Abstract
The Tigecycline In Vitro Surveillance in Taiwan (TIST) study, a nationwide, prospective surveillance during 2006 to 2010, collected a total of 7,793 clinical isolates, including methicillin-resistant Staphylococcus aureus (MRSA) (n = 1,834), penicillin-resistant Streptococcus pneumoniae (PRSP) (n = 423), vancomycin-resistant enterococci (VRE) (n = 219), extended-spectrum β-lactamase (ESBL)-producing Escherichia coli (n = 1,141), ESBL-producing Klebsiella pneumoniae (n = 1,330), Acinetobacter baumannii (n = 1,645), and Stenotrophomonas maltophilia (n = 903), from different specimens from 20 different hospitals in Taiwan. MICs of tigecycline were determined following the criteria of the U.S. Food and Drug Administration (FDA) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST-2011). Among drug-resistant Gram-positive pathogens, all of the PRSP isolates were susceptible to tigecycline (MIC90, 0.03 μg/ml), and only one MRSA isolate (MIC90, 0.5 μg/ml) and three VRE isolates (MIC90, 0.125 μg/ml) were nonsusceptible to tigecycline. Among the Gram-negative bacteria, the tigecycline susceptibility rates were 99.65% for ESBL-producing E. coli (MIC90, 0.5 μg/ml) and 96.32% for ESBL-producing K. pneumoniae (MIC90, 2 μg/ml) when interpreted by FDA criteria but were 98.7% and 85.8%, respectively, when interpreted by EUCAST-2011 criteria. The susceptibility rate for A. baumannii (MIC90, 4 μg/ml) decreased from 80.9% in 2006 to 55.3% in 2009 but increased to 73.4% in 2010. A bimodal MIC distribution was found among carbapenem-susceptible A. baumannii isolates, and a unimodal MIC distribution was found among carbapenem-nonsusceptible A. baumannii isolates. In Taiwan, tigecycline continues to have excellent in vitro activity against several major clinically important drug-resistant bacteria, with the exception of A. baumannii.
INTRODUCTION
Microbial resistance is a major public health issue worldwide but especially in Asia (5, 11, 12). It has a direct impact on mortality, morbidity, length of hospital stay, and health care costs (7). Multidrug resistance has been found among many pathogens, including Gram-positive and Gram-negative bacteria. Among the most clinically relevant Gram-positive pathogens, methicillin-resistant Staphylococcus aureus (MRSA), penicillin-resistant Streptococcus pneumoniae (PRSP), and vancomycin-resistant enterococci (VRE) are the most common threats (1, 13). Among the most clinically relevant Gram-negative bacteria, extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae (such as Escherichia coli and Klebsiella pneumoniae), multidrug-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia pose the greatest threats to health care systems (1, 4, 18). These infections pose a great challenge to clinicians in regard to the choice of appropriate antimicrobial therapy, especially the initial empirical treatment. Continuous surveillance of antimicrobial susceptibility would provide important information locally and globally.
Tigecycline, the first semisynthetic glycylcycline available for clinical application, is a novel 9-t-butyl-substituted minocycline derivative that overcomes several major tetracycline resistance mechanisms (3). It demonstrates broad-spectrum antimicrobial effects against multiple resistant Gram-positive, Gram-negative, anaerobic, and atypical pathogens (3). Tigecycline demonstrated excellent sustained in vitro activity against a wide spectrum of contemporary Gram-positive and Gram-negative pathogens from countries in the Asia-Pacific region in 2008 (10). Tigecycline has been approved as the agent of choice for treating complicated intra-abdominal infections, complicated skin and soft tissue infections, and community-acquired lower respiratory tract infections (3).
The Tigecycline In Vitro Surveillance in Taiwan (TIST) study, a nationwide, multicenter prospective surveillance study, was conducted by 20 medical centers and regional hospitals throughout Taiwan over a 5-year period (2006 to 2010) (10). This program was initiated to monitor the tigecycline susceptibilities of several major clinically relevant antibiotic-resistant isolates of bacteria in hospitals in Taiwan.
MATERIALS AND METHODS
Bacterial isolates.
During the period from 1 January 2006 to 31 December 2010, clinical isolates of MRSA (n = 1,834), PRSP (n = 423), VRE (n = 219), ESBL-producing E. coli (n = 1,141), ESBL-producing K. pneumoniae (n = 1,330), A. baumannii (n = 1,645), and S. maltophilia (n = 903) were collected from different specimens at different hospitals (Table 1). The isolates were identified as clinically relevant causative pathogens, and duplicate isolates from the same patients were excluded. These isolates were identified at each hospital and reconfirmed by the central laboratory at the National Taiwan University Hospital. Before testing, these isolates were stored at −70°C in Trypticase soy broth (BBL Microbiology Systems, Cockeysville, MD) supplemented with 15% glycerol.
Table 1.
Sources of bacterial isolates in the study from 2006 to 2010
| Bacterium(a) (no. of isolates) | No. of isolates from clinical specimen: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Blood | Pleural effusion | Ascites | Cerebrospinal fluid | Sputum | Urine | Pus | Pus (intra-abdominal infection) | Other | |
| MRSA (1,834) | 642 | 21 | 10 | 1 | 204 | 18 | 872 | 20 | 46 |
| PRSP (423) | 117 | 5 | 0 | 2 | 189 | 0 | 107 | 0 | 3 |
| VRE (219) | 48 | 4 | 8 | 3 | 2 | 66 | 67 | 6 | 15 |
| ESBL-producing E. coli (1,141) | 416 | 9 | 19 | 4 | 136 | 295 | 197 | 48 | 17 |
| ESBL-producing K. pneumoniae (1,330) | 377 | 20 | 21 | 5 | 414 | 202 | 224 | 49 | 18 |
| A. baumannii (1,645) | 491 | 14 | 19 | 9 | 674 | 78 | 305 | 17 | 38 |
| S. maltophilia (903) | 245 | 13 | 20 | 9 | 408 | 24 | 136 | 27 | 21 |
Antimicrobial susceptibility testing.
MICs of tigecycline were determined by the broth microdilution method according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (range, 0.008 to 16 μg/ml) (6). The U.S. Food and Drug Administration (FDA) and European Committee on Antimicrobial Susceptibility Testing (EUCAST-2011; www.eucast.org) criteria were used to interpret the results (Table 2). Quality control tests were performed using S. pneumoniae ATCC 49619, S. aureus ATCC 25923, S. aureus ATCC 29213, E. faecalis ATCC 29212, E. coli ATCC 25922 and 35218, and Pseudomonas aeruginosa ATCC 27853. Information about the susceptibility of A. baumannii isolates to carbapenems (either imipenem or meropenem), determined by the routine disk diffusion method, was available only for 2008 to 2010. There were no tigecycline breakpoints for A. baumannii recommended by the FDA and EUCAST. In this study, the tigecycline MIC interpretive breakpoints for Enterobacteriaceae recommended by the FDA (susceptible, ≤2 μg/ml; intermediate, 4 μg/ml; and resistant, ≥8 μg/ml) were applied to Acinetobacter spp. (18).
Table 2.
MIC interpretive criteria for Gram-positive and Gram-negative bacteria applied in this study
| Bacterium(a) | FDA/EUCAST-2011 MIC (μg/ml) interpretive criteria for tigecycline |
||
|---|---|---|---|
| Susceptible | Intermediate | Resistant | |
| MRSA | ≤0.5/≤0.5 | NAa | NA/>0.5 |
| PRSP | ≤0.06/NA | NA | >.012/NA |
| VRE | ≤0.25/≤0.25 | NA/0.5 | NA/>0.5 |
| ESBL-producing E. coli | ≤2/≤1 | 4/2 | ≥8/>2 |
| ESBL-producing K. pneumoniae | ≤2/≤1 | 4/2 | ≥8/>2 |
| A. baumanniib | ≤2/≤1 | 4/2 | ≥8/>2 |
NA, MIC interpretive breakpoint for tigecycline was not available.
The tigecycline MIC interpretive breakpoints for Enterobacteriaceae recommended by the FDA (susceptible, ≤2 μg/ml; intermediate, 4 μg/ml; and resistant, ≥8 μg/ml) were applied to Acinetobacter spp. for comparison (18).
Statistical analysis.
Linear regression analysis was used to analyze the trend in the rates of susceptibility of the tested organisms to tigecycline. A P value of ≤0.05 was considered statistically significant.
RESULTS
All PRSP isolates (100%; 423 isolates) were susceptible to tigecycline (Table 3). In addition, only one of the 1,834 isolates of MRSA and three of the 219 VRE isolates were nonsusceptible to tigecycline. Among the ESBL-producing Enterobacteriaceae species, 99.7% of ESBL-producing E. coli and 96.3% of ESBL-producing K. pneumoniae isolates were susceptible to tigecycline according to the FDA criteria. However, the susceptibility rates were 98.7% for ESBL-producing E. coli and 85.8% for ESBL-producing K. pneumoniae when EUCAST-2011 criteria were used. In addition, the susceptibility rate for A. baumannii was 68.2% according to the breakpoint criteria provided by the FDA and 35% according to criteria provided by EUCAST-2011.
Table 3.
Tigecycline susceptibilities of various antimicrobial-resistant isolates, obtained from 2006 to 2010, determined by the broth microdilution method
| Bacterium(a) (no. of isolates) | Range | MIC (μg/ml) |
% of isolates categorized by: |
||||||
|---|---|---|---|---|---|---|---|---|---|
| FDA MIC interpretive criteria |
EUCAST-2011 MIC interpretive criteria |
||||||||
| MIC50 | MIC90 | Susceptible | Intermediate | Resistant | Susceptible | Intermediate | Resistant | ||
| MRSA (1,834) | 0.03–1 | 0.125 | 0.5 | 100 | NAa | NA | 100 | NA | 0 |
| PRSP (423) | 0.008–0.06 | 0.016 | 0.03 | 100 | 0 | 0 | NA | NA | NA |
| VRE (219) | 0.016–1 | 0.03 | 0.125 | 98.6 | 0 | 0 | 98.6 | 0.9 | 0.5 |
| ESBL-producing E. coli (1,141) | 0.03–8 | 0.25 | 0.5 | 99.6 | 0.3 | 0.1 | 98.7 | 1.0 | 0.3 |
| ESBL-producing K. pneumoniae (1,330) | 0.125–16 | 0.5 | 2 | 96.3 | 2.9 | 0.8 | 85.8 | 10.5 | 3.7 |
| A. baumannii (1,645) | 0.03–64 | 2 | 4 | 68.2 | 23.1 | 8.8 | 35.0 | 33.1 | 31.9 |
| S. maltophilia (903) | 0.03–16 | 2 | 4 | NA | NA | NA | NA | NA | NA |
NA, MIC interpretive breakpoint for tigecycline was not available.
Annual tigecycline susceptibility changes for each pathogen are shown in Table 4. The only nonsusceptible MRSA isolate was found in 2007. The three nonsusceptible VRE isolates were isolated in 2008 (n = 1) and in 2010 (n = 2). The seven (1.7%) ESBL-producing E. coli isolates determined to be nonsusceptible by EUCAST-2011 criteria were found in 2008, and there was no significant change (P = 0.33 by FDA and P = 0.19 by EUCAST-2011 criteria) in susceptibility rates during the study period. However, the nonsusceptibility rate for ESBL-producing K. pneumoniae decreased from 98.5% to 89.8% (P = 0.21) during the study period according to FDA criteria and from 89.8% to 81.4% (P = 0.22) during the study period according to EUCAST-2011 criteria. In addition, there was a significant decline in susceptibility of A. baumannii to tigecycline from 2006 (80.9%) to 2009 (55.3%) (P < 0.001), but susceptibility rose in 2010 (73.4%) according to FDA criteria. A similar trend was noted when the EUCAST-2011 criteria were used. The rates of susceptibility of ESBL-producing K. pneumoniae to tigecycline were 8.7% when measured by FDA criteria and 12.9% when measured by EUCAST-2011 criteria. The rates of susceptibility of A. baumannii to tigecycline were 20.2% when measured US FDA criteria and 38.4% when measured by EUCAST-2011 criteria.
Table 4.
Annual rates of tigecycline susceptibility of isolates, determined using the broth microdilution method, from 2006 to 2010
| Bacterium(a) and yr (no. of isolates) | % of isolates categorized by: |
|||||
|---|---|---|---|---|---|---|
| FDA MIC interpretive criteria |
EUCAST-2011 MIC interpretive criteria |
|||||
| Susceptible | Intermediate | Resistant | Susceptible | Intermediate | Resistant | |
| MRSA (1,834) | ||||||
| 2006 (540) | 100 | NAa | NA | 100 | NA | 0 |
| 2007 (535) | 99.8 | NA | NA | 99.8 | NA | 0.2 |
| 2008 (382) | 100 | NA | NA | 100 | NA | 0 |
| 2009 (187) | 100 | NA | NA | 100 | NA | 0 |
| 2010 (190) | 100 | NA | NA | 100 | NA | 0 |
| PRSP (423) | ||||||
| 2006 (96) | 100 | 0 | 0 | NA | NA | NA |
| 2007 (101) | 100 | 0 | 0 | NA | NA | NA |
| 2008 (164) | 100 | 0 | 0 | NA | NA | NA |
| 2009 (33) | 100 | 0 | 0 | NA | NA | NA |
| 2010 (29) | 100 | 0 | 0 | NA | NA | NA |
| VRE (219) | ||||||
| 2006 (13) | 100 | NA | NA | 100 | 0 | 0 |
| 2007 (15) | 100 | NA | NA | 100 | 0 | 0 |
| 2008 (41) | 97.6 | NA | NA | 97.6 | 2.4 | 0 |
| 2009 (78) | 100 | NA | NA | 100 | 0 | 0 |
| 2010 (72) | 97.2 | NA | NA | 97.2 | 1.4 | 1.4 |
| ESBL-producing E. coli (1,141) | ||||||
| 2006 (275) | 99.6 | 0.4 | 0 | 98.9 | 0.7 | 0.4 |
| 2007 (264) | 99.6 | 0.4 | 0 | 99.2 | 0.4 | 0.4 |
| 2008 (416) | 100 | 0 | 0 | 98.3 | 1.7 | 0 |
| 2009 (94) | 100 | 0 | 0 | 98.9 | 1.1 | 0 |
| 2010 (92) | 97.8 | 1.1 | 1.1 | 97.8 | 0 | 2.2 |
| ESBL-producing K. pneumoniae (1,330) | ||||||
| 2006 (324) | 98.5 | 0.9 | 0.6 | 89.8 | 8.6 | 1.5 |
| 2007 (270) | 98.5 | 1.5 | 0 | 89.6 | 8.9 | 1.5 |
| 2008 (385) | 93.7 | 5.5 | 0.8 | 80.8 | 13.0 | 6.2 |
| 2009 (179) | 97.8 | 1.7 | 0.6 | 87.7 | 10.1 | 2.2 |
| 2010 (172) | 93.0 | 4.1 | 2.9 | 81.4 | 11.6 | 7.0 |
| A. baumannii (1,645) | ||||||
| 2006 (393) | 80.9 | 12.2 | 6.9 | 43.8 | 37.2 | 19.1 |
| 2007 (526) | 69.0 | 22.4 | 8.6 | 30.6 | 38.4 | 31.0 |
| 2008 (350) | 56.6 | 31.4 | 12.0 | 29.4 | 27.1 | 43.4 |
| 2009 (188) | 55.3 | 34.6 | 10.1 | 35.1 | 20.2 | 44.7 |
| 2010 (188) | 73.4 | 20.7 | 5.9 | 39.4 | 34.0 | 26.6 |
NA, MIC interpretive breakpoint for tigecycline was not available.
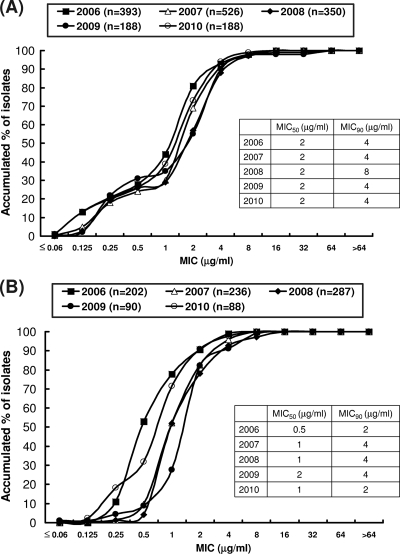
The annual cumulative MIC distributions for A. baumannii and S. maltophilia during the period from 2006 to 2010 are shown in Fig. 1. There were no significant changes in annual MIC50 or MIC90 values against A. baumannii (Fig. 1A). The MIC50 and MIC90 values were 2 and 4 μg/ml, respectively, for S. maltophilia. There was a shift to higher MIC values during the period from 2006 to 2009, but a shift to lower MIC values was found during the period from 2009 to 2010 (Fig. 1B).
Fig 1.
Distributions of tigecycline MIC, MIC50, and MIC90 values among 1,645 isolates of A. baumannii (A) and 903 isolates of S. maltophilia (B) as determined by the broth microdilution method by year from 2006 to 2010.
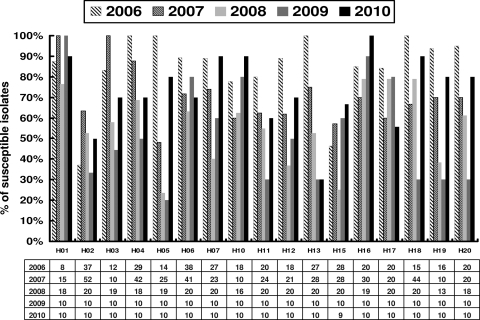
The rate of susceptibility of A. baumannii to tigecycline varied in different hospitals and with the year (Fig. 2). The susceptibility rates were higher in 2010 than in 2009 in 12 hospitals (H02, H03, H04, H05, H07, H10, H11, H12, H16, H18, H19, and H20).
Fig 2.
Rates of susceptibility to tigecycline among A. baumannii isolates from 16 hospitals that continuously participated in the study from 2006 to 2010 in Taiwan. Numbers in the table are the number of A. baumannii isolates studied in each hospital from 2006 to 2010.
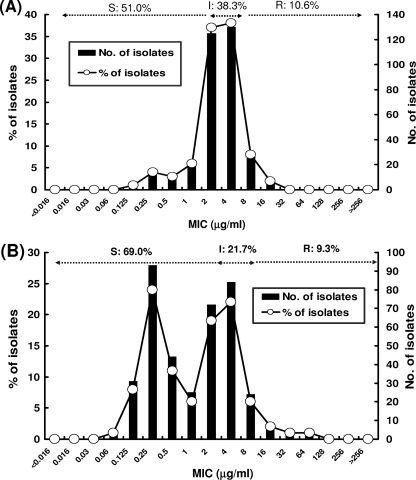
The MIC distributions for A. baumannii isolates collected during 2008 to 2010 are shown in Fig. 3. Among carbapenem-nonsusceptible isolates, a unimodal distribution was found, with a peak at 2 to 4 μg/ml (Fig. 3A), and the rate of susceptibility to tigecycline was 48.9%. Among carbapenem-susceptible isolates, a bimodal MIC distribution with peaks at 0.25 and 2 to 4 μg/ml was found (Fig. 3B), and the rate of susceptibility to tigecycline was 69%.
Fig 3.
Distributions of MICs of tigecycline among 2,339 isolates of carbapenem-nonsusceptible A. baumannii (A) and 387 isolates of carbapenem-susceptible A. baumannii (B) as determined by the agar dilution method and interpreted using tigecycline MIC interpretive breakpoints for Enterobacteriaceae recommended by the FDA (susceptible [S], ≤2 μg/ml; intermediate [I], 4 μg/ml; and resistant [R], ≥8 μg/ml), 2008 to 2010.
DISCUSSION
During the period from 2006 to 2010, there were no significant changes in the tigecycline susceptibility rates for drug-resistant Gram-positive and Gram-negative bacteria, with the exception of A. baumannii, in Taiwan. The drug-resistant Gram-positive bacteria were very susceptible to tigecycline, irrespective of the year of collection. Similar surveillance results were obtained in other countries. In Mexico, for example, all MRSA isolates (n = 482) collected during 2005 to 2009 were susceptible (MIC90, 0.125 μg/ml) (19). In addition, the Tigecycline Evaluation and Surveillance Trial (T.E.S.T.), a global surveillance study, showed that 99.98% of MRSA isolates (n = 8,249) were susceptible to tigecycline (MIC90, 0.25 μg/ml) during 2004 to 2009 (9). In Canada, a prospective nationwide surveillance study (CANWARD 2008) showed that all MRSA isolates (n = 272) were susceptible to tigecycline (21).
In this study, the MIC90 for VRE was low (0.125 μg/ml) and was similar to the MIC90 for VRE reported in the global T.E.S.T. study. In that study, both vancomycin-sensitive and vancomycin-resistant E. faecalis (8,576 and 201 isolates, respectively) showed the same MIC90 values (0.25 μg/ml). The present study also showed that all PRSP isolates were susceptible to tigecycline. However, the global T.E.S.T. study (2004 to 2008) revealed that 86.3% of PRSP isolates in Europe, 87% in Latin America, and 91.2% isolates in North America were susceptible to tigecycline (8). Another global study (2004 to 2009) showed that 87.2% of penicillin-susceptible S. pneumoniae isolates (9,019 isolates), 91.8% of penicillin-intermediate S. pneumoniae isolates (510 isolates), and 91% of penicillin-resistant S. pneumoniae isolates (89 isolates) were susceptible to tigecycline (9).
Among ESBL-producing Enterobacteriaceae, tigecycline still showed potent in vitro activity in our study. The susceptibility rate for ESBL-producing E. coli was more than 98% (99.6% by FDA criteria and 98.7% by EUCAST-2011 criteria). The susceptibility rate for ESBL-producing E. coli was higher than that for ESBL-producing K. pneumoniae, especially when measured using EUCAST-2011 breakpoint criteria. Similarly, in Mexico, 100% of ESBL-producing E. coli isolates and 94% of ESBL-producing K. pneumoniae isolates were susceptible to tigecycline during the period from 2005 to 2009, using the FDA interpretive criteria (19). Furthermore, a multicenter study conducted during the period from 2004 to 2007 in countries of the Asia-Pacific Rim region showed that 100% of ESBL-producing E. coli isolates (MIC90, 0.5 μg/ml) and 93.7% of ESBL-producing K. pneumoniae isolates (MIC90, 2 μg/ml) were susceptible to tigecycline by the FDA interpretive criteria (2). Although carbapenems are recommended for treating infections due to these ESBL-producing bacteria, tigecycline has been shown to be an alternative choice in the treatment of these infections according to data from the above-mentioned surveillance studies.
Data from the Asia-Pacific region in 2008 demonstrated a high rate of susceptibility (99.8%) to tigecycline among Acinetobacter spp., with an MIC90 of 2 μg/ml (10). The global T.E.S.T. study reported that A. baumannii isolates from blood cultures showed significant increases in tigecycline geometric mean MIC values in Europe (from 0.24 μg/ml in 2004 to 0.34 μg/ml in 2007; P = 0.02) and in countries in the Asia-Pacific Rim region (from 0.16 μg/ml in 2004 to 0.40 μg/ml in 2007; P = 0.02) but not in countries in North and South America (20). In addition, the T.E.S.T study also reported that the rates of resistance of isolates from intensive care unit (ICU) patients were higher than those of isolates from non-ICU patients (20).
In our study we found that the MIC distributions of A. baumannii differed among carbapenem-susceptible and carbapenem-nonsusceptible groups. In previous studies, different modes of MIC distribution were also found in different populations; however, those studies did not further analyze the difference according to the susceptibility of carbapenem (14, 20). A bimodal distribution of tigecycline MICs for Acinetobacter spp. was found in North America, with clear peaks at 0.12 μg/ml and 0.5 to 1 μg/ml (14), but a unimodal distribution was found in the European and Asia-Pacific Rim regions, both with a peak at 0.25 μg/ml (20).
Our study also addressed the differences in susceptibility rates obtained using FDA breakpoints and those obtained using EUCAST breakpoints. We found that the tigecycline susceptibility rates for ESBL-producing K. pneumoniae measured using FDA criteria were about 10% higher than those measured using EUCAST breakpoints. Similar differences in susceptibility rates obtained using the two criteria were noted in a study conducted in Greece, namely, that the susceptibility rates for K. pneumoniae were 99% according to FDA criteria and 91% as measured using EUCAST criteria (16). That study also reported a major difference in susceptibility of isolates of Enterobacter spp. (16). In a study conducted during the period from 2005 to 2009 in the United States, the rates of tigecycline susceptibility as measured by FDA and EUCAST criteria differed by 0.6% for ESBL-producing E. coli (100% versus 99.4%; n = 334; P = 0.499) and by 7.3% for K. pneumoniae (97.9% versus 90.6%; n = 426; P < 0.001) (18).
In our study, we found that the susceptibility rates determined by the two criteria for A. baumannii differed by about 30%. Certain resistance mechanisms (such as the overexpression of marA or ramA) in several Enterobacteriaceae species contribute to their increasing resistance to tigecycline (15, 17). These mechanisms might contribute to this difference among A. baumannii isolates; proof of this requires further investigation. Further studies to investigate the correlation between in vitro results and clinical outcomes for patients with various infections due to these isolates are warranted.
In conclusion, continuous surveillance provides invaluable information for local or global health care specialists. Our data reveal that tigecycline continued to have potent antimicrobial effects against most Gram-positive and Gram-negative bacteria, namely, MRSA, VRE, PRSP, and ESBL-producing E. coli, during the period from 2006 to 2010 in Taiwan.
Footnotes
Published ahead of print 27 December 2011
REFERENCES
- 1. Boucher HW, et al. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1–12 [DOI] [PubMed] [Google Scholar]
- 2. Bouchillon SK, Iredell JR, Barkham T, Lee K, Dowzicky MJ. 2009. Comparative in vitro activity of tigecycline and other antimicrobials against Gram-negative and Gram-positive organisms collected from the Asia-Pacific Rim as part of the Tigecycline Evaluation and Surveillance Trial (TEST). Int. J. Antimicrob. Agents 33:130–136 [DOI] [PubMed] [Google Scholar]
- 3. Cai Y, Wang R, Liang B, Bai N, Liu Y. 2011. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob. Agents Chemother. 55:1162–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen YH, et al. 2011. Antimicrobial susceptibility profiles of aerobic and facultative Gram-negative bacilli isolated from patients with intra-abdominal infections in the Asia-Pacific region according to currently established susceptibility interpretive criteria. J. Infect. 62:280–291 [DOI] [PubMed] [Google Scholar]
- 5. Chen WY, Jang TN, Huang CH, Hsueh PR. 2009. In vitro susceptibilities of aerobic and facultative anaerobic Gram-negative bacilli isolated from patients with intra-abdominal infections at a medical center in Taiwan: results of the Study for Monitoring Antimicrobial Resistance Trends (SMART) 2002-2006. J. Microbiol. Immunol. Infect. 42:317–323 [PubMed] [Google Scholar]
- 6. Clinical and Laboratory Standards Institute 2011. Performance standard for antimicrobial susceptibility testing (M100-S20). CLSI, Wayne, PA [Google Scholar]
- 7. Cosgrove SE. 2006. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin. Infect. Dis. 42(Suppl. 2):S82–S89 [DOI] [PubMed] [Google Scholar]
- 8. Darabi A, Hocquet D, Dowzicky MJ. 2010. Antimicrobial activity against Streptococcus pneumoniae and Haemophilus influenzae collected globally between 2004 and 2008 as part of the Tigecycline Evaluation and Surveillance Trial. Diagn. Microbiol. Infect. Dis. 67:78–86 [DOI] [PubMed] [Google Scholar]
- 9. Dowzicky MJ, Chmelarova E. 2011. Global in vitro activity of tigecycline and linezolid against Gram-positive organisms collected between 2004 and 2009. Int. J. Antimicrob. Agents 37:562–566 [DOI] [PubMed] [Google Scholar]
- 10. Farrell DJ, Turnidge JD, Bell J, Sader HSHS, Jones RN. 2010. The in vitro evaluation of tigecycline tested against pathogens isolated in eight countries in the Asia-Western Pacific region (2008). J. Infect. 60:440–451 [DOI] [PubMed] [Google Scholar]
- 11. Hsueh PR. 2008. Tigecycline In-Vitro Surveillance in Taiwan (TIST). Int. J. Antimicrob. Agents 32(Suppl. 3):S173. [DOI] [PubMed] [Google Scholar]
- 12. Jean SS, Hsueh PR. 2011. High burden of antimicrobial resistance in Asia. Int. J. Antimicrob. Agents 37:291–295 [DOI] [PubMed] [Google Scholar]
- 13. Johnson AP. 2011. Methicillin-resistant Staphylococcus aureus: the European landscape. J. Antimicrob. Chemother. 66(Suppl. 4):iv43–iv48 [DOI] [PubMed] [Google Scholar]
- 14. Jones RN, et al. 2007. Multicenter studies of tigecycline disk diffusion susceptibility results for Acinetobacter spp. J. Clin. Microbiol. 45:227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keeney D, Ruzin A, McAleese F, Murphy E, Bradford PA. 2008. MarA-mediated overexpression of the AcrAB efflux pump results in decreased susceptibility to tigecycline in Escherichia coli. J. Antimicrob. Chemother. 61:46–53 [DOI] [PubMed] [Google Scholar]
- 16. Papaparaskevas J, et al. 2010. In vitro activity of tigecycline against 2423 clinical isolates and comparison of the available interpretation breakpoints. Diagn. Microbiol. Infect. Dis. 66:187–194 [DOI] [PubMed] [Google Scholar]
- 17. Ruzin A, Visalli MA, Keeney D, Bradford PA. 2005. Influence of transcriptional activator RamA on expression of multidrug efflux pump AcrAB and tigecycline susceptibility in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 49:1017–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sader HS, Farrell DJ, Jones RN. 2011. Tigecycline activity tested against multidrug-resistant Enterobacteriaceae and Acinetobacter spp. isolated in US medical centers (2005-2009). Diagn. Microbiol. Infect. Dis. 69:223–227 [DOI] [PubMed] [Google Scholar]
- 19. Silva-Sanchez J, et al. 2011. In vitro activity of tigecycline against extended-spectrum beta-lactamase-producing Enterobacteriaceae and MRSA clinical isolates from Mexico: a multicentric study. Diagn. Microbiol. Infect. Dis. 70:270–273 [DOI] [PubMed] [Google Scholar]
- 20. Wang YF, Dowzicky MJ. 2010. In vitro activity of tigecycline and comparators on Acinetobacter spp. isolates collected from patients with bacteremia and MIC change during the Tigecycline Evaluation and Surveillance Trial, 2004 to 2008. Diagn. Microbiol. Infect. Dis. 68:73–79 [DOI] [PubMed] [Google Scholar]
- 21. Zhanel GG, et al. 2010. Prevalence of antimicrobial-resistant pathogens in Canadian hospitals: results of the Canadian Ward Surveillance Study (CANWARD 2008). Antimicrob. Agents Chemother. 54:4684–4693 [DOI] [PMC free article] [PubMed] [Google Scholar]