 6.72 ± 0.97 | 3.28 ± 0.37 | 1.54 ± 0.01 |
| A/Netherlands/230/2003 | HPAI H7N7 | Conjunctivitis | 3.36 ± 0.42 | 1.05 ± 0.05 | 0.62 ± 0.09 |
| A/Canada/504/2004 | HPAI H7N3 | Conjunctivitis | 2.95 ± 0.16 | 1.64 ± 0.22 | 1.32 ± 0.15 |
| A/Canada/444/2004 | LPAI H7N3 | Conjunctivitis | 3.41 ± 0.13 | 2.05 ± 0.11 | 1.56 ± 0.04 |
| A/New York/107/2003 | LPAI H7N2 | Respiratory | 2.58 ± 0.33 | 0.55 ± 0.06 | 0.76 ± 0.11 |
Source information for clinical isolates and patient information was published previously (3, 10, 25). Identical virus stocks were used for in vitro and in vivo experiments.
HPAI, highly pathogenic avian influenza virus; LPAI, low-pathogenicity avian influenza virus.
Mean IC50 ± standard deviation (SD) values were calculated from data collected from duplicate independent experiments.
MUNANA, 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid.
While previous work has identified strain-specific differences in NA inhibitor sensitivity within virus subtypes (27), only a few H7 subtype viruses have been evaluated to date. Numerous differences have been identified between the Eurasian and North American lineages of H7 influenza viruses, including their virulence in mammals, hemagglutinin receptor-binding preference, and induction of host innate immune responses (1, 2, 4, 6, 15). To determine the sensitivity to existing NA inhibitors of H7 viruses of both lineages associated with disease in humans, we performed an NA activity inhibition assay with the NA inhibitors oseltamivir carboxylate (Roche), zanamivir (GlaxoSmithKline), and perami-vir (BioCryst) (Table 1); this assay has been shown previously to be more predictive than cell-culture-based systems for the assessment of influenza NA inhibitor drug susceptibility (12, 23, 26). Fifty percent inhibitory concentration values (IC50s) of each virus were determined using a fluorescent NA inhibition assay performed as described previously (18). All H7 subtype viruses tested (covering multiple NA subtypes) were sensitive to all NA inhibitors examined, with levels of inhibitory enzyme activity comparable to those of other susceptible seasonal influenza A and B viruses (19).
To determine the sensitivity of H7 influenza viruses to oseltamivir in vivo, we inoculated BALB/c mice by the ocular route with two H7 viruses which exhibit divergent phenotypes in this model (5). A/Netherlands/219/2003 virus (H7N7 [NL/219]) replicates efficiently in both ocular and respiratory tract tissues without prior adaptation and causes 30% mortality following ocular inoculation. In contrast, infection of mice with A/Canada/504/2004 virus (H7N3 [Can/504]) does not cause substantial morbidity, although efficient replication in ocular tissue is detected. Oseltamivir (50 mg/kg body weight; Roche) was administered by oral gavage once daily for 8 days commencing 24 h before virus inoculation (24). Control mice (inoculated but untreated) received distilled water on the same schedule. Six-week-old BALB/c mice were inoculated with 106 50% egg infective doses (EID50) of NL/219 or Can/504 virus by the ocular route as previously described, and 5 to 6 mice/group were monitored daily for 2 weeks for morbidity (as measured by weight loss) and mortality (5). Four to five mice from each group were sacrificed on days 3 and 6 postinfection to collect eye, nose, and lung tissue for viral titration in eggs as previously described (5).
Oseltamivir administration was efficacious against both H7N7 and H7N3 viruses in mice, although complete inhibition of infection was not observed (Table 2). Mice inoculated by the ocular route with NL/219 virus and treated with oseltamivir were protected from weight loss and death, whereas untreated mice exhibited a mean weight loss of 14% on day 10 postinoculation (p.i.) with 30% mortality (Fig. 1). The administration of oseltamivir resulted in a decrease in both the titer and frequency of virus detection in all tissues examined in mice inoculated with H7 viruses by the ocular route. Strikingly, virus was not detected in the eyes of mice receiving oseltamivir when challenged with either NL/219 or Can/504 virus, while virus replication was detected in eyes of all untreated mice on day 6 p.i. These results suggest that oseltamivir can inhibit virus replication in ocular tissue in addition to the respiratory tract.
Table 2.
Effect of orally administered oseltamivir on virus titers in mice inoculated by the ocular route with H7 influenza viruses
| Tissue | Day p.i. | No. of mice with virus detected/totala |
|||
|---|---|---|---|---|---|
| NL/219 |
Can/504 |
||||
| Oseltamivirb | No treatmentc | Oseltamivirb | No treatmentc | ||
| Nose | 3 | 0/5* | 3/4 (4.3 ± 0.1) | 0/5 | 0/4 |
| Lung | 3 | 1/5 (5.5) | 4/4 (3.4 ± 1.8) | 0/5 | 0/4 |
| Eye | 6 | 0/5** | 4/4 (2.7 ± 0.4) | 0/4** | 5/5 (2.9 ± 0.8) |
| Nose | 6 | 1/5 (4.3)* | 4/4 (3.7 ± 1.2) | 0/4* | 4/5 (2.3 ± 1.1) |
| Lung | 6 | 2/5 (2.6 ± 1.6)** | 4/4 (5.7 ± 2.2) | 0/4 | 2/5 (1.1 ± 0.2) |
Values in parentheses reflect the mean viral titer in eggs ± SD of all positive samples, expressed as log10 EID50/ml. The limit of detection is 0.8 log10 EID50/ml, with tissues homogenized and titrated as previously described (5). *, P < 0.05, and **, P < 0.005, compared with untreated mice with homologous virus challenge. Significance was determined by Student's t test as previously described (5).
Oseltamivir was administered once daily by oral gavage (50 mg/kg), starting 24 h preinoculation for 8 days total.
Distilled water was administered by oral gavage as a control on the same schedule as oseltamivir.
Fig 1.
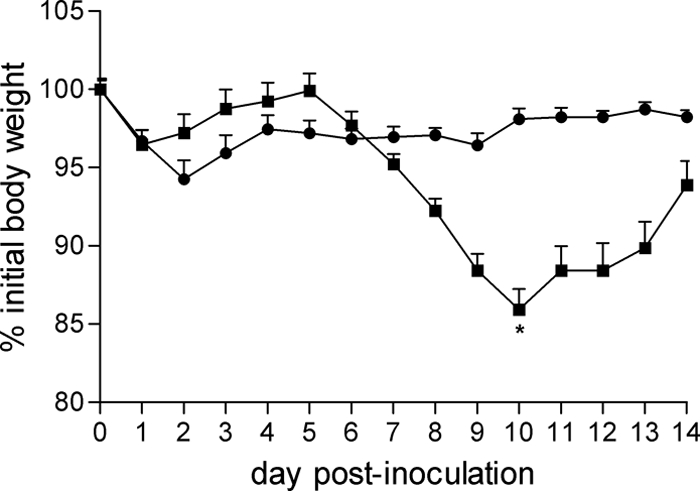
Efficacy of oseltamivir against NL/219 virus in mice following ocular inoculation. Oseltamivir was administered once daily by oral gavage (50 mg/kg), starting 24 h preinoculation for 8 days total. BALB/c mice were inoculated by the ocular route with 106 EID50/5 μl NL/219 virus and monitored daily for morbidity and mortality. Data are expressed as a percentage of mean starting weight plus standard deviation. *, two mice treated with distilled water did not survive.
Isolation of virus from conjunctival swabs of persons infected with H7 viruses suggests that the clinical management of human H7 disease should include suppression of virus replication in ocular tissue. Documented spread of virus from ocular to respiratory tract tissues following ocular exposure to influenza virus in a murine model provides a further rationale for reducing viral titers in the eye via the use of antiviral drugs (J. A. Belser, P. A. Rota, and T. M. Tumpey, unpublished data). In this study, we demonstrate that both lineages of H7 subtype viruses associated with disease in humans are sensitive to existing NA inhibitors in vitro and that intragastric administration of oseltamivir to mice challenged with influenza virus by the ocular route inhibits virus replication in both ocular and respiratory tissues. The reduction in H7 viral titers in respiratory tract tissues is similar to that in previous studies in mice infected with an H5N1 virus and administered oseltamivir prophylaxis (27). As mice inoculated by the ocular route with influenza virus do not present with conjunctivitis (5), further study is warranted to more fully evaluate the ability of antiviral treatments to mitigate ocular disease caused by influenza virus infection. However, this is the first report demonstrating the ability of an influenza antiviral treatment to reduce viral replication in ocular tissue with viruses known to cause conjunctivitis in humans. Collectively, this study provides experimental data in support of the use of oseltamivir during outbreaks of influenza resulting in ocular disease in addition to the use of recommended personal protection (7, 8).
ACKNOWLEDGMENT
The findings and conclusions in this report are those of the authors and do not necessarily reflect the views of the funding agency.
Footnotes
Published ahead of print 12 December 2011
REFERENCES
- 1. Banks J, Speidel EC, McCauley JW, Alexander DJ. 2000. Phylogenetic analysis of H7 haemagglutinin subtype influenza A viruses. Arch. Virol. 145:1047–1058 [DOI] [PubMed] [Google Scholar]
- 2. Belser JA, et al. 2008. Contemporary North American influenza H7 viruses possess human receptor specificity: implications for virus transmissibility. Proc. Natl. Acad. Sci. U. S. A. 105:7558–7563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Belser JA, Bridges CB, Katz JM, Tumpey TM. 2009. Past, present, and possible future human infection with influenza virus A subtype H7. Emerg. Infect. Dis. 15:859–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belser JA, et al. 2007. Pathogenesis of avian influenza (H7) virus infection in mice and ferrets: enhanced virulence of Eurasian H7N7 viruses isolated from humans. J. Virol. 81:11139–11147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belser JA, Wadford DA, Xu J, Katz JM, Tumpey TM. 2009. Ocular infection of mice with influenza A (H7) viruses: a site of primary replication and spread to the respiratory tract. J. Virol. 83:7075–7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belser JA, Zeng H, Katz JM, Tumpey TM. 2011. Infection with highly pathogenic H7 influenza viruses results in an attenuated proinflammatory cytokine and chemokine response early after infection. J. Infect. Dis. 203:40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. CDC 20 September 2010, posting date Guidelines and recommendations: prevention strategies for seasonal influenza in healthcare settings. Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 8. CDC 14 January 2006, posting date Interim guidance for protection of persons involved in U.S. avian influenza outbreak disease control and eradication activities. Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 9. Eames KT, et al. 2010. Assessing the role of contact tracing in a suspected H7N2 influenza A outbreak in humans in Wales. BMC Infect. Dis. 10:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fouchier RA, et al. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl. Acad. Sci. U. S. A. 101:1356–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gubareva LV, Kaiser L, Hayden FG. 2000. Influenza virus neuraminidase inhibitors. Lancet 355:827–835 [DOI] [PubMed] [Google Scholar]
- 12. Gubareva LV, Matrosovich MN, Brenner MK, Bethell RC, Webster RG. 1998. Evidence for zanamivir resistance in an immunocompromised child infected with influenza B virus. J. Infect. Dis. 178:1257–1262 [DOI] [PubMed] [Google Scholar]
- 13. Gubareva LV, Penn CR, Webster RG. 1995. Inhibition of replication of avian influenza viruses by the neuraminidase inhibitor 4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid. Virology 212:323–330 [DOI] [PubMed] [Google Scholar]
- 14. Ilyushina NA, Govorkova EA, Russell CJ, Hoffmann E, Webster RG. 2007. Contribution of H7 haemagglutinin to amantadine resistance and infectivity of influenza virus. J. Gen. Virol. 88:1266–1274 [DOI] [PubMed] [Google Scholar]
- 15. Joseph T, et al. 2007. Evaluation of replication and pathogenicity of avian influenza a H7 subtype viruses in a mouse model. J. Virol. 81:10558–10566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koopmans M, et al. 2004. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet 363:587–593 [DOI] [PubMed] [Google Scholar]
- 17. Morgan O, et al. 2009. Personal protective equipment and risk for avian influenza (H7N3). Emerg. Infect. Dis. 15:59–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nguyen HT, Sheu TG, Mishin VP, Klimov AL, Gubareva LV. 2010. Assessment of pandemic and seasonal influenza A (H1N1) virus susceptibility to neuraminidase inhibitors in three enzyme activity inhibition assays. Antimicrob. Agents Chemother. 54:3671–3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sheu TG, et al. 2008. Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob. Agents Chemother. 52:3284–3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Skowronski DM, et al. 2007. Protective measures and human antibody response during an avian influenza H7N3 outbreak in poultry in British Columbia, Canada. CMAJ 176:47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sleeman K, et al. 2010. In vitro antiviral activity of favipiravir (T-705) against drug-resistant influenza and 2009 A(H1N1) viruses. Antimicrob. Agents Chemother. 54:2517–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. te Beest DE, van Boven M, Bos ME, Stegeman A, Koopmans MP. 2010. Effectiveness of personal protective equipment and oseltamivir prophylaxis during avian influenza A (H7N7) epidemic, the Netherlands, 2003. Emerg. Infect. Dis. 16:1562–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tisdale M. 2000. Monitoring of viral susceptibility: new challenges with the development of influenza NA inhibitors. Rev. Med. Virol. 10:45–55 [DOI] [PubMed] [Google Scholar]
- 24. Tumpey TM, et al. 2002. Existing antivirals are effective against influenza viruses with genes from the 1918 pandemic virus. Proc. Natl. Acad. Sci. U. S. A. 99:13849–13854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tweed SA, et al. 2004. Human illness from avian influenza H7N3, British Columbia. Emerg. Infect. Dis. 10:2196–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Woods JM, et al. 1993. 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid is a highly effective inhibitor both of the sialidase (neuraminidase) and of growth of a wide range of influenza A and B viruses in vitro. Antimicrob. Agents Chemother. 37:1473–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yen HL, Monto AS, Webster RG, Govorkova EA. 2005. Virulence may determine the necessary duration and dosage of oseltamivir treatment for highly pathogenic A/Vietnam/1203/04 influenza virus in mice. J. Infect. Dis. 192:665–672 [DOI] [PubMed] [Google Scholar]


